Clinical Endocrinology (2004) 61, 88–93 doi: 10.1111/j.1365-2265.2004.02057.x
88
Blackwell Publishing, Ltd.
Cord plasma concentrations of adiponectin and leptin in
healthy term neonates: positive correlation with
birthweight and neonatal adiposity
Po-Jung Tsai*, Chun-Hsien Yu† ¶, Shih-Penn Hsu‡,Yu-Hsiang Lee‡, Chuen-Hua Chiou§, Yu-Wen Hsu§, Su-Chen Ho* and Chun-Hong Chu‡
*Department of Food Science, and Institute of Biotechnology, Yuanpei University of Science and Technology, Hsin-Chu, Departments of †Paediatrics,
‡Obstetrics and Gynaecology and §Nursing, Taipei Municipal Women’s and Children’s Hospital and ¶Graduate Institute of Clinical Medicine, College of Medicine, National Taiwan University, Taipei, Taiwan
(Received 18 November 2003; returned for revision 1 February 2004; finally revised 3 March 2004; accepted 19 April 2004)
Summary
OBJECTIVE Adiponectin is negatively associated with
leptin, insulin and obesity in children and adults. Whereas increases in fetal insulin and leptin are asso-ciated with increased weight and adiposity at birth, the role of adiponectin in fetal growth has not yet been determined. The aims of this study were to examine the relationships between adiponectin and insulin, leptin, weight and adiposity at birth in healthy term infants.
DESIGN AND METHODS Anthropometric parameters
including weight, length, circumferences and skinfold thickness were measured, and plasma lipid profiles, insulin, leptin and adiponectin concentrations in cord blood samples from 226 singleton infants born at term after uncomplicated pregnancies were assayed.
RESULTS Cord plasma adiponectin, leptin and insulin
levels correlated significantly and positively with birth-weight (P = 0·001, P < 0·001, P < 0·001, respectively) and the sum of skinfold thicknesses (P < 0·001, P < 0·001,
P < 0·001, respectively). Mean cord plasma adiponectin and leptin levels, but not insulin level, were signifi-cantly higher in large-for-gestational-age (LGA) infants compared with appropriate-for-gestational-age (AGA) infants. Cord plasma leptin concentration, but not
adiponectin concentration, was significantly higher in female infants than in male infants (P = 0·003 and P = 0·94, respectively). Cord plasma adiponectin concen-tration correlated positively with leptin level (P = 0·007) but not with insulin level (P = 0·78).
CONCLUSIONS High adiponectin levels are present in
the cord blood. Cord plasma adiponectin and leptin levels are positively correlated with birthweight and adiposity. This suggests that adiponectin may be involved in regulating fetal growth.
The weight and body composition of a fetus changes drastically during the third trimester of gestation. Fetal weight increases more than fourfold and over 90% of fetal body fat is deposited during this period (Happarty, 2002). Total body fat at birth is positively associated with birthweight and reflects intrauterine growth. Additionally, increased neonatal adiposity is associated with childhood obesity (Vohr & McGarvey, 1997), which is further associated with risk factors of chronic diseases, such as high blood
pressure, hyperlipidaemia or hyperinsulinaemia (Freedman et al.,
1999). Although the mechanisms controlling intrauterine growth and relating these observations are poorly understood, adipose tissue may plausibly play a role in linking events in fetal growth to the subsequent development of adult diseases.
In addition to its role as a storage depot for fat, adipose tissue produces and secrets a number of hormones, collectively called adipocytokines, of importance in modulating metabolism and energy homeostasis. Leptin is a member of the adipocytokine family. This hormone is detectable in cord blood from early in the second trimester and its level in the cord blood increases from the middle of the third trimester towards term, in parallel with
the development of fetal adipose tissue (Geary et al., 1999). Cord
blood leptin level has been positively correlated with fetal
adiposity at birth (Schubring et al., 1999). However, the early
appearance of this hormone during fetal development and the recognition of the placenta as another source of leptin production suggest that leptin is important in fetal growth, beyond its
func-tion as an indicator of fetal adipose tissue mass (Masuzaki et al.,
1997; Clapp & Kiess, 1998; Hoggard et al., 2001).
