Three-Dimensional Culture Systems
Po-Wei Lin,1Chi-Chen Wu,2Chien-Ho Chen,3 Hsiu-O Ho,4Yi-Chun Chen,5Ming-Thau Sheu4,5 1Taipei Municipal Wan Fang Hospital, Taipei, Taiwan, Republic of China
2Department of Anesthesiology, Taipei Medical University Hospital, and Department of Anesthesiology, School of Medicine, Taipei Medical University, Taipei, Taiwan, Republic of China
3Department of Medical Technology, Taipei Medical University, Taipei, Taiwan, Republic of China
4Graduate Institute of Pharmaceutical Sciences, Taipei Medical University, Taipei, Taiwan, Republic of China 5Graduate Institute of Biomedical Materials, Taipei Medical University, 250 Wu-Hsing Street,
Taipei, Taiwan 110, Republic of China
Received 26 July 2004; revised 11 October 2004; accepted 14 December 2004
Published online 6 July 2005 in Wiley InterScience (www.interscience.wiley.com). DOI: 10.1002/jbm.b.30276
Abstract: To improve the ability of regeneration by grafting living cells or by adding growth factor to a lesion site, it is important to find good biomaterials for neuron survival and regeneration. This study focused on two- and three-dimensional cultures in a matrix using biomaterials such as agarose, collagen, fibrin, and their mixtures, because these are considered to be suitable biomaterials for neuron outgrowth. Cortical neurons were dissected from E17 rat embryos and cultured in agarose gel, collagen gel, fibrin glue, and mixtures of collagen and fibrin. Results showed that neurons cultured in collagen gel and fibrin glue had longer periods of survival (more than 3 weeks) and better neurite extension than those observed in agarose gels. As to the survival rate according to the MTT and lactate dehydrogenase assays, fibrin glue was the most suitable biomaterial for neuron survival among the biomaterials examined. With two-dimensional fibrin plating, neuron cells exhibited cell aggregation and stress fibers, but the same results were not observed with collagen gel. There were no differences in neurite extension and survival in the mixtures of collagen and fibrin. The results suggest that collagen and fibrin can provide a suitable substrate for a three-dimensional culture matrix for neuronal survival and differentiation.© 2005 Wiley Periodicals, Inc. J Biomed Mater Res Part B: Appl Biomater 75B: 146 –157, 2005
Keywords: biomaterial; three-dimensional culture model; collagen; fibrin
INTRODUCTION
Nerve regeneration is a determinant repair process for restor-ing motor and sensory functions after such trauma as spinal cord injury, peripheral nerve destruction, and extensive burns. During normal nerve regeneration, regrowth of neu-rites is stimulated by the extracellular matrix (ECM), neuro-trophic factors, cell-to-cell contact, and spatial cues.1 The ECM is well known for its ability to regulate and organize axonal growth during development and regeneration.2 Mole-cules such as laminin in the ECM may be used to signal favorable nerve cell responses.3In addition to ECM signals, mobile neurite growth cones have the capacity to respond to diffusible neurotrophic factors.4,5 The slow release of nerve
growth factor (NGF) has been demonstrated to improve the repair potential of transected dorsal roots in vivo.6 Neurotro-phic factors, such as NGF and brain-derived neurotroNeurotro-phic factor, are proteins that have important roles in neuronal survival, function, and target innervation.7,8Their exogenous administration has been shown to improve nerve regenera-tion.9 –11 Schwann cells (SCs) are important for neuronal
survival and axonal specialization during development,12and SC transplantation has been shown to enhance peripheral and central nerve regeneration.13–16 This response is considered to occur through provision of ECM molecules, growth fac-tors, and specific cell-to-cell receptor interactions by SCs.17–20In terms of using spatial control as a cue for tissue regeneration, several approaches have been developed to advance the concept of cell patterning from two to three dimensions. Within this concept of three-dimensional (3D) patterning, the formation of tubular devices is of particular Correspondence to: M-T Sheu (e-mail: mingsheu@tmu.edu.tw)
© 2005 Wiley Periodicals, Inc.
relevance to repairing nervous systems because of the simi-larities in their architectures.
At present, there are two main surgical approaches for promoting tissue restoration and axonal recovery: cell re-placement and endogenous repair using artificial substrate implants.21To improve the ability for regeneration by
graft-ing livgraft-ing cells or addgraft-ing growth factor into a lesion site, it is important to find a good biomaterial for the in vitro study of neuron survival. A good biomaterial matrix has to supply the conditions for normal neuronal differentiation and out-growth.22The efficacies of many different types of matrices
for facilitating or promoting nerve repair have been tested in
vitro, as well as in experimental animals. These matrices can
be broadly categorized according to their chemical nature into protein-based polymers, carbohydrate-based polymers, artifi-cial materials, and combinations of these.
