New experimental evidence to
support roaming in the reaction
Cl
+ isobutene (i-C
4
H
8
)
Li-Wei Chen
1, Ching-Ming Hung
1, Hiroyuki Matsui
1& Yuan-Pern Lee
1,2The reaction Cl + isobutene (i-C4H8) was reported by Suits et al. to proceed via, in addition to
abstraction, an addition-elimination path following a roaming excursion of Cl; a near-zero translational energy release and an isotropic angular distribution observed at a small collision energy characterized this mechanism. We employed a new experimental method to further characterize this roaming mechanism through observation of the internal distribution of HCl (v, J) and their temporal behavior upon irradiation of a mixture of Cl2C2O2 and i-C4H8 in He or Ar buffer gas. With 1–3 Torr buffer gas added
to approach the condition of small collision energy, the intensities of emission of HCl (v = 1, 2) and the HCl production rates increased significantly; Ar shows a more significant effect than He because Ar quenches Cl more efficiently to reduce the collisional energy and facilitate the roaming path. According to kinetic modeling, the rate of addition-elimination (roaming) increased from kE ≈ 2 × 105 s−1 when
little buffer gas was present to ~1.9 × 106 s−1 when 2–3 Torr of Ar was added, and the branching ratio
for formation of [HCl (v = 2)]/[HCl (v = 1)] increased from 0.02 ± 0.01 for abstraction to 0.06 ± 0.01 for roaming.
The reactions of atomic chlorine (Cl) with alkenes (CnH2n) play important roles in the chemistry of the tropo-sphere1–5. These reactions are also of fundamental importance in understanding the reaction kinetics or dynam-ics that involve a competition between various paths and a site selectivity. The two most significant channels of the primary Cl + CnH2n reactions are the addition of a Cl atom to the C= C double bond to form a thermally stabilized adduct, chloroalkyl radical (•CnH2nCl), and the metathesis reaction to form HCl and an alkyl radical (•CnH2n−1); the metathesis includes a direct abstraction of a H atom of the alkene by the Cl atom and an elimina-tion of HCl from the energetic adduct •CnH2nCl6. It is generally accepted that the abstraction dominates at low pressure, whereas the addition-stabilization mechanism becomes more important at high pressure6–8.
Employing crossed molecular beams to investigate the dynamics of the reaction Cl + i-C4H8, Suits and cow-orkers detected C4H7 with a slice ion-imaging method9,10. These authors found that the addition-elimination path occurs from an abstraction-like Cl-H-C geometry rather than a conventional three-center or four-center tran-sition state, and this geometry is attained through roaming excursions of the Cl atom from the initially formed adduct. The experimental observations to support this roaming mechanism are limited: a release of translational energy with a maximal distribution near zero energy and a fully isotropic angular distribution of C4H7 product were observed when a small collisional energy was employed.
Preston et al. employed velocity-map imaging to measure the quantum states and velocity of HCl pro-duced from reactions Cl + propene, i-C4H8, and dimethylbutene; signals from both direct abstraction and addition-elimination were observed11. These authors simulated trajectories that indicated the importance of a large-amplitude excursion of the Cl atom far from equilibrium geometry within the chloroalkyl complex, which ultimately led to formation of HCl + allyl fragmentation12, but a clear distinction in the internal energy distribu-tion of HCl produced via abstracdistribu-tion from that via addidistribu-tion-eliminadistribu-tion of Cl + i-C4H8 was unavailable.
Roaming dynamics that involve reactions of a radical (or an atom) with a radical, resulting typically from photofragmentation of a suitable precursor, are now widely recognized as an important path in unimolecular decompositions13–17, but their roles in radical (or an atom) - molecule reactions remain less certain. Molecular dynamics simulations of the unimolecular dissociation of energetic C2H4OH radicals reveal a minor roaming channel of OH + C2H4 that leads to formation of H2O and C2H318. Roaming was observed in photolysis of NO3 1Department of Applied Chemistry and Institute of Molecular Science, National Chiao Tung University, 1001 Ta-Hsueh Rd., Hsinchu 30010, Taiwan. 2Institute of Atomic and Molecular Sciences, Academia Sinica, Taipei 10617, Taiwan. Correspondence and requests for materials should be addressed to Y.-P.L. (email: yplee@mail.nctu.edu.tw) received: 20 October 2016
accepted: 30 November 2016 Published: 12 January 2017
www.nature.com/scientificreports/
via both ground- and excited-state surfaces to form NO + O2 and supported by theoretical calculations19–22. Both these reactions are initiated from photodissociation of free radicals; reported roaming reaction initiated from reactions of radical (atom) and molecule is rare.