Adiponectin, one of the most abundant adipose tissue-specific proteins, is exclusively expressed and secreted from adipose tissue
and is important in glucose and lipid metabolism (Berg et al.,
Correspondence: Chun-Hsien Yu, Department of Paediatrics, Taipei Municipal Women’s and Children’s Hospital, 12 Fu Chou Street, Taipei 100, Taiwan. Tel.: 886-2-23416489; Fax: 886-2-23944134;
Adipocytokines, weight and adiposity at birth 89
2002). Plasma adiponectin concentrations are depressed in subjects with obesity, insulin resistance or type 2 diabetes (Weyer
et al., 2001; Stefan et al., 2002; Weiss et al., 2003; Yannakoulia
et al., 2003). Plasma adiponectin levels decrease as adiposity
increases in children (Stefan et al., 2002), and adiponectin
con-centration increases after weight reduction in obese adults (Yang
et al., 2001). Moreover, plasma adiponectin concentrations are negatively correlated with leptin and fasting insulin concentrations
(Matsubara et al., 2002; Stefan et al., 2002). Insulin plays a central
role in regulating fetal growth (Fowden, 1989) and may increase leptin levels. Increases in fetal insulin and leptin have been
asso-ciated with increased adiposity at birth (Ong et al., 1999). Given
that adiponectin is inversely related to insulin, leptin and body weight during fetal growth, neonates with a large birth size would have reduced adiponectin levels. Little information is available on the levels of adiponectin in the circulation of the fetus, and the role of adiponectin in fetal growth has not been clearly
deter-mined. Lindsay et al. (2003) recently reported that adiponectin
was unrelated to birthweight in infants of type 1 diabetic mothers and a small group of control infants. The aims of this study were to examine the relationships between cord blood adiponec-tin levels and insulin, lepadiponec-tin, weight and adiposity at birth in a large group of healthy term infants of normal, nondiabetic mothers.
Subjects and methods Subjects
This hospital-based study of the mechanism that underlies the development of macrosomia was conducted between January 2001 and December 2002 at Taipei Municipal Women’s and Children’s Hospital. The study was reviewed and approved by the Institutional Review Board of the hospital. The study’s pur-pose was fully explained to each pregnant woman, and written informed consent was obtained before enrolment. Cord blood samples were collected into ethylenediamine tetraacetic acid
(EDTA) tubes immediately after delivery and were stored at 4 °C
for a maximum of 12 h. They were then centrifuged, and the
plasma was aliquoted and stored at −70 °C until assayed. Routine
measurements of birthweight and length were recorded, and infants were categorized as appropriate for gestational age (AGA, birthweight between 10th and 90th percentiles) and large for ges-tational age (LGA, birthweight more than 90th percentile) (Hsieh
et al., 1991). We prospectively enrolled the consecutive term AGA and LGA infants. Infants who were small for gestational age were regarded as high-risk newborns and were not included in this study. Additionally, infants of mothers with diabetes or any other medical complications during their pregnancy and infants with anomalies or who required intensive care were also excluded. In this study, 226 consecutive healthy infants of
singleton pregnancies born at term (gestational age 37 weeks and higher), and whose stored plasma samples were available, were analysed. Gestational age at birth was calculated from the last menstrual period, and supported by ultrasound measurements.
