Syn the sis of Sym met ric and Unsymmetric 1,1 -Dialkenylferrocenes via
Sa mar ium Diiodide Pro moted Re ac tions of 1,1 -Diacetylferrocene
with Ha lides
Shean-Jeng Jong ( ), Jim-Min Fang* ( ), Yi-Hung Liu ( ) and Y. Wang ( )
De part ment of Chem is try, Na tional Tai wan Uni ver sity, Tai pei, Tai wan 106, R.O.C.
Monoalkenylferrocenes were pre pared from 1,1 -diacetylferrocene and ap pro pri ate benzyl bro mides by the pro mo tion of sa mar ium diiodide. A prac ti cal method for prep a ra tion of both sym met ric and unsymmetric dialkenylferrocenes was also ex plored. The re ac tions were stereoselective to give only (E) dou ble bonds. The unsymmetric dialkenylferrocene bear ing elec trondonating substituent (e.g. methoxy group) and elec tronwithdrawing substituent (e.g. cyano group) on dif fer ent phenyl rings likely ex hib its a large non lin ear op -ti cal prop erty.
IN TRO DUC TION
Ferrocenyl alkenes and dienes are im por tant sub strates for ap pli ca tions in ma te rial sci ence.1-3 For ex am ple, 1 ferro
-cenyl- 2-(4-nitrophenyl)ethene and the re lated ferrocenyl alkenes ex hibit large op ti cal nonlinearities use ful for the de -vel op ment of op ti cal in for ma tion pro cess ing.1 Ferrocenyl 1,3butadiene is an im por tant sub strate for man u fac tur ing co -pol y mer and homo-polymer,2 which are em ployed as the coat ing ma te rial for aero space trans por ta tion to en hance re sis -tance against photo deg ra da tion. Ferrocene-1,3-butadiene can also be used as an en hance ment fuel in solid pro pel lants.2
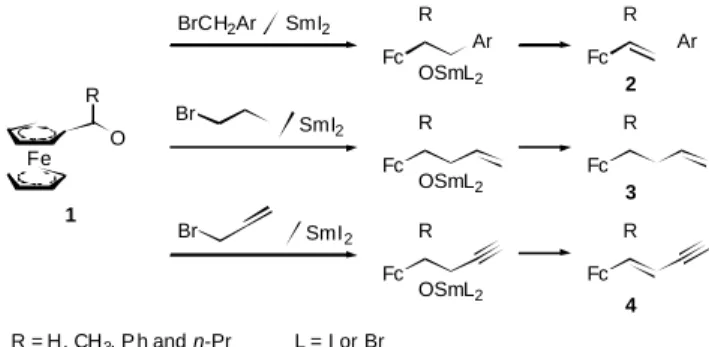
We have re cently re ported an ef fi cient method for the prep a ra tion of ferrocenyl alkenes 2, dienes 3 and enynes 4 by the SmI2 pro moted con den sa tion re ac tions of ferrocene car
-bon yls with benzyl bro mides, allyl bro mide and propargyl bro mide (Fig. 1).4 This SmI
2 pro moted onepot re ac tion pro
-ceeds with a Barbier-type ad di tion5 fol lowed by elim i na tion of HOSmL2 (L = I or Br)6 to fur nish the de sired prod ucts in
very high yields. By com par i son with the pre vi ous meth ods us ing Wittig re ac tions,7 organometallic ad di tion re ac tions,8
or Heck cou pling re ac tions,9 our method shows sev eral ad -van ta geous fea tures of sim ple op er a tion and high yields. We de scribe herein an ap pli ca tion of the SmI2 pro moted re ac tion
to 1,1 -diacetylferrocene (5) for for ma tion of sym met ric and unsymmetric 1,1 -dialkenylferrocenes (Scheme I).
