N A N O E X P R E S S
Open Access
Intensify the application of ZnO-based
nanodevices in humid environment: O
2
/H
2
plasma suppressed the spontaneous reaction of
amorphous ZnO nanowires
Chun-Yen Lai
1†, Tzu-Chiao Chien
1†, Ting-Yi Lin
1, Teng Ke
1, Shih-Han Hsu
1, Yun-Ju Lee
1, Chien-ying Su
2,
Jeng-Tzong Sheu
2and Ping-Hung Yeh
1*Abstract
In this work, we have demonstrated that amorphous ZnO nanobranches (a-ZnO NBs) could spontaneously react from the crystalline ZnO NWs (c-ZnO NWs) at specific humid environment. The spontaneous reaction mechanism and result can be analyzed by humidity controlling and optical microscope (OM)/scanning electron microscope (SEM)/Kelvin probe force microscopy (KPFM)/transmission electron microscopy (TEM) system. We can make the c-ZnO NWs spontaneous reaction happen at different humid environments and suppress the a-ZnO NBs spontaneous reaction by oxygen/hydrogen plasma surface passivation. The hydrogen plasma surface treatment also can improve the UV sensing sensitivity more than twofold. This work provides the mechanism and methods of the a-ZnO NBs spontaneous growth and offers the passivation treatment for strengthening and enhancing ZnO-based nanodevice application in humid environment and UV light detection, respectively.
Keywords: Spontaneous reaction; Humidity reaction; a-ZnO; Plasma passivation Background
As one of the most important materials, ZnO has been extensively applied in numerous purposes which include optics, energy [1,2], piezo-phototronics [3-6], Schottky contact nanosensors [7-9], biomedical sciences [10,11], and spintronics [12]. Due to diverse and abundant nano-structures and a great potential in nanotechnology, a great number of novel ZnO nanodevices such as piezo-electric power generators [13-16], field-effect transistors (FET) [17,18], ultraviolet photodetectors [19], Schottky diodes [6,20-22], switches [21], and flexible piezotronic strain sensors [23] are gradually under research. Those devices, moreover, are expected to operate in various environments; therefore, maintaining their great per-formance and stability for an extended period of time is required. Due to this reason, nanostructures of ZnO in different atmospheres have become an interesting topic to
study. According to several research articles, amorphous ZnCO3thin films and nanowires could be formed due to
the defacing of ZnO nanostructures by moisture and the small amount of CO2 in the atmosphere [24,25]. In this
work, we would figure out the mechanisms of the spon-taneous reaction and prove the efficacy of c-ZnO NWs surface passivation that would suppress the spontaneous reaction.
Methods
Crystalline ZnO NWs were prepared with the common procedures presented in a previous research article [26]. Briefly, a proper amount of ZnO powders, treated as the precursor and loaded on an alumina boat, were placed at the center of an alumina tube which was set in a fur-nace to serve as the reaction chamber. A furfur-nace was heated to 1,475°C and held at that temperature for 4.5 h and the gas, Argon, flowed through an alumina tube at a flow rate of 50 sccm to carry ZnO vapors to the end of an alumina tube for NWs growing. Then, the tube was cooled down to room temperature under a continuous
* Correspondence:phyeh331@mail.tku.edu.tw
†Equal contributors
1Department of Physics, Tamkang University, Tansui 25137, Taiwan Full list of author information is available at the end of the article
© 2014 Lai et al.; licensee Springer. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited.
argon flow. Crystalline-ZnO NWs were placed on the substrates (cleaned by standard processes) by homemade nanomanipulator. After that, the different samples were loaded into the various humidity conditions waiting for periodically observation. The samples were analyzed and measured by Zeiss SIGMA FESEM (Oberkochen, Germany)/Veeco Dimension 3100 SPM/JEM-2100 F FETEM (Plainview, NY, USA), and Agilent B1500A (Santa Clara, CA, USA).