Anthropometry
Anthropometric measurements were performed on the second day of life by one investigator, and included measurements of weight, length, circumferences and subcutaneous skinfold thick-ness. Head, chest, upper arm, abdomen, hip, thigh and calf circum-ferences were measured using a nonstretchable, plastic Ross insertion tape (Abbott Laboratories, Columbus, OH, USA). Skin-fold thickness of the triceps, subscapular, abdominal, suprailiac, thigh and medial calf were measured twice with a Lange skinfold calliper (Cambridge Scientific Industries Inc., Cambridge, MD, USA) on the right side, according to standard techniques, and an average of two measurements was determined. Because meas-urement of skinfold thickness from multiple sites is the simplest
method to estimate percentage total body fat (Wolfe et al., 1990),
the sum of skinfold thicknesses from the six sites measured was used to represent adiposity at birth in this study. Body weight was obtained using a standard electrical scale. Recumbent length was measured with an infantometer containing a stationary head-board, a movable footboard and a built-in centimetre scale.
Biochemical assays
Cord plasma leptin was assayed using human leptin radio-immunoassay (RIA) kits (HL-81K; Linco Research, St Charles, MO, USA), with interassay coefficient of variation (CV) 3·0 – 6·2% and intra-assay CV 3·9 – 4·7%. Adiponectin in cord plasma was assayed after 1 : 500 dilution using human adiponectin RIA kits (HADP-61HK; Linco Research), with interassay CV 6·9 – 9·3% and intra-assay CV 1·2 – 6·9%. All samples were analysed in duplicate. The concentrations of cord plasma total cholesterol, low density lipoprotein (LDL)-cholesterol, high density lipoprotein (HDL)-cholesterol and triglyceride were measured by using a Hitachi 7250 special autoanalyser (Hitachi, Tokyo, Japan). Cord plasma insulin concentration was measured by RIA using BioChem ImmunoSystems kits (BioChem Pharma Inc., Italy).
Statistical analyses
Data were presented as mean ± SD. Variables that were not
normally distributed (adiponectin, leptin and insulin) were log transformed before correlation and regression analyses. Pearson correlations and linear regression analysis were used to evaluate the relationships among the variables of interest. All statistical analyses were performed using the SPSS statistical package (SPSS, Chicago, IL, USA).
90 P.-J. Tsai et al.
Results
Full-term infants were included in this study (mean gestational
age 39·2 ± 1·2 weeks). Birthweight was 3·77 ± 0·45 kg, and
length was 51·3 ± 2·2 cm. Sixty-two per cent of study subjects
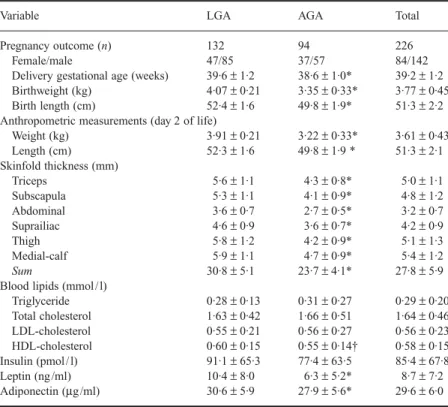
were male. Comparison of neonatal anthropometric measurements between LGA and AGA infants is shown in Table 1. As expected, all anthropometric measurements were significantly higher in LGA infants compared with AGA infants. LGA infants were delivered 1 week later on average than AGA infants. The dis-tribution of gender did not differ significantly between the LGA and AGA groups. Comparison of cord plasma concentrations of lipids and hormones between LGA and AGA infants is also shown in Table 1. Mean leptin and adiponectin were significantly
higher in the LGA group than in the AGA group (10·4 ± 8·0 vs.
6·3 ± 5·2 ng / ml, P < 0·001; 30·6 ± 5·9 vs. 27·9 ± 5·6 µg / ml, P =
0·001). The LGA group also exhibited significantly higher mean
HDL-cholesterol levels (P = 0·02), while insulin, triglyceride,
total cholesterol and LDL-cholesterol levels did not differ sig-nificantly between LGA and AGA infants.
Cord plasma leptin concentration (10·7 ± 8·4 vs. 7·5 ± 6·1 ng /
ml, P = 0·003), but neither adiponectin (29·6 ± 5·4 vs. 29·5 ± 6·2
µg / ml, P = 0·94) nor insulin (96·5 ± 70·4 vs. 79·0 ± 60·7, pmol /
l, P = 0·06) levels, was significantly higher in female infants than
in male infants.