RE SULTS AND DIS CUS SION
When a THF so lu tion of 1,1 -diacetylferrocene (1.2 mmol) and benzyl bro mide (2 mmol) was treated with SmI2
(3.6 mmol) at am bi ent tem per a ture for 24 h, the prod uct of monoalkenylferrocene 6a was ob tained in a quan ti ta tive yield. No dialkenylferrocene was formed. The sim i lar re ac tions with p and mmethylbenzyl bro mide also gave mono -alkenylferrocenes 6b and 6c in quan ti ta tive yields, but not the cor re spond ing dialkenylferrocenes. How ever, a dialkenyl -ferrocene 7d could be ob tained in 19% yield along with the
Ded i cated to Pro fes sor Sheng-lieh Liu on the oc ca sion of his nine ti eth birth day. * Cor re sponding au thor. E-mail: jmfang@ccms.ntu.edu.tw
Br Fc R Fe R O SmI2 BrCH2Ar SmI2 Fc R Ar Fc R Br SmI2 1 2 3 4 R = H, CH3, P h and n-Pr Ar = Ph, p-NCC6H4, m-MeOC6H4, 2-naphthyl, p-F3CC6H4 Fc R Fc R Ar Fc R OSmL2 OSmL2 OSmL2 L = I or Br
Fig. 1. Syn the sis of ferrocenyl alkenes, dienes and enynes via samaruim diiodide pro moted tan dem ad di tion and de hy dra tion of ferrocenyl car -bon yls with halides.
ma jor monoalkenylferrocene prod uct 6d (77%) by the SmI2
pro moted re ac tion with ocyanobenzyl bro mide. These re -sults might re flect the rel a tive re luc tance of the sec ond Barbier ad di tion to the monoalkenylferrocene.
For tu nately, the sec ond SmBarbier ad di tion to mono -alkenylferrocenes was achieved by a sub se quent treat ment with freshly pre pared SmI2 and ap pro pri ate benzyl bro mides.
Thus, both sym met ric and unsymmetric dialkenylferrocenes 7b and 8ac were syn the sized in an ex pe di ent man ner. A con den sa tion re ac tion of monoalkenylferrocene 6b with pro -pargyl bro mide was also car ried out by the pro mo tion of SmI2, giv ing com pound 9 in 88% yield.
The de tailed NMR and X-ray anal y ses in di cated that the dou ble bonds in the pre pared mono and dialkenyl ferro -cenes 6a-9 all ex isted as the ( E) con fig u ra tion, pre sum ably due to the sta bil ity of (E) iso mers over the (Z) iso mers. Crys tal data
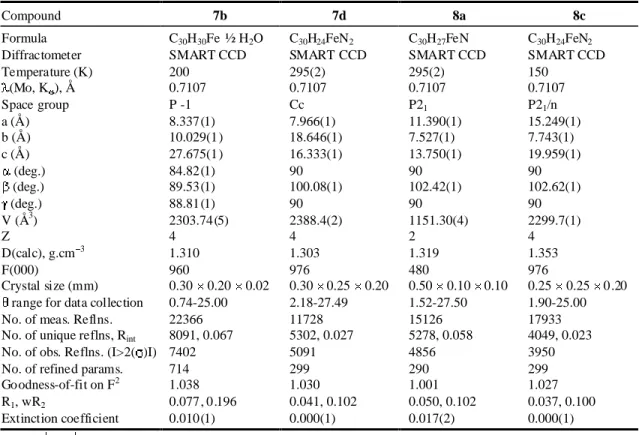
Dialkenylferrocenes 7b, 7d, 8a and 8c were re crys -tallized from CHCl3/hex ane. Es sen tial crys tal data of these
com pounds ap pear in Ta ble 1. The solid-state struc tures of 7b (R1 = R2 = p-Me), 8a (R1 = p-Me, R2 = p-CN) and 8c (R1 =
o-CN, R2 = p-CN) ex isted as the syn con for ma tion, whereas that of 7d (R1 = R2 = o-CN) ex isted as the anti con for ma tion. The syn con for ma tion of 7d might be disfavored by the steric
ef fect from the ortho-sub stitu ents. Sum mary
The dif fer ent re ac tiv ity of 1,1 -acetylferrocene against its monoalkenylferrocene de riv a tives (e.g. 6a-d) al lows us to syn the size a se ries of unsymmetric dialkenylferrocenes (e.g. 8ac and 9) with ex clu sive ( E) con fig u ra tion. The unsym -metric dialkenylferrocene 8a (R1 = p-Me, R2 = p-CN) with a do noracceptor char ac ter likely ex hib its a large non lin ear op -ti cal prop erty.1
EX PER I MEN TAL SEC TION
Melting points are un cor rected. Chem i cal shifts are re -ported rel a tive to CHCl3 ( 7.26) and CDCl3 [ C (cen tral line