Results and discussion
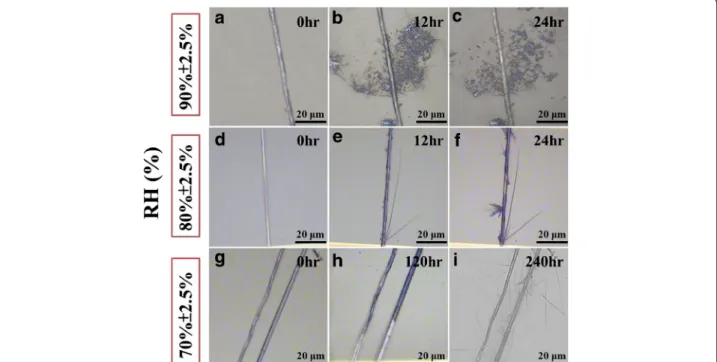
The spontaneous reaction of a-ZnO nanobranches (NBs) could be observed by optical microscopy (OM); the morphology of a-ZnO NBs was varied with time and humidity (70% ± 2.5%, 80% ± 2.5%, and 90% ± 2.5%), as shown in Figure 1, which implied that the reliable performance of ZnO nanodevices might be deteriorated or even broken down by absorbing abundant H2O
mole-cules. In high humidity (90% ± 2.5%), there are some ZnO particles that could be seen around the ZnO NWs, as illustrated in Figure 1a,b,c. In low humidity (70% ± 2.5%), a great number of thin and needle-like a-ZnO NBs formed from the c-ZnO NWs; the length and direction of the a-ZnO NBs were varied and random as shown in Figure 1g,h,i. Furthermore, when the value of humidity is around 80%, some flawed spots would become nucleate points; most a-ZnO NBs were grown from those nucleate points. Compare these three conditions; the a-ZnO NBs
could be grown much faster and thicker in humidity 80% ± 2.5% (within 12 h) than in humidity 70% ± 2.5% (almost 10 days). So the percentage of humidity will be an important parameter for the morphology of spon-taneous reaction.
The reaction mechanism of a-ZnO NBs can be studied by scanning electron microscopy (SEM) analysis as illus-trated in Figure 2a,b. The H2O molecules (light blue
bubbles) would be absorbed at the surface of c-ZnO NWs (the dark green rod) because the c-ZnO NWs are placed in the humid environment, as shown in the inset of Figure 2a. When the concentration of the H2O
mole-cules was reaching certain level, the moisture would dissolve the specific spots of the c-ZnO NWs surface. Due to the interaction between the surface of c-ZnO NWs and moisture solution, the radial concentration of Zn2+ ion would be changed because Zn2+ions gradually dissolve and diffuse from the original c-ZnO NWs sur-face into the moisture solution. When the concentration of Zn2+ ion in moisture solution meets the saturation condition, the Zn2+ ions start to segregate out from the moisture solution; the a-ZnO NBs cause to grow from the main body of the original c-ZnO NWs, which can be seen in Figure 2b. If the dimension of the original c-ZnO NWs is sufficient, the dissolving and diffusing effects can be maintained for a long period; the a-ZnO NBs will keep growing and forming ultra-long a-ZnO NBs. Nor-mally, a-ZnO NBs would be spontaneously grown from
Figure 1 The spontaneous reaction of ZnO nanobranches (NBs) can be observed by optical microscope (OM). The morphology of ZnO NBs is varied with time and humidity (70% ± 2.5%, 80% ± 2.5%, and 90% ± 2.5%). (a, b, c) In high humidity (90% ± 2.5%), plenty of ZnO particles can be found around the ZnO NWs about 12 h. (d, e, f) When the humidity is around 80% ± 2.5%, a few ZnO NBs can be found within 12 h. (g, h, i) In low humidity (70% ± 2.5%), there are no ZnO NBs can be formed until 240 h.
specific size of c-ZnO NWs, such as around hundreds of nanometers. In high humidity, however, it is difficult for a-ZnO NBs to segregate from the moisture solution, which means that the Zn2+ ion concentration in mois-ture solution is not high enough to meet the condition of saturation forming a-ZnO NBs. That is why the ultra-long a-ZnO NBs cannot be seen in high humidity (90% ± 2.5%).
ZnOð Þs þ H2Oð Þl ↔ Zn OHð Þ2 sð Þ ð1Þ
Zn OHð Þ2 sð Þþ H2Oð Þl ↔ Zn OHð Þ−3 aqð ÞþHþ ð2Þ
Zn OHð Þ2 sð Þþ 2H2Oð Þl ↔ Zn OHð Þ2−4 aqð Þþ2Hþ ð3Þ
The main reactions can be understood by the previous equations [27-29]; there are several reactive intermediates like Zn(OH)42−, Zn(OH)2, or Zn(OH)3−, which depend on
the specific parameters such as the concentration of Zn2+ ion, the amount of H2O molecules, and the pH value.