In the roaming mechanism of Cl + i-C4H8, because the reaction to form HCl + C4H7 has a small exother-micity (~68 kJ mol−1) and because the addition-elimination path occurs at the abstraction-like near-linear Cl− H− C geometry, to distinguish the internal distributions of HCl produced via abstraction from that via the addition-roaming-elimination path is difficult. In this work we demonstrate a new experimental method to characterize the roaming path in the atom-molecule reaction Cl + i-C4H8 → HCl + C4H7 by using a step-scan Fourier-transform infrared (FTIR) spectrometer to obtain time-resolved IR emission spectra of HCl23–25.
Results and Discussion
Emission spectra of HCl.
According to the results of Joalland et al.9,10, roaming of Cl + i-C4H8 was most prominent at a small collisional energy (~17 kJ mol−1). Because the Cl atoms produced upon photolysis still have significant kinetic energy, collisional quenching to a nearly thermal distribution of kinetic energy is expected to enhance the roaming path. We hence compared the effect of adding He or Ar up to a total pressure of 3 Torr to quench the kinetic energy of Cl atoms after photolysis.
Similar experiments with the same amount of Cl2C2O2 (10–11 mTorr) and laser fluence were performed for three sets of data; the conditions are listed in Table 1. Set A was for investigation of the dependence on the concen-tration of i-C4H8 with little buffer gas, set B was for investigation of the effect of adding He as a quencher, and set C was for investigation of the effect of adding Ar as a quencher. The absorption cross section of Cl2C2O2 at 248 nm is ~3.1 × 10−19 cm2 molecule−126. Typically, ~13% of Cl
2C2O2 was photodissociated with a fluence of 343 mJ cm−2. Emission spectra of HCl at resolution 0.7 cm−1 recorded 0–5 μ s after photolysis of Cl
2C2O2 (~11 mTorr),
i-C4H8 (~220 mTorr), and Ar (0.010 and 2.99 Torr) are presented in Fig. 1(a,b), respectively; lines of H35Cl and H37Cl are well resolved. The vibration-rotational assignments of each line based on spectral parameters reported by Arunan et al.27 and Coxon and Roychowdhury28 are shown as sticks for transitions of v′ = 1, J′ ≤ 13 and v′ = 2,
J′ ≤ 10. At ~3.2 Torr, formation of the HCl (v = 2) was enhanced slightly, as shown in Fig. 1(b). For comparison,
Preston et al. observed v′ = 1, J′ ≤ 7 and v′ = 2, J′ = 1 in their jet experiments using velocity-map ion imaging11. The energetics, discussed in Supplementary Sec. A, indicate that the available energy for formation of HCl + C4H7 is ~81 kJ mol−1 (6770 cm−1) when Cl atoms are thermalized by collisions with Ar or He; this energy is close to the energy of HCl (v = 2, J = 10) at 6743 cm−1.
Rotational temperature.
The rotational distributions of HCl are Boltzmann, as shown in Supplementary Fig. S1 for spectra recorded 0–1 μ s after photolysis. Derivation of nascent rotational temperature is discussed in Supplementary Sec. B. Nascent rotational temperatures and average rotational energies observed in three sets of experiments are listed in Supplementary Table S1. In all cases, the rotational temperatures are similar (deviations within 15%) and decrease slightly from ~370 K to ~340 K as the pressure increases to ~3.2 Torr; detailed data are in Supplementary Table S1. The average rotational energy is hence ~3 kJ mol−1, independent of pressure. The small rotational energy indicates that, if roaming occurs more significantly at greater pressure, the HCl product generated from this path has rotational excitation similar to that from abstraction. This condition is consist-ent with a prediction that the roaming (addition-elimination) path occurs from the abstraction-like near-linear Cl-H-C geometry9.Vibrational excitation and relative intensity of HCl.
Because only HCl (v = 1) and HCl (v = 2) were observed, to determine accurately the vibrational temperature and energy is difficult. We list Pv=2/Pv=1 and the relative intensity y of HCl (v = 1 and 2) in various experiments in Table 1; the relative intensity wasSet
Conditionsa Experiment Kinetic fit of HCl (v = 1) and HCl (v = 2) Pi-C4H8/Torr PM/Torr yb
[HCl(v = 2)]/
[HCl(v = 1)] kE/105 s−1 krev/105 s−1 kq/105 s−1 φ2/φ1 (abs.) ε2/ε1 (elim.)