Agarose is an attractive immobilization material because it is nontoxic, and gelation occurs under mild conditions. Aga-rose gels consist of a network of interconnected pores and have been investigated for the culture of neural cell types.23,24
But cells are almost completely unable to attach because of a lack of a peptide for cell adhesion.25Furthermore, it has poor
biodegradability; this property and the absence of appropriate enzymatic degradation systems in mammalian tissues make it an unattractive matrix candidate for nerve repair studies in
vivo. Collagen is regarded to be one of the most useful
biomaterials. It has excellent biocompatibility and safety be-cause of its biological characteristics, such as biodegradabil-ity and weak antigenicbiodegradabil-ity. It has been used for tissue engi-neering including skin replacement, bone substitutes, carti-lage, artificial blood vessels, and valves.26,27Furthermore, the
3D structure provides biological scaffolds for cell attachment and migration, and it supports the growth of PC12 cells, cortical neurons, astrocytes, as well as neural progenitor cells.28 –31Fibrin is the natural primary biomatrix for wound
healing. Fibrin glue supports fibroblast infiltration and pro-liferation, and thus enhances the wound-healing process. Therefore, fibrin, in the form of fibrin sealant or glue, has gained worldwide recognition as one of the most effective hemostatic agents available. The physiological composition of fibrin also makes it a particularly well-suited biomatrix for tissue engineering applications. It can be polymerized and easily formed from its basic protein constituents: fibrinogen and thrombin. Fibrin polymers represent a natural, biodegrad-able, and biocompatible matrix for keratinocytes and urothe-lial cells for in vivo tissue regeneration,32,33 and for the
creation of prefabricated tissue for reconstructive surgery, including bioartificial skin and skin products, cartilage, bone, and urological tissue.34 –36
The 3D structural scaffold provides good cell adherence, effectively guides tissue regeneration, and promotes cell growth and migration. It supports nerve regeneration and angiogenesis,37–39and improves axonal regeneration through
nerve guides in peripheral nerves.40 Consequently, it can
combine with other polymeric matrices to produce blended polymer scaffolds, or exogenous peptides and bioactive agents can be incorporated into the fibrin gel to enhance
biological activity.41,42We have proposed a system that uses
3D cultures of neural cells immobilized in hydrogel matrices. In this design, the viability and survival rate of PC12 cells and primary cortical neurons cultured in different matrices were evaluated in vitro. This study focused on 2- and 3D biomaterials such as agarose, collagen, and fibrin glue that are thought to be suitable biomaterials for neuronal outgrowth.
MATERIALS AND METHODS Reagents
Fetal calf serum was purchased from Biological Industries (Israel). Protein-assay dye was supplied by Bio-Rad. Rabbit anti-neurofilament H was provided by Chemicon. Basal Me-dium Eagle (BME, 1⫻), Dulbecco’s modified Eagle medium (DMEM), Dulbecco’s phosphate-buffered saline (PBS), fetal bovine serum, horse serum, L-glutamine, protein molecular weight marker, and trypsin-ethylenediaminetetraacetic acid were obtained from Gibco. NGF was supplied by Promega. The cytotoxicity detection kit was provided by Roche (Ger-many). Basal Medium Eagle (BME, 2⫻), bovine serum al-bumin (BSA), collagen type I (C-7661, from rat tail), D -glucose, DMEM (without phenol red), dimethyl sulfoxide, fibrinogen (F-4753), low melting point agarose (A-9414), MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide), poly-D-lysine, sodium bicarbonate, and thrombin (T-4648) were from Sigma. The ABC kit, biotinylated anti-rabbit immunoglobulin (Ig)G, blocking kit, fluorescein avidin D, and mount medium were purchased from Vector.
PC12 Cell Line
PC12 cells, an immortal cell line derived from the cancerous tissue of a rat adrenal gland pheochromocytoma, were pro-vided by the Food Industry Research and Development In-stitute (Hsinchu, Taiwan, ROC). PC12 cells were maintained in DMEM supplemented with 10% horse serum and 5% fetal calf serum. Cells were incubated at 37°C in a humidified, 5% CO2environment. Cells were passaged 1:3 once a week by
detachment from the culture flask using mild agitation with a stream of fresh culture medium. PC12 cells were trypsinized off, counted, and concentrated to 2⫻ 104/well. The control
was cells plated on a poly-D-lysine-coated culture dish. The growth medium (0.5 mL, containing NGF 30 ng/mL) covered on the above.
Matrix Preparation
All matrices were prepared at the same time. The preparation of different matrices was performed as follows.
Agarose. Low melting temperature agarose was prepared from 0.25, 0.4, 0.5, and 1% w/v solutions in PBS. The solution was liquefied and sterilized by autoclaving and then allowed to cool to 37°C before use for cell immobilization. Cells were immobilized in those gels by mixing agarose and
cell medium with cells at the desired density. The pre-gel solution was transferred by pipette into wells of a 24-well culture plate which was placed on ice for approximately 1–2 min to allow gel formation. Cell growth medium (0.5 mL) was added to the gels, and they were transferred to an incubator.
Agaroseⴙ Collagen (From Porcine Skin). Collagen (0.5 mg/mL) produced from porcine skin following the method reported previously43 was dissolved in 0.1% acetic acid and
adjusted to a neutral pH with NaOH. Cells were immobilized by adding medium with cells at the desired density to a mixture containing equal volumes of agarose and the collagen solution. The pre-gel solution was transferred by pipetting into wells of a 24-well culture plate which was placed on ice for approximately 1–2 min to allow gel formation. Cell growth medium (0.5 mL) was added on top of the gels, and the culture plate was transferred to an incubator.
Agarose ⴙ ECM (From Porcine Skin). The ECM (0.5 mg/mL) produced from porcine skin following the method reported previously44was dissolved in pH 7.4 PBS. The other
steps were the same as that for preparing the “agarose ⫹ collagen” gel.
Primary Culture of Cortical Neurons
Pregnant Wistar rats were purchased from the National Lab-oratory Animal Center (Taiwan, ROC). Ad libitum access to food and water was allowed, and the light/dark cycle was maintained at 12/12 h. The isolation procedures of cortical neurons were performed according to that reported by Lee et al.45In brief, pregnant Wistar rat bearing embryonic day 17 (E17) embryos was deeply anesthetized with 80 mg/kg body weight of sodium pentobarbital. Embryos were removed and placed into a GME-containing (BME ⫹ serum) glass dish, and the cerebral cortex was dissected. Then the cortex was transferred into a GME-containing 35-mm dish, and a can-nula was used to connect to a 10-mL syringe to aspirate the cortical tissue. Then the cortical tissue was dissociated back and forth through the cannula, and centrifuged at 800 rpm for 5 min to collect cell pellet. After three cycles of dissociation, the pellet was resuspended in an appropriate volume of GME, and the cell suspension was distributed into each dish with desired volumes. Cortical neurons were incubated in the humidified CO2incubator with a 5% CO2setting at 37°C for
45– 60 min for attachment. After that time, the GME was exchanged with BME, and incubation was continued. The day of plating was counted as 0 days in vitro (DIV).