Bivariate correlations between the cord plasma hormones and anthropometric measurements of all the infants in the study were examined (Table 2). Log cord plasma adiponectin, leptin and insulin levels correlated significantly and positively with
birthweight (r = 0·21, P = 0·001; r = 0·37, P < 0·001; r = 0·26,
P < 0·001, respectively) and with birth length (r = 0·14, P = 0·04;
r = 0·25, P < 0·001; r = 0·22, P = 0·001, respectively). Log cord
plasma levels of adiponectin, leptin and insulin also correlated significantly and positively with the sum of skinfold thicknesses
(r = 0·25, P < 0·001; r = 0·46, P < 0·001; r = 0·28, P < 0·001,
respectively). Whereas cord plasma insulin levels was unrelated
Variable LGA AGA Total
Pregnancy outcome (n) 132 94 226
Female/male 47/85 37/57 84/142
Delivery gestational age (weeks) 39·6 ± 1·2 38·6 ± 1·0* 39·2 ± 1·2
Birthweight (kg) 4·07 ± 0·21 3·35 ± 0·33* 3·77 ± 0·45
Birth length (cm) 52·4 ± 1·6 49·8 ± 1·9* 51·3 ± 2·2
Anthropometric measurements (day 2 of life)
Weight (kg) 3·91 ± 0·21 3·22 ± 0·33* 3·61 ± 0·43 Length (cm) 52·3 ± 1·6 49·8 ± 1·9 * 51·3 ± 2·1 Skinfold thickness (mm) Triceps 5·6 ± 1·1 4·3 ± 0·8* 5·0 ± 1·1 Subscapula 5·3 ± 1·1 4·1 ± 0·9* 4·8 ± 1·2 Abdominal 3·6 ± 0·7 2·7 ± 0·5* 3·2 ± 0·7 Suprailiac 4·6 ± 0·9 3·6 ± 0·7* 4·2 ± 0·9 Thigh 5·8 ± 1·2 4·2 ± 0·9* 5·1 ± 1·3 Medial-calf 5·9 ± 1·1 4·7 ± 0·9* 5·4 ± 1·2 Sum 30·8 ± 5·1 23·7 ± 4·1* 27·8 ± 5·9
Blood lipids (mmol / l)
Triglyceride 0·28 ± 0·13 0·31 ± 0·27 0·29 ± 0·20 Total cholesterol 1·63 ± 0·42 1·66 ± 0·51 1·64 ± 0·46 LDL-cholesterol 0·55 ± 0·21 0·56 ± 0·27 0·56 ± 0·23 HDL-cholesterol 0·60 ± 0·15 0·55 ± 0·14† 0·58 ± 0·15 Insulin (pmol / l) 91·1 ± 65·3 77·4 ± 63·5 85·4 ± 67·8 Leptin (ng /ml) 10·4 ± 8·0 6·3 ± 5·2* 8·7 ± 7·2 Adiponectin (µg/ml) 30·6 ± 5·9 27·9 ± 5·6* 29·6 ± 6·0
Values are presented as mean ± SD. *P ≤ 0·001, †P < 0·05 compared to LGA group by t-test.
Table 1 Subject characteristics of total infants
Table 2 Pearson correlation coefficients between cord plasma hormones and weight and length at birth and anthropometric measurements at day 2 of life
At birth Day 2 of life
Weight Length Weight Sum of skinfold thicknesses Adiponectin 0·21† 0·14‡ 0·25* 0·25*
Leptin 0·37* 0·25* 0·34* 0·46*
Insulin 0·26* 0·22† 0·21† 0·28*
*P < 0·001; †P < 0·01; ‡P < 0·05. Adiponectin, leptin and insulin were logarithmically transformed.