A 0.213 0.010 (Ar) 0.06 0.015 ± 0.004 0.5 19.8 0.6 0.00 (0.02)c 0.23 0.482 0.010 (Ar) 0.20 0.024 ± 0.004 2.4 43.5 1.0 0.05 (0.03) 0.00 0.687 0.011 (Ar) 0.17 0.023 ± 0.005 2.1 63.4 1.5 0.01 (0.03) 0.20 0.914 0.011 (Ar) 0.16 0.026 ± 0.007 1.5 85.6 2.1 0.05 (0.04) 0.00 B 0.224 0.940 (He) 0.57 0.035 ± 0.002 9.0 11.4 1.0 0.04 (0.02) 0.04 0.225 1.910 (He) 0.47 0.034 ± 0.002 8.8 10.9 1.1 0.00 (0.02) 0.06 0.226 2.990 (He) 0.37 0.031 ± 0.002 7.8 10.9 1.2 0.00 (0.01) 0.06 C 0.224 0.940 (Ar) 0.75 0.035 ± 0.002 10.9 9.5 1.2 0.00 (0.00) 0.05 0.225 1.910 (Ar) 0.72 0.038 ± 0.001 19.6 0.0 2.1 0.00 (0.00) 0.05 0.226 2.990 (Ar) 1.00 0.045 ± 0.001 18.7 0.0 3.1 0.01 (0.05) 0.07
Table 1. Experimental conditions, relative intensities y of HCl, observed ratios of [HCl(v = 2)]/
[HCl(v = 1)], fitted rate coefficients of elimination kE and their associated krev, rate coefficient of quenching
kq, branching ratios for formation of HCl (v = 2) over HCl (v = 1) φ2/φ1 (from abstraction) and ε2/ε1
(from elimination) in three sets of experiments. aPartial pressure of Cl
2C2O2 is 10–11 mTorr. bIntensity of HCl relative to that from the experiment with PAr = 2.99 Torr. cFitted results with ε2/ε1 fixed at 0.05 are listed in parentheses.
compared to the results of PT = 3.23 Torr (Ar) in set C. When little buffer gas was present, [HCl (v = 2)]/[HCl (v = 1)] = 0.015 ± 0.004, whereas when ~3 Torr of Ar was added, [HCl (v = 2)]/[HCl (v = 1)] = 0.045 ± 0.001. Because of the excellent ratio of signal to noise in the spectra, the error in the determination of relative popula-tion was estimated to be less than 25% of the value when the signal is small; the difference in these ratios is hence significant, as can also be seen from the consistency in ratios determined in various experiments (Table 1). The small difference is partly because the abstraction path still contributes when the buffer gas was added and partly because the difference in [HCl (v = 2)]/[HCl (v = 1)] between abstraction and addition-elimination is not so large. Nevertheless, the vibrational excitation of HCl was clearly enhanced when the buffer gas was added.
Because we could not measure the population of HCl (v = 0), these relative intensities consequently provide only a rough estimate of the extent of HCl produced. Nevertheless, it is clear from Table 1 that the intensity of (vibrationally excited) HCl was enhanced by as much as 16 times when a buffer gas was added, and adding Ar showed a greater enhancement than adding He.
Rate coefficient derived from kinetic fitting of vibrational temporal profiles.
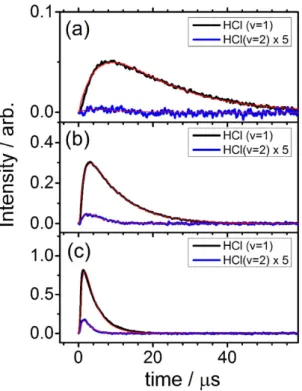
Because the rota-tional temperature of HCl is near 360 K and independent of pressure, the rotarota-tional temporal profile provides little information about the kinetics of the reaction. We thus analyzed only the vibrational temporal profiles; some representative plots in experimental set A for PT = 0.23 Torr, set B for PT = 3.23 Torr (He), and set C forPT = 3.23 Torr (Ar) are shown in Fig. 2. Temporal profiles for other experiments in sets A–C are presented in Supplementary Figs S2–S4, respectively.