Cell Immobilization in Hydrogel Matrices and Cell Plating
Cortical neurons were immobilized by preparing a suspension of cells in a pre-gel solution of the selected gel material. Unless otherwise indicated, matrices were prepared at a cell density of 5⫻ 105/well. The conditions for matrix network
formation varied depending on the gel type. The control was plated on a poly-L-lysine-coated culture dish.
Agarose. Low melting temperature agarose was prepared as a 1% w/v solution in PBS. The solution was liquefied and sterilized by autoclaving and then allowed to cool to 37°C before use for cell immobilization. Cells were immobilized in a 0.5% w/v gel by mixing equal volumes of agarose and 2⫻ BME. The bottoms of the wells of a 24-well tissue culture dish were coated with 200L of the 0.5% agarose solution. The dish was then placed at 4°C for approximately 15 min to allow the agarose to gel. For 2D plating, cells were plated on the agarose gel. For 3D immobilization, cells were added to 400 L of the 0.5% agarose solution and carefully mixed. The cell-agarose solution was added to the well containing the previously gelled agarose on the bottom. Dishes were placed at 4°C to allow the solution to gel. BME (0.5 mL) was added to the top of the gels.
Collagen (From Rat Tail). Collagen purchased as a sterile and lyophilized powder was dissolved to a final concentration of 2 mg/mL with sterile 0.1% v/v acetic acid. Collagen gels were formed by the self-assembly of collagen molecules upon warming to 37°C. The collagen solution was diluted with an equal volume of 2⫻ BME and an amount of 1⫻ BME to achieve a final collagen concentration of 0.5 mg/mL. The bottoms of wells of a 24-well tissue culture dish were coated with 200L of the 0.5 mg/mL collagen solution. The dish was then placed at 37°C for approximately 1 h to allow the collagen to gel. For 2D plating, cells were plated onto the collagen film. For the 3D immobilization, the collagen solu-tion previously chilled in an ice bath to prevent gel formasolu-tion was allowed to warm to room temperature, and cells were added to 400 L of the 0.5 mg/mL collagen solution and carefully mixed. The cell-collagen solution was added to the well containing the previously gelled collagen. Dishes were placed in an incubator for approximately 1–2 h until gel formation. Once the collagen gel had set, 0.5 mL of BME was added to the dishes, and the matrix was returned to the incubator.
Fibrin. Fibrinogen was dissolved in 1⫻ BME and filter-sterilized with 0.45-m syringe filters. Twenty microliters of a thrombin solution (2 U/mL in Tri buffer) was added to 0.2 mL of the fibrinogen solution (5 mg/mL), and then the mixture was transferred to wells of a 24-well culture dish. The dish was then placed at 37°C for approximately 1 h to allow gelling to occur. Cells were plated on the fibrin glue for 2D plating. Steps for the 3D immobilization were the same as those described in the preparation of “collagen.”
Fibrin ⴙ Collagen (From Rat Tail). Fibrinogen was dis-solved in 2⫻ BME (10 mg/mL) and 1⫻ BME (5 mg/mL), and filter-sterilized with 0.45-m filters. The 2 mg/mL col-lagen solution was diluted with an equal volume of 2⫻ fibrinogen-BME solution and an amount of 1⫻
fibrinogen-BME solution to achieve a final collagen concentration of 0.5 mg/mL. Twenty microliters of the thrombin solution (2 U/mL) was well mixed with 0.2 mL of the fibrin-collagen solution, and this was added to wells of a 24-well culture plate. The plate was then placed at 37°C for approximately 1 h to allow gelling to occur. Other steps were the same as those described for “fibrin.”
Fibrinⴙ Collagen (From Porcine Skin). The steps were the same as those described for “fibrin⫹ collagen gel (from rat tail).”
Fibrin ⴙ ECM (From Porcine Skin). The steps were the same as those described for “fibrin⫹ collagen gel (from rat tail).”
Analysis and Quantification of Neurite Extension In vitro analysis and quantification of neurite extension in the
gel scaffolds were performed during 1–5 days of culture using an Olympus (Tokyo, Japan) DP50 digital camera fol-lowing the procedure reported.1Light microscopic images of cells in 3D culture were captured, and the neurites were traced. This process was repeated for at least 20 neurites, and the mean value reported for each sample was obtained by averaging the measured neurite lengths.
Determination of Cell Death
MTT Assay. On day 5 of culture, medium from each condition was withdrawn, and the matrices were incubated with 250L of MTT solution for approximation 4 h. Then, 200 L of dimethyl sulfoxide was added to dissolve the formazan. After incubation for 16 h, 100L of supernatant was removed and transferred to a 96-well microplate. Absor-bance using an enzyme-linked immunosorbent assay plate reader was measured at 595 nm.
Lactate Dehydrogenase (LDH) Assay. Using a cytotox-icity detection kit, LDH activity was measured to quantify cell death. On day 5 of culture, 100L of medium of each culture sample was withdrawn and transferred to a 96-well microplate. The reaction mixture (100L/well) of the detec-tion kit for LDH activity was added to each medium and incubated for 30 min. Then, the absorbance at 490/630 nm was measured using an enzyme-linked immunosorbent assay plate reader.
Immunocytochemistry. An antibody for neurofilament protein (NFP), a specific marker for neurons, was used for identification of the neuronal population. On 5 DIV, cultures were washed three times in 37°C PBS, and then fixed in 4% paraformaldehyde for 30 min at room temperature. The fix-ative was removed by rinsing three times with PBS for 15 min. Cultures were exposed to a blocking solution consisting of 3% BSA in PBS for 60 min, followed by overnight incubation with polyclonal rabbit anti-NFP serum at 4°C (a 1:200 dilution of antiserum in blocking solution plus 0.3% Triton X-100). After washing three times with PBS, cultures were processed for antibody binding using an avidin-biotin peroxidase system (Vectastain ABC kit, anti-rabbit IgG), incubated in fluorescent anti-rabbit secondary antibodies (flu-orescein isothiocyanate) for 1 h at room temperature, and rinsed again with PBS; photos were taken with an Olympus DP50 digital camera.