Adipocytokines, weight and adiposity at birth 91
to gestational age across the entire group (r = −0·10, P = 0·18),
cord plasma leptin and adiponectin level were positively related to gestational age (r = 0·15, P = 0·03; r = 0·14, P = 0·03, respectively). Cord plasma adiponectin concentration correlated positively with leptin level (r = 0·18, P = 0·007), but not with insulin level (r = 0·02, P = 0·78), and cord plasma leptin concentration cor-related positively with insulin level (r = 0·25, P < 0·001).
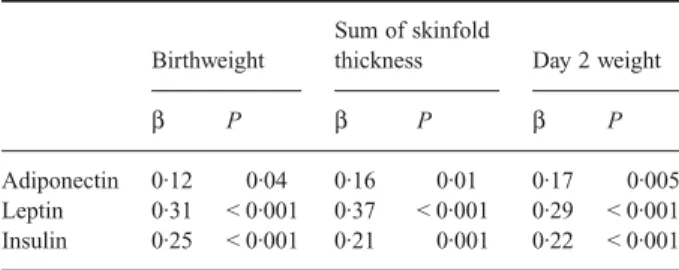
Table 3 presents a summary of the multivariate regression ana-lysis for birthweight and anthropometric measurements at day 2 of life in relation to the cord plasma hormones, adjusted for gender and gestational age. The significantly positive correlation between cord plasma adiponectin level and birthweight and the sum of skinfold thicknesses persisted after adjustment for gender, gestational age and cord plasma leptin and insulin (r = 0·12, P = 0·04; r = 0·16, P = 0·01, respectively). Cord plasma leptin level also correlated strongly with birthweight and the sum of skinfold thicknesses after adjustment for the same variables (r = 0·31,
P < 0·001; r = 0·29, P < 0·001, respectively).
Discussion
This study clearly shows that cord plasma adiponectin levels correlate significantly and positively with birthweight, neonatal adiposity and cord plasma leptin concentrations, but not with cord plasma insulin levels in the fetus. These results are in contrast to the previously reported negative correlation of adiponectin lev-els with obesity, adiposity, leptin and insulin levlev-els in children (Stefan et al., 2002) and adults (Weyer et al., 2001; Weiss et al., 2003; Yannakoulia et al., 2003). Another important observation in the present study is the relatively high adiponectin levels in the fetus, two to six times higher than those in adolescents and young adults using the same assay (Weiss et al., 2003; Yannak-oulia et al., 2003).
The mechanism of the regulation of plasma adiponectin level and its negative association with body weight and adiposity is not yet fully understood. In rodent studies, mice that were fed
on a high-fat diet exhibited reduced adiponectin levels, while caloric restriction increased the levels of adiponectin (Berg et al., 2001; Yamauchi et al., 2001). Taken together, these studies imply that dynamic changes of adiponectin levels might represent an adaptive response of adipose tissue to environmental energy supply. Accordingly, increase in plasma adiponectin levels after weight reduction in obese humans who underwent gastric partition surgery (Yang et al., 2001) may be due to reduced caloric intake following surgery, while reduced plasma adiponectin levels with increased body weight and adiposity in children (Stefan et al., 2002) may result from chronic overfeeding on high-fat diets, a common diet pattern in Western industrial societies with more than 40% of the total calories supplied from fat, rather than from weight loss or gain. This notion would explain the high adi-ponectin concentrations in cord blood found in this study, at least partially, because, in contrast to postnatal growth and development, prenatal growth is substrate and calorie limited (Sparks et al., 1998). Recent studies have shown that adiponectin has substantial effects on glucose homeostasis, by inhibiting hepatic glucose output, and on improvement in insulin sensitivity (Berg et al., 2001, 2002). During fetal growth, glucose is the main energy source of the fetus and is supplied continuously from the maternal circulation (Sparks et al., 1998). Insulin plays a significant role in increasing the uptake of circulating glucose by fetal muscle and adipose tissue. Therefore, although little information is available regarding the role of adiponectin in fetal growth, a high concen-tration of adiponectin in the fetus may be crucial to enhance the growth-promoting effect of insulin through its insulin-sensitizing action. This idea may explain the positive correlation between cord plasma adiponectin level and weight and adiposity at birth observed in this study, as well as the finding that the mean insulin concentration in the LGA infants was not significantly higher than that in AGA infants, but sufficed to cause the significant dif-ferences between the mean weights and adiposities of the two groups of infants at birth. Such a notion is supported by recent studies that have shown increased adiponectin levels following treatment with thiazolidinedione in conjunction with improved insulin sensitivity and weight gain in mice and humans (Berg
et al., 2001; Yang et al., 2002a).