We consider the reaction mechanism depicted in Fig. 3. The reaction of Cl + i-C4H8 forms energetic adduct ClC4H8* with a rate coefficient kfor; ClC4H8* might be stabilized by collision with the third-body M (rate coeffi-cient kM [M]), dissociate back to the reactants (rate coefficient krev), or eliminate HCl via roaming-elimination (rate coefficient kE). The abstraction and elimination channels might produce HCl with distinct vibrational dis-tributions. The abstraction reaction (rate coefficient kabs) has three channels to produce HCl (v = 0), HCl (v = 1), and HCl (v = 2) with branching ratios φ0, φ1, and φ2, respectively. The elimination reaction has, similarly, three channels with branching ratios ε0, ε1, and ε2 for production of HCl (v = 0–2), respectively. The decay rate coeffi-cients kq (v) are also separated for each vibrational level of HCl and include both quenching and loss. The solution of [HCl(t)] contains three exponential terms, but they are complex functions of rate coefficients, as discussed in Supplementary Sec. C. Hence, instead of fitting the temporal profiles to three exponential terms, we simulated the temporal profile with given rate coefficients and compare with experimental results. We systematically varied the rate coefficients using mathematical tools to derive the best fit between the experimental data and the simulated temporal profiles with the least deviations.
Because eight rate coefficients are involved in this mechanism, it is unlikely to derive a unique fit of these parameters from the temporal profiles. We thus fixed some well-known rate coefficients and varied only kE and
krev, and the vibrational branching ratios of abstraction (φ2/φ1) and elimination (ε2/ε1) reactions. The total rate coefficient kT for reaction Cl + i-C4H8 at 1 bar (air) was determined to be kT = (3.40± 0.28)× 10−10 cm3 molecule−1 s−1 by Ezell et al.29. Following the empirical additivity rules based on data of reactions of Cl with alkanes and alkenes, these authors proposed that kabs = 6.8× 10−11 cm3 molecule−1 s−1 for the formation of HCl and kadd = 2.7× 10−10 cm3 molecule−1 s−1 for the formation of stabilized ClC
4H8; the value of kabs should be kmeta, rate coefficient Figure 1. Emission spectra of HCl in spectral region 2500–3200 cm−1 recorded 0–5 μs after photolysis at
248 nm. (a) A flowing mixture of Cl2C2O2 (10 mTorr), i-C4H8 (213 mTorr) and Ar (10 mTorr). (b) A flowing mixture of Cl2C2O2 (11 mTorr), i-C4H8 (226 mTorr) and Ar (2.99 Torr). Spectral resolution is 0.7 cm−1. The assignments are shown as sticks; numbers indicate rotational quantum number J′ .
www.nature.com/scientificreports/
of metathesis, that includes abstraction and addition-elimination. Considering that abstraction reactions occur only on the methyl groups of propene and isobutene, one estimates kabs = 4.6× 10−11 cm3 molecule−1 s−1 because
i-C4H8 has two methyl moieties and kabs was determined to be 2.3× 10−11 cm3 molecule−1 s−1 for Cl + propene7. The value of kfor for the reaction Cl + i-C4H8 can be taken as the difference between total rate coefficient kT and
kabs, that is, kfor = 2.94× 10−10 cm3 molecule−1 s−1, which corresponds satisfactorily with the value for Cl + pro-pene, k∞ = (2.7 ± 0.4)× 10−10 cm3 molecule−1 s−1. The rate coefficient at the low-pressure limit for Cl + i-C4H8 is unreported, but the corresponding value for Cl + propene is k0 = (4.0 ± 0.4)× 10−28 cm6 molecule−2 s−17. If we assume that the termolecular reaction Cl + i-C4H8 + M to form ClC4H8 is still in the linear regime for PT ≤ 3 Torr, and use k0 of the reaction of Cl + propene for Cl + i-C4H8, kM = (4.0 ± 0.4)× 10−28 [i-C4H8] [M] cm6 moleule−2 s−1.
Because HCl (v = 0) is unobserved in emission, we fit only the temporal profiles of HCl (v = 1) and HCl (v = 2) simultaneously on varying kq, φ2/φ1, kE, and ε2/ε1 to derive the best fit between the experimental data and the sim-ulated temporal profiles. In these fits, kabs = 4.6× 10−11 cm3 molecule−1 s−1, kfor = 2.9× 10−10 cm3 molecule−1 s−1,
kM = 4.0× 10−28 [i-C4H8] [M] cm6 molecule−2 s−1 were fixed, and krev + kM + kE = kfor [i-C4H8]. The representative fitted temporal profiles of HCl (v) for experiments in set A (0.22 Torr), set B (3.23 Torr), and set C (3.23 Torr, Ar) are shown in Fig. 2; additional profiles are shown in Supplementary Figs S2–S4. We plot kE as a function of density [M] of i-C4H8 or He or Ar in Fig. 4 for experimental sets A–C. Fitted results of kE, kq, φ2/φ1, and ε2/ε1 are listed in Table 1.