Total Protein Preparation
On 5 DIV, 100 L of lysis buffer was added to one well. Cells were scraped from the well, and transferred to a micro-centrifuge tube where samples were briefly sonicated. Sam-ples were centrifuged to pelletize the insoluble material, and then the supernatant was withdrawn and stored at ⫺20°C. The protein assay for determining the concentration of solu-Figure 1. PC12 cells cultured in (A) agarose, (B) agarose/collagen,
bilized protein was based on the method of Bradford.46Three to five dilutions of a protein standard, which was represen-tative of the protein solution to be tested, were prepared. The
linear range of the assay for BSA was 1.2–10.0g/mL. Eight hundred microliters of each standard and sample solution were pipetted into a clean, dry test tube. Dye reagent con-centrate (200L) was added to each tube and vortexed. After incubation at room temperature for at least 5 min, the absor-bance was measured at 595 nm.
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis
The 6% separating gel and 4% stacking gel were prepared. An equal amount of 2⫻ treatment buffer was added to each sample, and dried in a bath at 95°C for 15 min. Samples were loaded into each well and electrophoresed until the bromo-phenol blue in the samples reached the bottom of the gel. Then the power supply was switched off. Gels were main-tained in the running buffer until ready to transfer.
Western Blotting
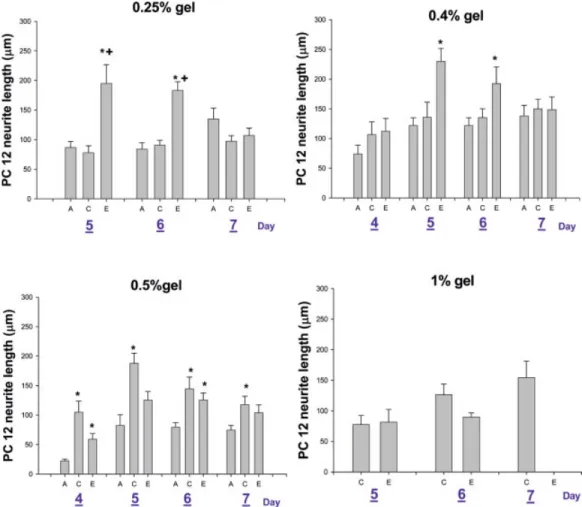
The separated proteins were transferred to Hybond ECL nitrocellulose membranes using a Bio-Rad Criterion Blotter Figure 2. Average neurite length of PC12 cells cultured in 0.25 and 1.0% gel for 5, 6, and 7 days, and
in 0.04 and 0.5% gels for 5, 6, and 7 days. (A) agarose, (C) agarose/collagen (containing 0.5 mg/mL collagen), and (E) agarose/ECM (containing 0.5 mg/mL ECM). (*,⫹: p ⬍ 0.05 compared with Agarose, Collagen Group, respectively). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 3. MTT OD values for PC12 cells cultured in agarose,
aga-rose/collagen (A/C), and agarose/ECM (A/E) gels (0.25, 0.40, 0.50, and 1.0%) for 7 days.
set at⫺400 mA for 4 h. Membranes were rinsed in dd-H2O
and blocked in the blocking solution (5% skim milk in Tris-buffered saline containing 50 mM Tris-HCl, 0.5M NaCl, and 0.1% NaN3) for 1 h at room temperature. Afterward, the
blocking membranes were probed with polyclonal rabbit anti-neurofilament protein H [NFP, H 200 kD, diluted 1:1000 (v/v)], and with the monoclonal anti-glyceraldehydes-3-phos-phate dehydrogenase antibody (diluted 1:3000 in the blocking solution) for 1 h at room temperature or overnight at 4°C. The membranes were washed three times for 15 min in the block-ing solution before incubation at room temperature with horseradish peroxidase-conjugated goat anti-rabbit IgG [di-luted 1:5000 (v/v)] and goat anti-mouse IgG [di[di-luted 1:10,000 (v/v)] in the blocking solution. The membranes were washed again three times for 15 min, and the blots were developed with the horseradish peroxidase-reactive chemilu-minescence reagents and exposed to autoradiographic film. The relative density of the protein band in the Western blot was further analyzed with an electrophoresis image analysis system.
RESULTS AND DISCUSSION PC12 Cell Immobilization in 3D Cultures
Morphology and Neurite Outgrowth of PC12 Cells. The morphology of PC12 cells entrapped in agarose-based gels is shown in Figure 1. After 5 days of culture, PC12 cells had differentiated into a neuronal-like cell type. Neurite extension in agarose was significantly shorter than those in the agarose/ collagen and agarose/ECM gels. But most of the PC12 cells did not differentiate when entrapped in the agarose-based gel, and cells exhibited no neurite extension and remained small and rounded, even after having been exposed to NGF for 7 days. The different concentrations and substrates of the aga-rose gels also influenced the neurite extension of PC12 cells as shown in Figure 2. In 0.25 and 0.4% gels, the neurite lengths in the agarose/ECM gel with 5 and 6 days of culture were greater than those in the agarose and agarose/collagen gels. In 0.5% gel, neurite lengths in the agarose/collagen gel after 4, 5, 6, and 7 days of culture were greater than those in the agarose gel, and the neurite lengths in the agarose/ECM gel were greater than those in the agarose gel after 4 and 6 days of culture. In 1% gel, PC12 cells cultured in the agarose gel were almost completely undifferentiated, and the neurite lengths in agarose/collagen and agarose/ECM after 5 and 6 days showed statistically insignificant differences.