of t) 77.0]. All re ac tions re quir ing an hy drous con di tions were con ducted in flame-dried ap pa ra tus un der an at mo sphere of ni tro gen. Sy ringes and nee dles for the trans fer of re agents were dried at 120 C and al lowed to cool in a des ic ca tor over P2O5 be fore use. THF was dis tilled from so dium ben zo phe
-none ketyl. Col umn chro ma tog ra phy was gen er ally car ried out on Kieselgel 60 (40-63 m) un less spec i fied. Merck sil ica gel 60F sheets were used for an a lyt i cal thinlayer chro ma tog -ra phy. Fe Me O SmI2, THF, rt, 24 h 5 Me O Fe Me Me O R1 Br R1 Fe Me Me + R1 R1 6a R1= H; 99% 6b R1 = p-Me; 100% 6c R1 = m-Me; 98% 6d R1= o-CN; 77% 7d R1= o-CN; 19% Fe Me Me R1 7b R1= R2= p-Me; 95% 8a R1= p-Me, R2= p-CN; 88% 8b R1 = m-Me, R2= o-Me; 88% SmI2, THF, rt, 24 h R2 Br SmI2, THF, rt, 24 h Br Fe Me Me 9 (88%) H H R2 8c R1 = o-CN, R2= p-CN; 90% Me (for 6b) Scheme I
Rep re sen ta tive Pro ce dure for the SmI2 Pro moted Re ac
-tions of 1,1 -Diacetylferrocene with Benzyl Bro mides and Propargyl Bro mide
Un der an at mo sphere of ar gon, a deep blue SmI2 so lu
-tion (0.1 M) was pre pared by treat ment of Sm (661 mg, 4.4 mmol) with 1,2diiodoethane (1.01 g, 3.6 mmol) in an hy -drous THF (20 mL) for 1.5 h at room tem per a ture (27 C). To the SmI2 so lu tion (cooled in an ice bath) were added a THF
so lu tion (10 mL) of ap pro pri ate bro mide (2.0 mmol) and 1,1 diacetylferrocene (1.2 mmol). The ice bath was re -moved, and the mix ture was kept stir ring at 0-27 C for 24 h. The sep tum was re moved, and the mix ture was stirred at room tem per a ture for ad di tional 48 h. The mix ture was passed through a short sil ica gel col umn by rinse with EtOAc/hex ane (1:1). The fil trate was con cen trated, and chromatographed by elu tion with EtOAc/hex ane (5:95) to give the de sired con den -sa tion prod ucts.
1-Acetyl-1 -(1-methyl-2-phenyl)ethenylferrocene (6a) Ac cord ing to the rep re sen ta tive pro ce dure, the SmI2
pro moted re ac tion of 1,1 -acetylferrocene (324 mg, 1.2 mmol) with benzyl bro mide (342 mg, 2.0 mmol) gave the ti tle com pound (409 mg) in quantivative yield (99 %). 6a: brown solid, mp 92-94 C; IR (KBr) 1612 cm-1; 1H NMR (CDC13, 300 MHz) 2.15 (3H, d, J = 1.0 Hz), 2.32 (3H, s), 4.29 (2H, t , J = 1.7 Hz), 4.45 (2H, t, J = 1.7 Hz), 4.49 (2H, t, J = 1.7 Hz), 4.72 (2H, t. J = 1.7 Hz), 6.73 (1H, d, J = 1.0 Hz), 7.37-7.20 (5H, m); 13C NMR (CDCl3, 75 MHz) 16.8, 27.5, 67.0 (2 ×), 70.5 (4 ×), 73.3 (2 ×), 80.1, 90.7, 104.7, 126.3, 128.1 (2 ×), 129.0 (2 ×), 133.2, 137.8, 201.8; FAB-MS m/z 344 (M+); HRMS Calcd for C21H2 0FeO: 344.0864. Found:
344.0876.
1-Acetyl-1 -[1-methyl-2-(4-methylphenyl)]ethenylferrocene (6b)
Ac cord ing to the rep re sen ta tive pro ce dure, the SmI2
pro moted re ac tion of 1,1 -acetylferrocene (324 mg, 1.2 mmol) with 4-methylbenzyl bro mide (370 mg, 2.0 mmol) gave the ti tle com pound (409 mg) in quantivative yield (100%). 6b: Red-brown solid; mp 60-62 C; IR (KBr) 1658 cm-1; 1H NMR (CDCl3, 200 MHz) 2.15 (3H,d, J = 1.3 Hz), 2.32 (3H, s), 3.35 (3H, s), 4.28 (2H, t, J = 2.0 Hz), 4.45 (2H, t, J = 2.0 Hz), 4.49 (2H, t, J = 2.0 Hz), 4.72 (2H, t, J = 2.0 Hz), 6.71 (1H, s), 7.26-7.13 (4H, m); 1 3C NMR (CDCl3, 50 MHz) 16.8, 21.1, 27.5, 66.9 (2 ×), 70.4 (4 ×), 73.2 (2 ×), 80.5, 90.9, 124.5, 128.8 (4 ×), 132.3, 134.9, 135.9, 201.8; FAB-MS m/z 358 (M+); HRMS Calcd for C22H2 2FeO: 358.1020. Found:
358.1023.