Fur-ther investigation, the spontaneous growth mechanism of a-ZnO NBs can be studied through the c-ZnO NWs sur-face potential measurement by using Kelvin probe force
microscope (KPFM) tapping mode. The surface potential of c-ZnO NWs can be changed due to the humidity ab-sorption. Before humidity treatment, the surface morph-ology and potential were smooth and almost constant (around 10 to 25 mV variation) by SEM and KPFM ana-lysis, respectively (Figure 2c). After humidity treatment, the surface morphology and potential were rough and var-ied (around 198.26 mV variation), respectively (Figure 2d). This surface potential variation might induce the a-ZnO NBs spontaneous growth.
The spontaneous growth of a-ZnO NBs can be studied by the transmission electron microscopy (TEM) system, as illustrated in Figure 3. The a-ZnO NBs can be con-firmed as an amorphous structure; the a-ZnO NBs will become new growth areas to keep extending the length of the a-ZnO NBs or growing extra a-ZnO NBs, as illus-trated in Figure 3a, and there are amorphous layers around the c-ZnO NW near the roots of a-ZnO NBs, as shown in Figure 3b. The c-ZnO NW exhibit good crys-talline feature with the growth along [001] direction, as shown in Figure 3c. The surface caves can be found on the c-ZnO NWs surface, and those caves might be the humidity influence; the dissolution direction is along [010], as shown in Figure 3d.
For general condition, the spontaneous reaction is loath to reveal in the ZnO NWs application; therefore, we have suppressed the spontaneous reaction from our
Figure 2 The spontaneous reaction mechanism of a-ZnO NBs is illustrated. (a) A uniform c-ZnO NWs (dark green rod) placed in the moisture environment surrounded by H2O molecules (light blue bubbles). The c-ZnO NW has uniform ZnO concentration which can be seen from the inset (ZnO concentration versus radius). (b) After H2O molecules absorbed at the surface of c-ZnO NWs, the Zn2+ions would be dissolved from the surface of c-ZnO NWs and became aqueous solution diffused away from the c-ZnO NWs. When the Zn2+ions and the ZnO NBs start to segregate out from the moisture solution and cause to grow from the main body of the original ZnO NWs, respectively (inset). (c, d) The surface potential was measured before and after moisture treatment.
Figure 3 The spontaneous growth of a-ZnO NBs. (a) The a-ZnO NBs became new growth areas; amorphous nanostructures are around the a-ZnO NBs. (b) There are also amorphous layers on the c-ZnO NW near the roots of a-ZnO NBs. (c) ZnO NWs exhibit a single crystalline feature with the growth along [001] direction. (d) There are surface caves can be found on the c-ZnO NW due to the humidity influence; the dissolution direction is along [010].
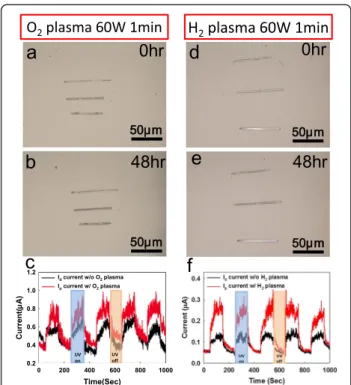
c-ZnO NWs devices by using surface oxygen/hydrogen plasma treatment [30]. Due to dangling bonds on the surface of c-ZnO NWs, H2O molecules would be
absor-bed on the c-ZnO NWs surface much easier. If we can prevent the H2O molecule from the surface of the
c-ZnO NWs, the spontaneous reaction might not happen and the ZnO nanodevices would maintain the functional-ity and performance. The c-ZnO NWs surface passivation can slow down the interaction between the moisture solu-tion and c-ZnO NWs surface; the passive c-ZnO NWs would not have the spontaneous reaction in the same humidity treatment, as seen in Figure 4a,b,c,d). Using oxygen/hydrogen plasma (60 mW) to occupy the oxygen vacancy, the a-ZnO NBs spontaneous reaction can be sup-pressed, compared with the unpassive c-ZnO NWs. Both O2and H2plasma can improve the UV detection ability,
but the H2 plasma treatment has stronger
enhance-ment, compared with O2plasma treatment, as shown in
Figure 4e,f. The UV sensing ability of ZnO NWs device also can be enhanced more than twofold by H2 plasma
treatment, as shown in Figure 4f. The plasma treatment not only can suppress the spontaneous reaction but also can enhance the UV sensing ability of the ZnO NWs devices.