PC12 Cell Survival in Matrices. The MTT assay was used to determine living cells, and the results are shown in Figure 3. The MTT OD values of various concentrations of agarose gel were similar. However, values of the 0.5% aga-rose/collagen and agarose/ECM gels were higher than those of the other concentrations. Concerning these results, it is clear that concentrations of gels and substrates are important factors influencing neuron outgrowth, and 0.5% agarose gel is
a suitable concentration for PC12 cell growth. Cell growth in the agarose gel decreased with increasing agarose concentra-tion, and the pore size of the agarose gel may have been a potential limitation. The average pore size in the range of 0.5–1% has been reported to be 200 –310 nm,23which is quite small compared with the 10- to 30-m size of PC12 cells. After culturing for 5 days, the neurite length had decreased. Because cell death causes the traced cell neurites to become increasingly fewer and shorter, the average neurite length decreased. Because agarose lacks the peptide for cell adhe-sion, the addition of collagen and ECM to the agarose gels provides good adhesion to compensate for this deficiency of agarose, leading to increased neurite outgrew and cell sur-vival by PC12 cells. But PC12 cells are not stable, and sometimes cells exhibit unregulated growth and often lose their functionality,47 so primary cells may be an ideal cell source for cell immobilization.
Cortical Neurons Cultured on 2D Film
Morphology of Cortical Neurons. The morphology of cortical neurons cultured on 2D film for 5 DIV is shown in Figure 4. As demonstrated, the cortical neurons were almost completely unable to attach and grow when they were plated on the surface of agarose [Figure 4(B)], especially when Figure 4. Cortical neuron cells plated on (A) poly-lysine-coated
dishes (control), or (B) agarose, (C) collagen, (D) fibrin, (E) fibrin/ collagen (rat), (F) fibrin/collagen (pig), and (G) fibrin/ECM films for 5 DIV. Scale bar⫽ 100m.
compared with control poly-lysine-coated dishes where cells adhered very well onto the surface forming a network of neurite processes [Figure 4(A)]. Cortical cells plated on the
collagen surface were similar in morphology to the control [Figure 4(C)], and cells were well attached and formed a neuronal network. Cells plated on the fibrin-based gel also Figure 5. Immunocytochemical staining of NFP at 5 DIV for cortical neurons cultured on (A, B)
poly-lysine-coated dishes (control), (C, D) collagen, (E, F) fibrin, (G, H) fibrin/collagen (rat), (I, J) fibrin/collagen (pig), and (K, L) fibrin/ECM gels. (A, C, E, G, I, K) Phase contrast; (B, D, F, H, J, L) activated NFP. Scale bar⫽ 100m. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
were adhesive and formed a neuronal network with neurites, but they had a large number of cell aggregations and “stress fibers” [Figure 4(D,F,G)]. But for cells cultured on the fibrin/ collagen (rat) surface, the amounts of aggregations and “stress fibers” were fewer, and their morphology was similar to that of cells plated on collagen gel [Figure 4(E)].
Expression of NFP. Immunocytochemistry results for de-tecting neurons are shown in Figure 5. The results are similar to those of light microscopic images, and neural cell bodies and neurites are distinguishable by fluorescein isothiocyanate fluorescence. The control group’s image [Figure 5(B)] was clearer than those of the other conditions, and those images for the matrices [Figure 5(D,F)], especially on fibrin-based gels [Figure 5(H,J,L)], were very fuzzy. The fluorescence message was weak, so the events of immunocytochemistry are not as expected. The reason could be that cell aggregation forms a barrier preventing the NFP antibody from binding to the cell.
Results of Western blot analysis expressed by the band density are shown in Figure 6(A). The expressions of neuro-filaments by cells plated on collagen, fibrin, and fibrin/ECM were higher than those at the other conditions. Cortical cells plated on the fibrin/collagen (rat) and fibrin/collagen (pig) gels exhibited good neurite extension, but the neurofilament expression was lower. The neurofilament expression in aga-rose was the lowest as a result of the lack of cell adherence as expected.
Cortical Neuron Survival on 2D Film. Survival rates are shown in Figure 7(A–C). Cortical cells cultured on agarose had the lowest MTT and LDH OD values, because the cortical cells were almost completely unable to attach. The MTT OD value of cortical neurons plated on fibrin-based matrices was higher than those of neurons plated on collagen and agarose [p⬍ 0.05; Figure 7(A)]. The LDH OD values for collagen and fibrin-based matrices did not differ compared with the control, poly-lysine-coated dish, and the value for agarose was lower than those of the other conditions [p ⬍ 0.05; Figure 7(B)]. From the MTT/LDH ratio, the survival rate of fibrin-based matrices was significant higher than those for the control, agarose, and collagen [p⬍ 0.05; Figure 7(C)]. The higher survival rate may have resulted from cortical cells plated on fibrbased gels forming large aggregates to in-crease cell survivability, thus increasing the MTT/LDH ratio.
Cortical Neuron Immobilization in 3D Gels Morphology of Cortical Neurons Cultured in 3D Gels.
When cortical cells immobilized in 3D matrices were cul-tured for 5 days, cell morphology of neurons in the matrices obviously differed (Figure 8). In the control, cell neurites extended and formed a neuron network [Figure 8(A)]. In the agarose gel, cells only exhibited short neurite outgrowth [Figure 8(B)]. In the collagen gel, cells displayed long neurite process outgrowth [Figure 8(C)], and in the fibrin and fibrin/ collagen (rat) gels, cells showed long neurite extension, but no cell aggregation compared with cells plated on 2D film [Figure 8(D,E)]. Cells cultured in the fibrin/collagen (pig) gel showed neurite outgrowth on days 0 –3, but with increasing culture days, the neurite outgrowth rate decreased and the neurites began to break into pieces [Figure 8(F)]. Cells in the fibrin/ECM gel were similar to those cultured in fibrin/colla-gen (pig). After culturing for 3 days, neurites began to break into pieces, and the cells appeared dead [Figure 8(G)].