Figure 2. Comparison of observed temporal profiles of HCl (v) with simulations. (a) A flowing mixture of Cl2C2O2 (10 mTorr), i-C4H8 (213 mTorr) and Ar (10 mTorr). (b) A flowing mixture of Cl2C2O2 (10 mTorr),
i-C4H8 (226 mTorr) and He (2.99 Torr). (c) A flowing mixture of Cl2C2O2 (10 mTorr), i-C4H8 (226 mTorr) and Ar (2.99 Torr). HCl (v = 1) is in black and HCl (v = 2), multiplied by a factor of 5, is in blue. The kinetic simulations are in red.
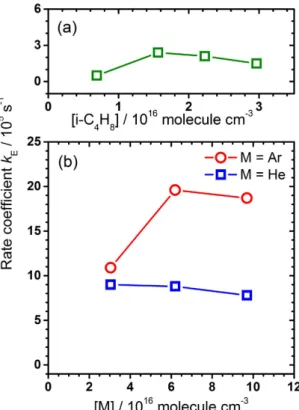
For experiments in set A with varying i-C4H8 and little buffer gas, kE remains small and is nearly constant with kE = (1.6 ± 0.8) × 105 s−1 (Fig. 4(a)). For He as a buffer gas, kE is much greater than those without buffer gas and remains nearly constant with kE = (8.5 ± 0.7) × 105 s−1 for [He] = (3.0–9.7) × 1016 molecule cm−3, whereas kE increases from 1.1 × 105 s−1 at [Ar] = 3.0 × 1016 molecule cm−3 to (1.9 ± 0.1) × 106 s−1 at [Ar] = (6.2–9.7) × 1016 molecule cm−3 (Fig. 4(b)). Such a significant increase in k
E for experiments with an added quencher, especially Ar, can be explained only with an enhanced addition-elimination channel induced by the collisional quenching of the kinetic energy of Cl; because the mass of Ar is similar to that of Cl, Ar is a much more efficient quencher than He for kinetic energy of Cl.
Detailed sensitivity and error analysis are available in Supplementary Sec. D. The deviations derived in these analysis are much smaller than the enhancement of kE observed in experimental sets B and C as compared to those in experiments with little buffer gas.
For experimental sets B and C (in which roaming is more important), ε2/ε1 are consistent with average (5.5 ± 0.9) %. We thus fixed ε2/ε1 = 0.06 to fit the data once more; a much more consistent value of φ2/φ1 with average (3.0 ± 0.7) % for set A (in which abstraction is more important) was derived, as shown in parentheses in Table 1 (other fitted parameters are listed in Supplementary Table S2). The φ2/φ1 value of 0.02 when little buffer gas was added is the smallest and is taken as the φ2/φ1 value for abstraction. This value is consistent with the average ratio of [HCl(v = 2)]/[HCl(v = 1)] = 0.022 in experimental set A derived from integrated intensities. The ratio of ε2/ε1 = 0.06 ± 0.01 for roaming, derived from kinetic fitting, is greater than values [HCl(v = 2)]/ [HCl(v = 1)] = 0.035–0.045 in experimental set C because the latter values include contributions from both abstraction and roaming.
These results indicate that the roaming path generates more vibrationally excited HCl than the abstraction path, consistent with the expectation according to consideration of the structures of the transition states, because the roaming transition state has an H− Cl distance of 2.37 Å9, much greater than the H− Cl distance of 1.59 Å for the transition state of H-abstraction30 and the equilibrium distance 1.275 Å of HCl. In the case of photolysis of acetaldehyde, the roaming transition state has a C− H distance of 1.722 Å, much greater than the equilibrium C− H distance of 1.093 Å of CH431. The CH4 product after roaming of CH3 around HCO was found to have extreme significant vibrational excitation, with the vibrational distribution peaked at ~95% of the total available energy32. For the reaction of Cl + i-C
4H8, the average available energy for formation of HCl + C4H7 is ~81 kJ mol−1 (6770 cm−1) when Cl atoms are thermalized by collisions with Ar or He. This energy can populate HCl only up to
v = 2, J = 10, so the extent of vibrational excitation is not as great as that of the roaming of CH3 + HCO.