Conclusions
We have demonstrated that c-ZnO NWs would spon-taneously react in humid environment; a-ZnO NBs structure can be grown with the appropriate humidity (around 70% to 85%). The spontaneous reaction is due to the interaction between the H2O molecules and the
surface of c-ZnO NWs. The spontaneous reaction mech-anism also can be proved by OM, SEM, KPFM, and TEM analyses. Finally, the a-ZnO NBs spontaneous reaction also can be suppressed by oxygen/hydrogen plasma sur-face passivation treatment; the plasma treatment could passivate the surface of the c-ZnO NWs from the H2O
molecule. The spontaneous reaction would not happen, and the ZnO NWs devices would maintain the functional-ity; for UV sensing, the sensitivity could be enhanced more than twofold by using H2plasma treatment. This research
not only provides the mechanism and methods of the a-ZnO NBs spontaneous reaction but also offers the passivation treatment for intensifying ZnO NWs device application in humid environment and enhancing the UV light detection sensitivity.
Competing interests
The authors declare that they have no competing interests. Authors’ contributions
The work presented here was performed in collaboration of all authors. CYL and TCC figured out the mechanism about this research. TYL and TK did the O2/ H2plasma treatment on the c-ZnO NWs. CYL, SHH and YJL did the FESEM and HRTEM analysis. CYS and JTS did the KPAFM analysis. PHY organized the article. All authors read and approved the final manuscript. Acknowledgements
This research was also supported by the National Science Council of Taiwan under Contracts No. NSC-101-2112-M-032-004-MY3.
Author details
1
Department of Physics, Tamkang University, Tansui 25137, Taiwan. 2Department of Materials Science and Engineering, National Chiao Tung University, Hsinchu 30050, Taiwan.
Received: 7 April 2014 Accepted: 17 May 2014 Published: 2 June 2014
References
1. Law M, Greene LE, Johnson JC, Saykally R, Yang P: Nanowire dye-sensitized solar cells. Nat Mater 2005, 4:455–459.
2. Zhang Q, Dandeneau CS, Zhou X, Cao G: ZnO nanostructures for dye-sensitized solar cells. Adv Mater 2009, 21:4087–4108. 3. Hu Y, Zhang Y, Chang Y, Snyder RL, Wang ZL: Optimizing the power
output of a ZnO photocell by piezopotential. ACS Nano 2010, 4:4220–4224.
4. Yang Q, Wang W, Xu S, Wang ZL: Enhancing light emission of ZnO microwire-based diodes by piezo-phototronic effect. Nano Lett 2011, 11:4012–4017.
5. Wang ZL: Progress in piezotronics and piezo-phototronics. Adv Mater 2012, 24:4632–4646.
6. Zhang Y, Wang ZL: Theory of piezo-phototronics for light-emitting diodes. Adv Mater 2012, 24:4712–4718.
7. Wei T-Y, Yeh P-H, Lu S-Y, Wang ZL: Gigantic enhancement in sensitivity using Schottky contacted nanowire nanosensor. J Am Chem Soc 2009, 131:17690–17695.
8. Zhou J, Gu Y, Hu Y, Mai W, Yeh P-H, Bao G, Sood AK, Polla DL, Wang ZL: Gigantic enhancement in response and reset time of ZnO UV
µ
Figure 4 The c-ZnO NWs have been passivated by O2/H2 plasma treatment. (a, b) c-ZnO NW with O2plasma (60 mW, 1 min) passivation has maintained the original forms after 48 h humidity (80% ± 2.5%) treatment. (c, d) ZnO NWs with H2plasma (60 mW, 1 min) passivation also have no a-ZnO NBs spontaneous reaction from the ZnO NWs. (e) For O2plasma treatment, the UV sensing ability can be improved. (f) For H2plasma treatment, the UV sensing ability of ZnO nanodevice also enhanced more than two fold.
nanosensor by utilizing Schottky contact and surface functionalization. Appl Phys Lett 2009, 94:191103.
9. Yeh P-H, Li Z, Wang ZL: Schottky-gated probe-free ZnO nanowire biosensor. Adv Mater 2009, 21:4975–4978.
10. Zhou J, Xu NS, Wang ZL: Dissolving behavior and stability of ZnO wires in biofluids: a study on biodegradability and biocompatibility of ZnO nanostructures. Adv Mater 2006, 18:2432–2435.
11. Li Z, Yang R, Yu M, Bai F, Li C, Wang ZL: Cellular level biocompatibility and biosafety of ZnO nanowires. J Phys Chem C 2008, 112:20114–20117. 12. Liang W, Yuhas BD, Yang P: Magnetotransport in Co-doped ZnO
nanowires. Nano Lett 2009, 9:892–896.