Neurite Length of Cortical Neurons. After cells were incubated in the different matrices for 1–5 days, the images were captured and the neurite lengths were traced. Neurite length was calculated by averaging the measured neurite lengths. Results are shown in Figure 9. In the agarose gel, neurites were short, and only a few neurons had differenti-ated; after culturing for 4 and 5 days, cells had almost all died, and neurites could not be traced. The same situation occurred with cells cultured in the fibrin/ECM gel. In the fibrin/collagen (pig) gel, neurites extended when initially Figure 6. Western blot analysis of NFP. (A) Expression of NFP by
cortical neuron cells at 5 DIV cultured on agarose, collagen, fibrin, fibrin/collagen (rat), fibrin/collagen (pig), and fibrin/ECM surfaces; (B) expression of NFP by cortical neuron cells at 5 DIV cultured in agarose, collagen, fibrin, fibrin/collagen (rat), fibrin/collagen (pig), and fibrin/ECM gels.
immobilized; however, neurites began to break into pieces after 4 –5 days of culture. In the collagen and fibrin/collagen (rat) gels, cortical neurons exhibited very long neurite exten-sion after culturing for 5 days. Neurites in the fibrin gel were a little shorter than those in the collagen and fibrin/collagen (rat) gels, but the neurite outgrowth was still better than those in the agarose, fibrin/collagen (pig), and fibrin/ECM gels.
Expression of NFP. Results of immunocytochemistry ap-peared cloudy (data not shown). This may have been because of the nonspecific binding of antibody to matrices, which caused higher background fluorescence. It was difficult to distinguish among cell bodies, neurites, and matrices. Results of Western blot analysis are shown in Figure 6(B). The NFP expres-sion in agarose gel was the lowest as a result of less neurite outgrowth. However, because neurites grew very well in the collagen, fibrin, and fibrin/collagen (rat) gels, expressions of neurofilaments in those gels were higher than others.
Cortical Neuron Survival in 3D Gels. When cortical cells were cultured in 3D matrices for 5 days, the MTT and LDH
assays were used to determine cell survival. Results are shown in Figure 7(D–F). Cell survival in fibrin-based gels was significant higher than that in the agarose and collagen gels ( p⬍ 0.05). Results were similar to cell survival on 2D film (Figure 8). Cells survived for more than 17 days in the control, collagen, fibrin, and fibrin/collagen (pig) gels (Figure 10), until day 25 when no surviving cells were observed (data not shown). Results showed that cells in the collagen, fibrin, and fibrin/collagen (rat) gel cultures lasted for at least 17 days with abundant neurite outgrowth.
Cell immobilization on the agarose gel presented two potential problems concerning its physical properties. First, hydrophilic chains of agarose do not promote cell adherence. Although it is likely that cell adherence issues can be over-come by the covalent modification of the gel with cell adhe-sive peptides,23,25 in its native state, the agarose gel is not
adhesive to attachment-dependent cells such as neurons. The second problem is the pore size of agarose gels. For the 0.5% w/v formulation, the average pore size was reported to be 310 nm,23which is quite small compared with the 10- to 30-m
size of neural cells. So it could be that the physical properties Figure 7. Survival rate of cortical neuron cells at 5 DIV cultured on 2D film (A–C) (*, #,⫹, ˆ: p ⬍ 0.05
compared with the poly-lysine group, agarose, collagen, or fibrin glue groups, respectively) and in 3D gel (D–F) (*, #, ˆ,⫹, ⫺: p ⬍ 0.05 compared with the agarose, collagen, fibrin glue, F/C (rat), and F/C (pig) groups, respectively). (A, D) MTT OD value; (B, E) LDH OD value; (C, F) MTT/LDH ratio.
of the agarose gel confined the entrapped neural cells and created a physical barrier to cell growth.
Compared with the agarose gel, collagen is an attractive material for cell immobilization because it has large pores. At a concentration of 0.5 mg/mL, the pore size was reported to be about 1.6 m,29 and the pore size of fibrin gel was
Figure 8. Cortical neuron cells 3D-immobilized on (A)
poly-lysine-coated dishes (control), and embedded in (B) agarose, (C) collagen, (D) fibrin, (E) fibrin/collagen (rat), (F) fibrin/collagen (pig), and (G) fibrin/ECM gels at 5 DIV. Scale bar⫽ 100m.
Figure 9. Average neurite length in different matrices. [Color figure can be viewed in the online issue,
which is available at www.interscience.wiley.com.]
Figure 10. Cortical neuron cells cultured in 3D gel after 17 days in (A)
poly-lysine-coated dishes (control), (B) collagen, (C) fibrin, and (D) fibrin/collagen (rat) gels. Scale bar⫽ 100m.
reported to be about 0.1– 0.5m, with fibrinogen and throm-bin concentrations of 5 mg/mL and 2 U/mL, respectively.48
This observation is consistent with that reported previously by Herbert et al.48It is hypothesized that the proteases at or
near the neurite tip degrade the fibrin strands to create a pathway for the growing neurites. Outgrowing neurites are able to degrade the fibrin around the growth cone to create large gaps between the matrix strands, but the agarose that cannot be degraded inhibits neurite outgrowth.
In a comparison of neurite outgrowth and cell survival in different matrices, the collagen, fibrin, and fibrin/collagen (rat) gels were better materials for neural cell immobilization. Cells cultured in the fibrin/collagen (pig) and fibrin/ECM gels did not work as well as those cultured in the fibrin/collagen (rat) gel, which may have been the result of the collagen and ECM being purified from pig skin contaminated with higher extent of dimers or higher, which may have had different effects on rat cortical neurons.
In the present study, agarose and collagen gels were used to culture E18 rat cortical neurons. The results were similar to reported results, in that cells could be maintained in culture for up to 24 days; that cell survival in the collagen gel was higher than that in the agarose gel31; and that collagen gels
also supported the growth of neonatal rat astrocytes, neural progenitor cells, and cortical cells.30,49 In addition, the
via-bility and growth of SCs immobilized in collagen gel and fibrin glue did not significantly differ.50 Fibrin glue also
supports elongation of neurites of dorsal root ganglia in
vitro,37,38,51 and accelerates regeneration of axons in
vivo.37,39In those studies, fibrin glue was not used to culture
other cells except for dorsal root ganglia; in addition, those matrices were often used alone, and differences between 2-and 3D culture systems were not reported. In our study, we chose primary cortical neurons cultured in agarose, collagen, fibrin, and a mix of collagen and fibrin gels with 2- and 3D culture systems. We found that cell survival and neurite extension of neurons immobilized in the mixture of collagen and fibrin were better than those in collagen and fibrin gels.