Consistent with previous studies (Clapp & Kiess, 1998; Schubring et al., 1999), this study found that cord plasma leptin levels correlated significantly and positively with weight and adiposity at birth, and with cord plasma insulin levels. Although leptin has been positively associated with intrauterine growth, the mechan-ism underlying this association remains unknown. Notably, in contrast to studies in adolescents and adults (Matsubara et al., 2002; Stefan et al., 2002), this work found a significantly positive correlation between cord plasma levels of leptin and adiponectin. It has been shown that the administration of both leptin and adiponectin is required to fully normalize the insulin resistance in lipotrophic mice (Berg et al., 2001). Accordingly, high levels
Table 3 Multivariate regression analysis for birthweight and anthropometric measurements at day 2 of life in relation to cord plasma hormones
Birthweight
Sum of skinfold
thickness Day 2 weight
β P β P β P
Adiponectin 0·12 0·04 0·16 0·01 0·17 0·005 Leptin 0·31 < 0·001 0·37 < 0·001 0·29 < 0·001 Insulin 0·25 < 0·001 0·21 0·001 0·22 < 0·001 β, standardized regression coefficient adjusted for gender, gestational age and cord plasma hormones. Adiponectin, leptin and insulin were logarithmically transformed.
92 P.-J. Tsai et al.
of circulating leptin and adiponectin in the fetus can be reason-ably expected to act in synergy, or at least in compensation for each other, to enhance further the anabolic effect of insulin under normal conditions of pregnancy.
Lindsay et al. (2003) reported recently that adiponectin was high in cord blood and was unrelated to birthweight and adiposity, cord blood leptin and insulin levels. These results seem to be inconsistent with our findings except with respect to the high cord blood adiponectin levels and the relationship between the cord blood levels of adiponectin and insulin. The discrepancy between that study and our data may be related to the different subjects studied. Lindsay’s study was conducted in the infants, including preterm and term, of mothers with type 1 diabetes (n = 134) and a small group of infants of control mothers (n = 45). By contrast, our study involved a large group of healthy term infants of normal, nondia-betic mothers, and thus offers the advantage of minimizing the potential effects of gestational age, maternal diseases and /or related treatments. Notably, a similar trend of positive correlations between cord blood adiponectin levels and weight and adiposity at birth was observed in the smaller group of control infants in that study. This study also found that cord plasma leptin levels were sig-nificantly higher in female infants than in male infants, consistent with results of previous studies that found that the leptin level differed with gender in utero (Hassink et al., 1997; Freedmann
et al., 1999). Adiponectin level has been reported to be
influ-enced by gender in both animals and humans (Nishizawa et al., 2002; Yanakoulia et al., 2003); however, other studies have not found any effect of gender on adiponectin (Stefan et al., 2002; Yang et al., 2002b; Weiss et al., 2003). The result herein showed that gender does not affect adiponectin levels at birth.
In conclusion, we show that high adiponectin levels are present in the cord blood. Cord plasma adiponectin and leptin levels are significantly and positively correlated with weight and adiposity at birth, independently of cord plasma insulin levels. This sug-gests that adiponectin may be involved in some mechanisms that regulate fetal growth.
Acknowledgements
We thank the mothers who participated in this study, and the labour room staff of the Taipei Municipal Women’s and Children’s Hospital for their assistance. We also thank Dr Wei-Shiung Yang, Department of Internal Medicine, National Taiwan University Hospital, for carefully reviewing this manuscript and offering constructive advice. This study was supported by a grant from the Department of Health, Taipei City Government, Taipei, Taiwan.