This competition between abstraction and addition-elimination via a long-lived complex is similar to the early work on H + ICl by Polanyi et al.33. In their 3D trajectory studies, they reported that the HCl formed with a small internal energy resulted from reaction of H directly at the Cl-end of ICl, whereas the HCl formed with high internal energy was produced by migration of H from the I-site to the Cl-site, following a lingering interaction of H with I.
Figure 4. Fitted kE in three sets of experiments. (a) A flowing mixture of Cl2C2O2 (10 mTorr), Ar (10 mTorr), and i-C4H8 (213–914 mTorr). (b) A flowing mixture of Cl2C2O2 (10 mTorr), i-C4H8 (226 mTorr) and buffer gas He (0.94–2.99 Torr, symbol □ ) or Ar (0.94–2.99 Torr, symbol ○ ).
www.nature.com/scientificreports/
Conclusion
In summary, experimental evidence of three types supports the involvement of the roaming mechanism that is expected to be enhanced at small collisional energy as more buffer gas is added to thermalize the kinetic energy of Cl after photolysis; Ar is expected to be more effective than He in quenching the kinetic energy of Cl. The evi-dence follows. (1) The intensity of HCl (v = 1 and 2) was enhanced by as much as sixteen times when buffer gas at 1–3 Torr was added, more so when the same amount of Ar than when He was added. (2) The observed temporal profiles indicate a significantly increased rate for the formation of HCl when the buffer gas was added; Ar was more effective than He. According to the kinetic modeling, the rate of addition-elimination (roaming) increased from kE ≈ 2 × 105 s−1 when little buffer gas was present to ~8.5 × 105 s−1 when 1–3 Torr of He was added, and ~1.9 × 106 s−1 when 2–3 Torr of Ar was added. (3) Ratio [HCl (v = 2)]/[HCl (v = 1)] increased when Ar (1–3 Torr) was added relative to when little buffer gas was present. According to the kinetic modeling, we derived a branch-ing ratio ε2/ε1 = 0.06 ± 0.01 from roaming and φ2/φ1 = 0.02 ± 0.01 from abstraction. This result is consistent with an expectation that HCl produced from the roaming mechanism to have greater vibrational excitation, even though the excitation is limited by the small exothermicity.
The rotational temperature of HCl near 360 K shows no significant variation under varied pressure, indicating that the rotational excitation of HCl from abstraction and roaming is similarly small, consistent with the proposal that roaming (addition-elimination) occurs from the abstraction-like linear Cl-H-C geometry.
Methods
The step-scan Fourier-transform infrared (FTIR) spectrometer coupled with a set of Welsh mirrors to obtain time-resolved IR emission spectra has been described23–25. A gaseous flowing mixture of isobutene and oxalyl chloride (Cl2C2O2) was irradiated with an excimer laser at 248 nm for production of Cl to initiate the reaction of Cl + i-C4H8. We used Cl2C2O2 instead of Cl2 as a source of Cl atoms because the secondary reactions of C4H7 with Cl2 might interfere.
The sizes of the photolysis beams at the detection center were ~11.0 × 5.7 mm2 with a fluence 345− 380 mJ cm−2 from a KrF laser (Coherent, COMPexPro-50) at 248 nm. The transient signal detected with an InSb detector (rise time 0.22 μ s) was further amplified 20‒ 160 times (bandwidth 1 MHz) before being digitized and recorded with an external data-acquisition board (12-bit) at resolution 25 ns. For survey spectra, data were typically averaged over 60 laser pulses at each scan step; 1332 scan steps were performed to yield an interferogram resulting in a spectrum in a region 1800–7800 cm−1 at resolution 12 cm−1. To detect emission of HCl, we used undersampling with two IR filters (Spectrogon SP-4300 and OCLI W03999-4) to allow passage of light in the region 2350− 3250 cm−1. Data were typically averaged over 30 laser pulses at each scan step; 3578 scan steps were performed to yield an interferogram resulting in a spectrum of resolution 0.7 cm−1. To improve the ratio of signal to noise (S/N) of the spectra of HCl, four spectra recorded under nearly the same experimental conditions were averaged. To improve further the S/N ratio, n consecutive time-resolved spectra were summed to yield spectra representing emission at intervals of n × 25 ns; typically n = 40 and spectra at 1-μ s intervals were used.