CONCLUSIONS
In this study, the plating of cortical cells on 2D matrices of collagen and fibrin had no effect on neurite length. When cells were immobilized in 3D matrices of collagen, fibrin, and a mixture of collagen and fibrin gels, they exhibited good neurite outgrowth and cell survival. This establishes a 3D culture system for neural cell outgrowth, and collagen and fibrin are potential biomaterials in this system for tissue engineering in neurobiology. In future studies, it is important to examine the effects of various mixing concentrations of collagen and fibrin and to optimize gel density and pore size for cortical neuron extension. Ultimately, the respective un-derlying mechanism responsible for regenerating cell growth and propagation of neurite by either collagen or fibrin should be elucidated for accomplishing the optimal design of nerve regeneration devices.
REFERENCES
1. Pearson RG, Molino Y, Williams PM, Tendler SJB, Davis MC, Roberts CJ, Shakesheff KM. Spatial confinement of neurite regrowth from dorsal root ganglia within nonporous microcon-duits. Tissue Eng 2003;9:201–208.
2. Chernousov MA, Carey DJ. Schwann cell extracellular matrix molecules and their receptors. Histol Histopathol 2000;15:593– 601.
3. Weeks BS, Nomizu M, Ramchandran RS, Yamada Y, Kleinman HK. Laminin-1 and the RKRLQVQLSIRT laminin-1␣1 glob-ular domain peptide stimulate matrix metalloproteinase secre-tion by PC12 cells. Exp Cell Res 1998;243:375–382.
4. Paves H, Saarma M. Neurotrophins as in vitro growth cone guidance molecules for embryonic sensory neurons. Cell Tissue Res 1997;290:285–297.
5. Song HJ, Poo MM. Signal transduction underlying growth cone guidance by diffusible factors. Curr Opin Neurobiol 1999;9: 355–363.
6. Bloch J, Fine EG, Bouche N, Zurn AD, Aebischer P. Nerve growth factor- and neurotrophin-3-releasing guidance channels promote regeneration of the transected rat dorsal root. Exp Neurol 2001;172:425– 432.
7. Terenghi G. Peripheral nerve regeneration and neurotrophic factors. J Anat 1999;194:1–14.
8. Yao M, Moir MS, Wang MZ, To MP, and Terris DJ. Peripheral nerve regeneration in CNTF knockout mice. Laryngoscope 1999;109:1263–1268.
9. Newman JP, Verity AN, Hawatmeh S, Fee WEJ, Terris DJ. Ciliary neurotrophic factors enhance peripheral nerve regener-ation. Arch. Otolaryngol. Head Neck Surg. 1996;122:399 – 403. 10. Sterne G, Brown R, Green C, Terenghi G. Neurotrophin-3 delivered locally via fibronectin mats enhances peripheral nerve regeneration. Eur J Neurosci 1997;9:1388 –1396.
11. Whitworth IH, Brown RA, Dore CJ, Anand P, Green CJ, Terenghi G. Nerve growth factor enhances nerve regeneration through fibronectin grafts. J Hand Surg [Br] 1996;21:514 –522. 12. Jessen KR, Mirsky R. Schwann cells and their precursors emerge as major regulators of nerve development. Trends Neu-rosci 1999;22:402– 410.
13. Guenard V, Kleitman N, Morrissey TK, Bunge RP, Aebischer P. Syngeneic Schwann cells derived from adult nerves seeded in semipermeable guidance channels enhance peripheral nerve re-generation. J Neurosci 1992;12:3310 –3320.
14. Levi AD, Guenard V, Aebischer P, Bunge RP. The functional characteristics of Schwann cells cultured from human peripheral nerve after transplantation into a gap within the rat sciatic nerve. J Neurosci 1994;14:1309 –1319.
15. Guest JD, Rao A, Olson L, Bunge MB, Bunge RP. The ability of human Schwann cell grafts to promote regeneration in the transected nude rat spinal cord. Exp Neurol 1997;148:502–522. 16. Smith GV, Stevenson JA. Peripheral nerve grafts lacking viable Schwann cells fail to support central nervous system axonal regeneration. Exp Brain Res 1988;69:299 –306.
17. Bryan DJ, Holway AH, Wang KK, Silva AE, Trantolo DJ, Wise D, Summerhayes IC. Influence of glial growth factor and Schwann cells in a bioresorbable guidance channel on periph-eral nerve regeneration. Tissue Eng 2000;6:129 –138.
18. Oudega M, Gautier SE, Chapon P, Fragoso M, Bates ML, Parel JM, Bunge MB. Axonal regeneration into Schwann cell grafts within resorbable poly(␣-hydroxy-acid) guidance channels in the adult rat spinal cord. Biomaterials 2001;22:1125–1136. 19. Hadlock T, Sundback C, Hunter D, Cheney M, Vacanti JP. A
polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng 2000;6:119 – 127.
20. Evans GRD, Brandt K, Katz S, Chauvin P, Otto L, Bogle M, Wang B, Meszlenyi RK, Lu LC, Mikos AG, Patrick CW. Bioactive poly(L-lactic acid) conduits seeded with Schwann
cells for peripheral nerve regeneration. Biomaterials 2002;23: 841– 848.
21. Woerly S. Restorative surgery of the central nervous system by means of tissue engineering using NeuroGel implants. Neuro-surg Rev 2000;23(2):59 –77.
22. Woerly S, Plant GW, Harvey AR. Neural tissue engineering: from polymer to biohybrid organs. Biomaterials 1996;17(3): 301–310.
23. Bellamkonda R, Ranieri JP, Aebischer P. Laminin oligopeptide derivatized agarose gels allow three-dimensional neurite exten-sion in vitro. J Neurosci Res 1995;41:501–509.
24. Balgude AP, Yu X, Szymanski A, Bellamkonda RV. Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials 2001;22(10):1077–1084.
25. Borkenhagen M, Clemence JF, Sigrist H, Aebischer P. Three-dimensional extracellular matrix engineering in the nervous system. J Biomed Mater Res 1998;40(3):392– 400.