ORTEP draw ing of com pound 8c ORTEP draw ing of com pound 7b
ORTEP draw ing of com pound 7d
1-Acetyl-1 -[1-methyl-2-(3-methylphenyl)]ethenylferrocene (6c)
Ac cord ing to the rep re sen ta tive pro ce dure, the SmI2
pro moted re ac tion of 1,1 -acetylferrocene (324 mg, 1.2 mmol) with 3-methylbenzyl bro mide (370 mg, 2.0 mmol) gave the ti tle com pound (420 mg) in 98% yield. 6c : brown oil; IR (KBr) 1671 cm-1; 1H NMR (CDCl 3, 200 MHz) 2.16 (3H, s), 2.33 (3H, s), 3.37 (3H, s), 4.29 (2H, t, J = 1.9 Hz), 4.45 (2H, t, J =1.9 Hz), 4.49 (2H, t, J = 1.9 Hz), 4.72 (2H, t, J = 1.9 Hz), 6.71 (1H, s), 7.29-7.03 (4H, m); 13C NMR (CDCl3, 50 MHz) 16.8, 21.4, 27.5, 66.9 (2 ×), 70.4 (4 ×), 73.3 (2 ×), 79.9, 90.8, 124.7, 126.0, 127.0, 128.0, 129.6, 132.9, 137.6, 137.7; FAB-MS m/z 358 (M+); HRMS Calcd for C22H22FeO: 358.1020. Found: 358.1028.
1-Acetyl-1 -[1-methyl-2-(2-cyanophenyl)]ethenylferrocene (6d) and 1,1 Bis[1methyl2(2cyanophenyl)]ethenyl -ferrocene (7d)
Ac cord ing to the rep re sen ta tive pro ce dure, the SmI2
pro moted re ac tion of 1,1 -acetylferrocene (324 mg, 1.2
mmol) with 2-cyanobenzyl bro mide (392 mg, 2.0 mmol) gave 6d (420 mg, 77%) and 7d (110 mg, 19%). 6d: Red-brown oil; IR (KBr) 2217, 1669 cm-1; 1H NMR (CDCl3, 300 MHz) 2.06 (3H, d, J = 1.3 Hz), 2.32 (3H, s), 4.31 (2H, t, J = 3.7 Hz), 4.77-4.87 (6H, m), 6.85 (1H, m), 7.29 (1H, t, J = 7.5 Hz), 7.44 (1H, d, J = 7.5 Hz), 7.53 (1H, t, J = 7.5 Hz), 7.60 (1H, d, J = 7.5 Hz); 13C NMR (CDC1 3, 75 MHz) 16.9, 27.6, 67.3 (2 ×), 70.6 (2 ×), 70.9 (2 ×), 73.7 (2 ×), 80.0, 88.6, 112.0, 118.2, 120.2, 126.6, 129.8, 131.9, 132.2, 133.7, 138.5, 141.5, 201.7; FAB-MS m/z 369 (M+); HRMS Calcd for C22H19FeNO: 369.0816. Found: 369.0811. 7d: Red-brown solid; mp 122-124 C; IR (KBr) 2227, 1620 cm-1; 1H NMR (CDCl3, 300 MHz) 2.14 (6H, d, J = 1.2 Hz), 4.35 (4H, t, J = 1.5 Hz), 4.54 (4H, t, J = 1.5 Hz), 6.88 (2H, s), 7.26 (2H, t, J = 7.5 Hz), 7.35 (2H, d, J = 7.5 Hz), 7.47 (2H, t, J = 7.5 Hz), 7.60 (2H, d, J = 7.5 Hz); 13C NMR (CDCl3, 75 MHz) 17.3 (2 ×), 67.4 (4 ×), 70.9 (4 ×), 88.0 (2 ×), 112.1 (2 ×), 118.4 (2 ×), 119.3 (2 ×), 126.2 (2 ×), 129.7 (2 ×), 132.1 (2 ×), 133.8 (2 ×), 139.7 (2 ×), 141.9 (2 ×); FAB-MS m/z 468 (M+); HRMS Calcd for C 30H2 4FeN2: 468.1289. Found: Table 1. Crystal Data of Compounds 7b, 7d, 8a and 8c