References
Berg, A.H., Combs, T.P., Du, X., Rownlee, M. & Scherer, P.E. (2001) The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nature Medicine, 7, 947–953.
Berg, A.H., Combs, T.P. & Scherer, P.E. (2002) ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends in Endo-crinology and Metabolism, 13, 84 – 89.
Clapp, J.F. & Kiess, W. (1998) Cord blood leptin reflects fetal fat mass. Journal of the Society for Gynecologic Investigation, 5, 300 –303. Fowden, A.L. (1989) The role of insulin in prenatal growth. Journal of
Developmental Physiology, 12, 173 –182.
Freedman, D.S., Dietz, W.H., Srinivasan, S.R. & Berernson, G.S. (1999) The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart Study. Pediatrics, 103, 1175 –1182.
Geary, M., Herschkovitz, R., Pringle, P.J., Rodeck, C.H. & Hindmarsh, P.C. (1999) Ontogeny of serum leptin concentrations in the human. Clinical Endocrinology, 51, 189 –192.
Happarty, P. (2002) Placental regulation of fatty acids delivery and its effect on fetal growth – a review. Placenta, 23, S28 –S38.
Hassink, S.G., Lancey, E., Sheslow, D.V., Smith-Kirwin, S.M., O’Connor, D.M., Considine, R.V., Opentanova, I., Dostal, K., Spear, M.L., Leef, K., Ash, M., Spitzer, A.R. & Funanage, V.L. (1997) Placental leptin: an important new growth factor in intrauterine and neonatal development? Pediatrics, 100, 1– 6.
Hoggard, N., Hoggard, P., Thomas, L. & Lea, R.G. (2001) Leptin expres-sion in placental and fetal tissue: does not leptin have a functional role? Biochemical Society Transactions, 29, 57– 62.
Hsieh, T.T., Hsu, J.J., Chen, C.J., Chiu, T.H., Liou, J.D., Hsieh, C.C., Lo, L.M., Kuo, D.M. & Soong, Y.K. (1991) Analysis of birth weight and gestational age in Taiwan. Journal of the Formosan Medical Association, 90, 382–387.
Lindsay, R.S., Walker, J.D., Hovel, P.J., Hamilton, B.A., Calder, A.A. & Johnston, F.D. (2003) Adiponectin is present in cord blood but is unrelated to birth weight. Diabetes Care, 26, 2244 –2249.
Masuzaki, H., Mise, H., Nishimura, H., Yoshimasa, Y., Tanaka, I., Mori, T. & Nakao, K. (1997) Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in human. Nature Medicine, 3, 1029 –1033.
Matsubara, M., Maruoka, S. & Katayose, S. (2002) Inverse relationship between plasma adiponectin and leptin concentrations in normal weight and obese women. European Journal of Endocrinology, 147, 173 –180.
Nishizawa, H., Shimomura, I., Kishida, K., Maeda, N., Kuriyama, H., Nagaretani, H., Matsuda, M., Kondo, H., Furuyama, N., Kihara, S., Nakamura, T., Tochino, Y., Funahashi, T. & Matsuzawa, Y. (2002) Androgens decrease plasma adiponectin, an insulin sensitizing adipocyte derived protein. Diabetes, 51, 2734 –2741.
Ong, K.K., Ahmed, M.L., Sherriff, A., Woods, K.A., Watts, A., Golding, J. & Dunger, D.B. (1999) Cord blood leptin is associated with size at birth and predicts infancy weight gain in human. ALSPAC study team. Avon longitudinal study of pregnancy and childhood. Journal of Clinical Endocrinology and Metabolism, 84, 1145 –1148.
Schubring, C., Siebler, T., Kratzsch, J., Englaro, P., Blim, W.F., Triep, K. & Kiess, W. (1999) Leptin serum concentrations in healthy neonates within the first week of life: relation to insulin and growth hormone levels, skin fold thickness, body mass index and weight. Clinical Endo-crinology, 51, 199 –204.