Samples of Cl2C2O2 and Ar (or He) were injected into the vacuum chamber as a diffusive beam through a slit-shaped inlet. The i-C4H8 sample has vapor pressure ~340 Torr at 298 K. Additional He or Ar in a minimal pressure (~10 mTorr) was added near the entrance of the photolysis port to suppress the formation of a solid deposit on the quartz window. The partial pressures of each species were calculated by the flow rates of each spe-cies, the total flow rate, and the total pressure.
Cl2C2O2 (> 98%, Lancaster) and i-C4H8 (99%, Sigma-Aldrich) were purified using the freeze-pump-thaw method. Ar (Specialty Gases of America, 99.9995%) and He (Specialty Gases of America, 99.9995%) were used as received.
References
1. Finlayson-Pitts, B. J. & Pitts, J. N. Chemistry of the Upper and Lower Atmosphere—Theory, Experiments, and Applications (Academic Press, San Diego, CA, USA, 2000).
2. Singh, H. B. & Kasting, J. F. Chlorine-hydrocarbon photochemistry in the marine troposphere and lower stratosphere. J. Atmos.
Chem. 7, 261–285 (1988).
3. Walavalkar, M. et al. Cl atom initiated oxidation of 1-alkenes under atmospheric conditions. Atoms. Environ. 67, 93–100 (2013). 4. Timerghazin, Q. K. & Ariya, P. A. Kinetics of the gas phase reactions of atomic chlorine with selected monoterpenes. Phys. Chem.
Chem. Phys. 3, 3981–3986 (2001).
5. Atkinson, R. & Arey, J. Gas-phase tropospheric chemistry of biogenic volatile organic compounds: a review. Atmos. Environ. 37, 197–219 (2003).
6. Taatjes, C. A. Time-resolved infrared absorption measurements of product formation in Cl atom reactions with alkenes and alkynes.
Int. Rev. Phys. Chem. 18, 419–458 (1999).
7. Kaiser, E. W. & Wallington, T. J. Pressure dependence of the reaction Cl + C3H6. J. Phys. Chem. 100, 9788–9793 (1996).
8. Pilgrim, J. S. & Taatjes, C. A. Infrared absorption probing of the Cl + C3H6 reaction: rate coefficients for HCl production between
290 and 800 K. J. Phys. Chem. A 101, 5776–5782 (1997).
9. Joalland, B., Shi, Y., Kamasah, A., Suits, A. G. & Mebel, A. M. Roaming dynamics in radical addition–elimination reactions. Nat.
Commun. 5, 4064 (2014).
10. Joalland, B., Camp, R. V., Shi, Y., Patel, N. & Suits, A. G. Crossed-beam slice imaging of Cl reaction dynamics with butene isomers.
J. Phys. Chem. A 117, 7589–7594 (2013).
11. Preston, T. J., Dunning, G. T., Orr-Ewing, A. J. & Vázquez, S. A. Direct and indirect hydrogen abstraction in Cl + alkene reactions. J.
Phys. Chem. A 118, 5595–5607 (2014).
12. Hornung, B., Preston, T. J., Pandit, S., Harvey, J. N. & Orr-Ewing, A. J. Computational study of competition between direct abstraction and addition-elimination in the reaction of Cl atoms with propene. J. Phys. Chem. A 119, 9452–9464 (2015).
13. Goncharov, V., Herath, N. & Suits, A. G. Roaming dynamics in acetone dissociation. J. Phys. Chem. A 112, 9423–9428 (2008). 14. Suits, A. G. Roaming atoms and radicals: a new mechanism in molecular dissociation. Acc. Chem. Res. 41, 873–881 (2008). 15. Hause, M. L., Herath, N., Zhu, R., Lin, M. & Suits, A. G. Roaming-mediated isomerization in the photodissociation of nitrobenzene.
Nat. Chem. 3, 932–937 (2011).
17. Bowman, J. M. Roaming. Mol. Phys. 112, 2516–2528 (2014).
18. Kamarchik, E., Koziol, L., Reisler, H., Bowman, J. M. & Krylov A. I. Roaming pathway leading to unexpected water + vinyl products in C2H4OH dissociation. J. Phys. Chem. Lett. 1, 3058–3065 (2010).
19. Grubb, M. P., Warter, M. L., Suits, A. G.& North, S. W. Evidence of roaming dynamics and multiple channels for molecular elimination in NO3 photolysis. J. Phys. Chem. Lett. 1, 2455–2458 (2010).
20. Grubb, M. P. et al. No straight path: roaming in both ground- and excited-state photolytic channels of NO3 → NO + O2. Science 335,
1075–1078 (2012).