Compound 7b 7d 8a 8c
Formula C30H30Fe ½ H2O C30H24FeN2 C30H27FeN C30H24FeN2
Diffractometer SMART CCD SMART CCD SMART CCD SMART CCD
Temperature (K) 200 295(2) 295(2) 150 (Mo, K ), Å 0.7107 0.7107 0.7107 0.7107 Space group P -1 Cc P21 P21/n a (Å) 8.337(1) 7.966(1) 11.390(1) 15.249(1) b (Å) 10.029(1) 18.646(1) 7.527(1) 7.743(1) c (Å) 27.675(1) 16.333(1) 13.750(1) 19.959(1) (deg.) 84.82(1) 90 90 90 (deg.) 89.53(1) 100.08(1) 102.42(1) 102.62(1) (deg.) 88.81(1) 90 90 90 V (Å3) 2303.74(5) 2388.4(2) 1151.30(4) 2299.7(1) Z 4 4 2 4 D(calc), g.cm3 1.310 1.303 1.319 1.353 F(000) 960 976 480 976 Crystal size (mm) 0.30 0.20 0.02 0.30 0.25 0.20 0.50 0.10 0.10 0.25 0.25 0.20 range for data collection 0.74-25.00 2.18-27.49 1.52-27.50 1.90-25.00
No. of meas. Reflns. 22366 11728 15126 17933
No. of unique reflns, Rint 8091, 0.067 5302, 0.027 5278, 0.058 4049, 0.023
No. of obs. Reflns. (I>2( )I) 7402 5091 4856 3950
No. of refined params. 714 299 290 299
Goodness-of-fit on F2 1.038 1.030 1.001 1.027 R1, wR2 0.077, 0.196 0.041, 0.102 0.050, 0.102 0.037, 0.100 Extinction coefficient 0.010(1) 0.000(1) 0.017(2) 0.000(1) Rint= Ii- I / Rl= Fo-Fc / Fo wR2= ( w Fo 2 -Fc 2 2 / wFo 4 )½
468.1290. The struc ture of 7d (recrystallized from CHCl3/
hex ane) was con firmed by an X-ray dif frac tion anal y sis. 1,1 -Bis[1-methyl-2-(4-methylphenyl)]ethenylferrocene (7b)
Ac cord ing to the rep re sen ta tive pro ce dure, the SmI2
(0.9 mmol) pro moted re ac tion of the sub sti tuted acetyl ferro -cene 6b (215 mg, 0.6 mmol) with 4-methylbenzyl bro mide (185 mg, 1.0 mmol) gave the ti tle com pound (255 mg) in 95% yield. 7b: Red-brown solid; mp 127-129 C; IR (KBr) 1626 cm-1; 1H NMR (CDCl3, 200 MHz) 2.19 (3H, s), 2.20 (3H, s),
2.36 (6H, s), 4.26 (4H, t, J = 1.9 Hz), 4.44 (4H, t, J = 1.9 Hz), 6.69 (2H, s), 7.24-7.10 (8H, m); 1 3C NMR (CDCl3, 50 MHz)
17.0 (2 ×), 21.2 (2 ×), 66.7 (4 ×), 69.7 (5 ×), 90.0 (2 ×), 123.3 (2 ×), 128.8 (8 ×), 133.7 (2 ×), 135.4 (2 ×), 135.5, 152.2; FAB-MS m/z 446 (M+); HRMS Calcd for C30H30Fe: 446.1693,
Found: 446.1697. The struc ture of 7b (recrys tallized from CHCl3/hex ane) was con firmed by an Xray dif frac tion anal y
-sis.