13. Wang ZL: Towards self-powered nanosystems: from nanogenerators to nanopiezotronics. Adv Funct Mater 2008, 18:3553–3567.
14. Lu M-P, Song J, Lu M-Y, Chen M-T, Gao Y, Chen L-J, Wang ZL: Piezoelectric nanogenerator using p-type ZnO nanowire arrays. Nano Lett 2009, 9:1223–1227.
15. Hu CJ, Lin YH, Tang CW, Tsai MY, Hsu WK, Kuo HF: ZnO-coated carbon nanotubes: flexible piezoelectric generators. Adv Mater 2011, 23:2941–2945.
16. Sohn JI, Cha SN, Song BG, Lee S, Kim SM, Ku J, Kim HJ, Park YJ, Choi BL, Wang ZL, Kim JM, Kim K: Engineering of efficiency limiting free carriers and an interfacial energy barrier for an enhancing piezoelectric generation. Energy Environ Sci 2013, 6:97–104.
17. Wang X, Zhou J, Song J, Liu J, Xu N, Wang ZL: Piezoelectric field effect transistor and nanoforce sensor based on a single ZnO nanowire. Nano Lett 2006, 6:2768–2772.
18. Fei P, Yeh P-H, Zhou J, Xu S, Gao Y, Song J, Gu Y, Huang Y, Wang ZL: Piezoelectric potential gated field-effect transistor based on a free-standing ZnO wire. Nano Lett 2009, 9:3435–3439.
19. Liang S, Sheng H, Liu Y, Huo Z, Lu Y, Shen H: ZnO Schottky ultraviolet photodetectors. J Cryst Growth2001, 225:110–113.
20. Chatman S, Poduska KM: The effect of synthesis conditions and humidity on current–voltage relations in electrodeposited ZnO-based Schottky junctions. ACS Appl Mater Interfaces 2009, 1:552–558.
21. Zhou J, Fei P, Gu Y, Mai W, Gao Y, Yang R, Bao G, Wang ZL:
Piezoelectric-potential-controlled polarity-reversible Schottky diodes and switches of ZnO wires. Nano Lett 2008, 8:3973–3977.
22. Liu X-Y, Shan C-X, Wang S-P, Zhao H-F, Shen D-Z: Intense emission from ZnO nanocolumn Schottky diodes. Nanoscale 2013, 5:7746–7749. 23. Zhou J, Gu Y, Fei P, Mai W, Gao Y, Yang R, Bao G, Wang ZL: Flexible
piezotronic strain sensor. Nano Lett 2008, 8:3035–3040.
24. Chang S-Y, Yang N-H, Huang Y-C, Lin S-J, Kattamis TZ, Liu C-Y: Spontaneous growth of one-dimensional nanostructures from films in ambient atmosphere at room temperature: ZnO and TiO2. J Mater Chem 2011, 21:4264–4271.
25. Pan Z, Tao J, Zhu Y, Huang J-F, Paranthaman MP: Spontaneous growth of ZnCO3 nanowires on ZnO nanostructures in normal ambient environment: unstable ZnO nanostructures. Chem Mater 2009, 22:149–154.
26. Pan ZW, Dai ZR, Wang ZL: Nanobelts of semiconducting oxides. Science 2001, 291:1947–1949.
27. Yamabi S, Imai H: Growth conditions for wurtzite zinc oxide films in aqueous solutions. J Mater Chem 2002, 12:3773–3778.
28. Peterson RB, Fields CL, Gregg BA: Epitaxial chemical deposition of ZnO nanocolumns from NaOH solutions. Langmuir 2004, 20:5114–5118. 29. Dem'yanets LN, Kostomarov DV, Kuz'mina IP: Chemistry and kinetics of
ZnO growth from alkaline hydrothermal solutions. Inorg Mater 2002, 38:124–131.
30. Hsu JK, Lin TY, Lai CY, Chien TC, Song JH, Yeh PH: Tunable Schottky barrier height and surface potential by using hydrogen ions. Appl Phys Lett 2013, 103:123507.
doi:10.1186/1556-276X-9-281
Cite this article as: Lai et al.: Intensify the application of ZnO-based nanodevices in humid environment: O2/H2plasma suppressed the spontaneous reaction of amorphous ZnO nanowires. Nanoscale Research Letters 2014 9:281.
Submit your manuscript to a
journal and benefi t from:
7 Convenient online submission 7 Rigorous peer review
7 Immediate publication on acceptance 7 Open access: articles freely available online 7 High visibility within the fi eld
7 Retaining the copyright to your article