21. Grubb, M. P., Warter, M. L., Johnson, K. M. & North, S. W. Ion imaging study of NO3 radical photodissociation dynamics:
characterization of multiple reaction pathways. J. Phys. Chem. A 115, 3218–3226 (2011).
22. Fernando, R. et al. Visible/Infrared dissociation of NO3: roaming in the dark or roaming on the ground. J. Phys. Chem. A 119,
7163–7168 (2015).
23. Lin, S.-R. & Lee, Y.-P. Photodissociation of 1,1-Difluoroethene(CH2CF2) at 193 nm monitored with step-scan time-resolved
Fourier-transform infrared emission spectroscopy. J. Chem. Phys. 111, 9233–9241 (1999).
24. Wu, C.-Y., Lee, Y.-P., Ogilvie, J. F. & Wang, N. S. Photolysis of oxalyl chloride (ClCO)2 at 248 nm: emission of CO(v′ ≤ 3, J′ ≤ 51)
detected with time-resolved Fourier-transform spectroscopy. J. Phys. Chem. A 107, 2389–2393 (2003).
25. Lee, P.-W., Scrape, P. G., Butler, L. J. & Lee, Y.-P. Two HCl-elimination channels and two CO-formation channels detected with time-resolved infrared emission upon photolysis of acryloyl chloride [CH2CHC(O)Cl] at 193 nm. J. Phys. Chem. A 119, 7293–7304
(2015).
26. Ghosh, B., Papanastasiou, D. K. & Burkholder, J. B. Oxalyl chloride, ClC(O)C(O)Cl: UV/VIS spectrum and Cl atom photolysis quantum yields at 193, 248, and 351 nm. J. Chem. Phys. 137, 164315 (2012).
27. Arunan, E., Setser, D. W. & Ogilvie, J. F. Vibration-rotational Einstein coefficients for HF/DF and HCl/DCl. J. Chem. Phys. 97, 1734–1741 (1992).
28. Coxon, J. A. & Roychowdhury, U. K. Rotational analysis of the B1Σ + → X1Σ + system of H35Cl. Can. J. Phys. 63, 1485–1497 (1985).
29. Ezell, M. J., Wang, W., Ezell, A. A., Soskin, G. & Finlayson-Pitts, B. J. Kinetics of reactions of chlorine atoms with a series of alkenes at 1 atm and 298 K: structure and reactivity. Phys. Chem. Chem. Phys. 4, 5813–5820 (2002).
30. Chu, G. et al. Investigation on addition and abstraction channels in Cl reactions with 1-butene and isobutene. Int. J. Mass Spectrom. 375, 1–8 (2015).
31. Houston, P. L. & Kable, S. H. Photodissociation of acetaldehyde as a second example of the roaming mechanism. Proc. Nat. Acad. Sci. 103, 16079–16082 (2006).
32. Heazlewood, B. R. et al. Roaming is the dominant mechanism for molecular products in acetaldehyde photodissociation. Proc. Nat.
Acad. Sci. 105, 12719–12724 (2008).
33. Polanyi, J. C., Schreiber, J. L. & Skrlac, W. J. Distribution of reaction products (theory) Part 12.− microscopic branching in H + XY → HX + Y, HY + X (X, Y = halogens). Faraday Discuss. Chem. Soc. 67, 66–89 (1979).
Acknowledgements
Ministry of Science and Technology of Taiwan (grant MOST105-2745-M-009-001-ASP) and Ministry of Education of Taiwan (“Aim for the Top University Plan” of National Chiao Tung University) supported this work. The National Center for High-performance Computing provided computer time.
Author Contributions
L.-W.C. wrote the program and performed the kinetic analysis. C.-M.H. performed the experiments and analyzed the spectra. H.M. conceived and designed the kinetic analysis. Y.-P.L. conceived and designed the experiments and wrote the paper.
Additional Information
Supplementary information accompanies this paper at http://www.nature.com/srep Competing financial interests: The authors declare no competing financial interests.
How to cite this article: Chen, L.-W. et al. New experimental evidence to support roaming in the reaction Cl + isobutene ( i -C4 H 8 ). Sci. Rep. 7, 40105; doi: 10.1038/srep40105 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
![Table 1. Experimental conditions, relative intensities y of HCl, observed ratios of [HCl(v = 2)]/](https://thumb-ap.123doks.com/thumbv2/9libinfo/7689555.143115/2.892.227.832.69.294/table-experimental-conditions-relative-intensities-hcl-observed-ratios.webp)