Sparks, J.W., Ross, J.C. & Cetin, I. (1998) Intrauterine growth and nutrition. In: Fetal and Neonatal Physiology (eds R. A. Polin & W. W. Fox), 2nd edn, pp. 267–289. W.B. Saunders, Philadelphia. Stefan, N., Bunt, J.C., Salbe, A.D., Funahashi, T., Matsuzawa, Y. &
Tatarnni, P.A. (2002) Plasma adiponectin concentrations in children: relationships with obesity and insulinemia. Journal of Clinical Endo-crinology and Metabolism, 87, 4652– 4656.
Adipocytokines, weight and adiposity at birth 93
Vohr, B.R. & McGarvey, S. (1997) Growth patterns of large for ges-tational age and appropriate for gesges-tational age infants of gesges-tational diabetes mothers and control mothers at 1 year. Diabetes Care, 20, 1066 –1072.
Weiss, R., Dufour, S., Groszmann, A., Petersen, K., Dziura, J., Taksali, S.E., Shulman, G. & Caprio, S. (2003) Low adiponectin levels in adolescent obesity: a marker of increased intramyocellular lipid accumulation. Journal of Clinical Endocrinology and Metabolism, 88, 2014 –2018.
Weyer, C., Funahashi, T., Tanaka, S., Hotta, K., Matsuzawa, Y., Pratley, R.E. & Tataranni, P.A. (2001) Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyper-insulinemia. Journal of Clinical Endocrinology and Metabolism, 86, 1930 –1935.
Wolfe, H.M., Brans, Y.W., Gross, T.L., Bhatia, R.K. & Sokol, R.J. (1990) Correlation of commonly used measures of intrauterine growth with estimated neonatal body fat. Biology of the Neonate, 57, 167–171. Yamauchi, T., Kamon, J., Waki, H., Terauchi, Y., Kubota, N., Hara, K.,
Mori, Y., Ide, T., Murakami, K., Tsuboyama-Kasaoka, N., Ezaki, O., Akanuma, Y., Gavrilova, O., Vinson, C., Reitman, M.L., Kagechika, H., Shudo, K., Yoda, M., Nakano, Y., Tobe, K., Nagai, R., Kimura, S.,
Tomita, M., Froguel, P. & Kadowaki, T. (2001) The fat-derived hor-mone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature Medicine, 7, 941– 946.
Yang, W.S., Lee, W.J., Funahashi, T., Tanaka, S., Matsuzawa, Y., Chao, C.L., Chen, C.L., Tai, T.Y. & Chung, L.M. (2001) Weight reduc-tion increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. Journal of Clinical Endocrinology and Metabolism, 86, 3518–3519.
Yang, W.S., Jeng, C.Y., Wu, T.J., Tanaka, S., Funahashi, T., Matsuzawa, Y., Wang, J.P., Chen, C.L., Tai, T.Y. & Chuang, L.M. (2002a) Synthetic peroxisome proliferator-activated receptor-γ agonist, rosiglitazone, increases plasma levels of adiponectin in type 2 diabetic patients. Dia-betes Care, 25, 376 –380.
Yang, W.S., Lee, W.J., Funahashi, T., Tanaka, S., Matsuzawa, Y., Chao, C.L., Chen, C.L., Tai, T.Y. & Chuang, L.M. (2002b) Plasma adiponectin levels in overweight and obese Asians. Obesity Research, 10, 1104 –1110. Yannakoulia, M., Yiannakouris, N., Bluher, S., Matalas, A.L.,
Klimis-Zacas, D. & Mantzoros, C.S. (2003) Body fat mass and macronutrient intake in relation to circulating soluble leptin receptor, free leptin index, adiponectin, and resistin concentrations in healthy humans. Journal of Clinical Endocrinology and Metabolism, 88, 1730 –1736.