26. van Susante JLC, Pieper J, Buma P, van Kuppevelt TH, van Beuningen H, van Der Kraan PM, Veerkamp JH, van den Berg WB, Veth RPH. Linkage of chondroitin-sulfate to type I colla-gen scaffolds stimulates the bioactivity of seeded chondrocytes in vitro. Biomaterials 2001;22(17):2359 –2369.
27. Kaps C, Bramlage C, Smolian H, Haisch A, Ungethum U, Burmester GR, Sittinger M, Gross G, Haupl T. Bone morpho-genetic proteins promote cartilage differentiation and protect engineered artificial cartilage from fibroblast invasion and de-struction. Arthritis Rheum 2002;46(1):149 –162.
28. Baldwin SP, Krewson CE, Saltzman WM. PC12 cell aggrega-tion and neurite growth in gels of collagen, laminin and fi-bronectin. Int J Dev Neurosci 1996;14(3):351–364.
29. Krewson CE, Chung SW, Dai W, Saltzman WM. Cell aggre-gation and neurite outgrowth in gels of extracellular matrix molecules. Biotechnol Bioeng 1994;43:555–562.
30. O’Connor SM, Stenger DA, Shaffer KM, Maric D, Barker JL, Ma W. Primary neural precursor cell expansion, differentiation and cytosolic Ca(2⫹) response in three-dimensional collagen gel. J Neurosci Methods 2000;102(2):187–195.
31. O’Connor SM, Stenger DA, Shaffer KM, Ma W. Survival and neurite outgrowth of rat cortical neurons in three-dimensional agarose and collagen gel matrices. Neurosci Lett 2001;304(3): 189 –193.
32. Wechselberger G, Schoeller T, Stenzl A, Ninkovic M, Lille S, Russell RC. Fibrin glue as a delivery vehicle for autologous urothelial cell transplantation onto a prefabricated pouch. J Urol 1998;160(2):583–586.
33. Bach AD, Bannasch H, Galla TJ, Bittner KM, Stark GB. Fibrin glue as matrix for cultured autologous urothelial cells in urethral reconstruction. Tissue Eng 2001;7(1):45–53.
34. Isogai N, Landis WJ, Mori R, Gotoh Y, Gerstenfeld LC, Upton J, Vacanti JP. Experimental use of fibrin glue to induce site-directed osteogenesis from cultured periosteal cells. Plast Re-constr Surg 2000;105(3):953–963.
35. Currie LJ, Sharpe JR, Martin R. The use of fibrin glue in skin grafts and tissue-engineered skin replacements: a review. Plast Reconstr Surg 2001;108(6):1713–1726.
36. Hunziker EB. Articular cartilage repair: basic science and clin-ical progress. A review of the current status and prospects. Osteoarthritis Cartilage 2001;10:432– 463.
37. Herbert CB, Nagaswami C, Bittner GD, Hubbell JA, Weisel JW. Effects of fibrin micromorphology on neurite growth from dorsal root ganglia cultured in three-dimensional fibrin gels. J Biomed Mater Res 1998;40(4):551–559.
38. Zeng L, Huck S, Redl H, Schlag G. Fibrin sealant matrix supports outgrowth of peripheral sensory axons. Scand J Plast Reconstr Surg Hand Surg 1995;29(3):199 –204.
39. Robinson GA, Madison RD. Survival of adult rat retinal gan-glion cells with regrown axons in peripheral nerve grafts: a comparison of graft attachment with suture of fibrin glue. J Neu-rosurg 2000;93(2):275–278.
40. Aebischer P, Guenard V, Valentini RF. The morphology of regenerating peripheral nerves is modulated by the surface microgeometry of polymeric guidance channels. Brain Res 1990;531:211–218.
41. Schense JC, Hubbell JA. Cross-linking exogenous bifunctional peptides into fibrin gels with factor XIIIa. Bioconjug Chem 1999;10(1):75– 81.
42. Schense JC, Bloch J, Aebischer P, Hubbell JA. Enzymatic incorporation of bioactive peptides into fibrin matrices enhances neurite extension. Nat Biotechnol 2000;18(4):415– 419. 43. Ho H-O, Lin C-W, Sheu M-T. Diffusion characteristics of
collagen film. J Controlled Release 2001;77:97–105.
44. Chen R-N, Ho H-O, Tsai Y-T, Sheu M-T. Process development of an acellular dermal matrix (ADM) for biomedical applica-tions. Biomaterials 2004;25(13):2679 –2686.
45. Lee YH, Deupree DL, Chen SC, Kao LS, Wu JY. Role of Ca2⫹ in alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-mediated polyphosphoinositide turnover in primary neuronal cultures. J Neurochem 1994;62(6):2325–2332.
46. Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248 –254. 47. Ma W, Pancrazio JJ, Coulombe M, Dumm J, Sathanoori R,
Barker JL, Kowtha VC, Stenger DA, Hickman JJ. Neuronal and glial epitopes and transmitter-synthesizing enzymes appear in parallel with membrane excitability during neuroblastoma x glioma hybrid differentiation. Brain Res Dev Brain Res 1998; 106(1–2):155–163.
48. Herbert CB, Bittner GD, Hubbell JA. Effects of fibrinolysis on neurite growth from dorsal root ganglia cultured in two- and three-dimensional fibrin gels. J Comp Neurol 1996;365:380 – 391.
49. O’Shaughnessy TJ, Lin HJ, Ma W. Functional synapse forma-tion among rat cortical neurons grown on three-dimensional collagen gels. Neurosci Lett 2003;340:169 –172.
50. Mosahebi A, Simon M, Wiberg M, Terenghi G.. A novel use of alginate hydrogel as Schwann cell matrix. Tissue Eng 2001; 7(5):525–534.
51. Dubey N, Letourneau PC, Tranquillo RT. Neuronal contact guid-ance in magnetically aligned fibrin gels: effect of variation in gel mechano-structural properties. Biomaterials 2001;22:1065–1075.