1-[1-Methyl-2-(4-cyanophenyl)]ethenyl-1 -[1-methyl-2-(4-methylphenyl)]ethenylferrocene (8a)
By a pro ce dure sim i lar to that for com pound 7b, the SmI2 pro moted re ac tion of the sub sti tuted acetylferrocene 6b
(215 mg, 0.6 mmol) with 4-cyanobenzyl bro mide (196 mg, 1.0 mmol) gave the ti tle com pound 8a (241 mg) in 88% yield. 8a: Red-brown solid, mp 157-159 C; IR (KBr) 2223, 1606 cm-1; 1H NMR (CDCl 3, 300 MHz) 2.18 (3H, d, J = 1.1 Hz), 2.20 (3H, d, J = 1.1 Hz), 2.38 (3H, s), 4.28 (2H, t, J = 2.0 Hz), 4.33 (2H, t, J = 2.0 Hz), 4.48-4.46 (4H, m), 6.64 (1H, s), 6.68 (lH, s), 7.17-7.10 (3H, m), 7.27 (2H, d, J = 8.5 Hz); 1 3C NMR (CDCl3, 75 MHz) 17.0, 17.2, 21.1, 66.7 (2 ×), 66.9 (2 ×), 69.7 (2 ×), 70.2 (2 ×), 88.6, 90.3, 108.7, 119.2, 121.6, 123.6, 128.7, 128.8 (2 ×), 129.2 (2 ×), 131.7 (2 ×), 131.9, 133.1, 135.1, 135.7, 138.6, 142.8; FAB-MS m/z 457 (M+); HRMS Calcd for C30H27FeN: 457.1493, Found: 457.1496. The struc
-ture of 8a (recrystallized from CHCl3/hex ane) was con firmed
by an X-ray dif frac tion anal y sis.
1-[1-Methyl-2-(2-methylphenyl)]ethenyl-1 -[1-methyl-2-(3-methylphenyl)]ethenylferrocene (8b)
By a pro ce dure sim i lar to that for com pound 7b, the SmI2 pro moted re ac tion of the sub sti tuted acetylferrocene 6c
(215 mg, 0.6 mmol) with 2-methylbenzyl bro mide (185 mg, 1.0 mmol) gave the ti tle com pound 8b (241 mg) in 88% yield. 8b: Red-brown oil; IR (KBr) 1630, 1602 cm- 1; 1H NMR (CDCl3, 300 MHz) 1.97 (3H, s), 2.14 (3H, s), 2.17 (3H, s), 2.25 (3H, s), 4.18 (4H, m), 4.36 (4H, m), 6.63 (2H, s), 6.93 (8H, m); 1 3C NMR (CDCl 3, 75 MHz) 16.8, 17.1, 20.1, 21.5, 66.7 (2 ×), 66.8 (2 ×), 70.0 (2 ×), 70.1 (2 ×), 89.4, 89.9, 122.4, 123.6, 125.3, 126.0, 126.4, 126.7, 128.0, 129.4, 129.6, 129.8, 134.3, 134.4, 136.3. 137.5, 137.6, 138.3; FAB-MS m/z 446 (M+); HRMS Calcd for C30H3 0Fe: 446.1697. Found:
446.1678.
1-[1-Methyl-2-(2-cyanophenyl)]ethenyl-1 -[1-methyl-2-(4-cyanophenyl)]ethenylferrocene (8c)
By a pro ce dure sim i lar to that for com pound 7b, the SmI2 pro moted re ac tion of the sub sti tuted acetylferrocene 6d
(221 mg, 0.6 mmol) with 4-cyanobenzyl bro mide (196 mg, 1.0 mmol) gave the ti tle com pound 8c (253 mg) in 90% yield. 8c: Red-brown solid, mp 123-125 C; IR (KBr) 2226, 1622, 1602 cm-1; 1H NMR (CDC13, 300 MHz) 2.10 (3H, d, J = 1.1 Hz), 2.20 (3H, d, J = 1.1 Hz), 4.30 (2H, t, J = 1.7 Hz), 4.35 (2H, t, J = 1.7 Hz), 4.50 (4H, m), 6.67 (1H, s), 6.85 (1H, s), 7.63-7.28 (8H, m); 13C NMR (CDCl3, 75 MHz) 17.1,17.3, 67.2 (5 ×), 70.4 (2 ×), 70.7 (2 ×), 88.0, 88.8, 108.7, 111.9, 118.3, 119.2, 119.3, 121.8, 126.3, 129.2 (2 ×), 129.4, 131.7 (2 ×), 132.0, 132.7, 138.4, 139.3, 141.6, 142.8; FAB-MS m/z 468 (M+); HRMS Calcd for C30H24FeN2: 468.1289. Found:
468.1304. The struc ture of 8c (recrystallized from CHCl3/
hex ane) was con firmed by an X-ray dif frac tion anal y sis. 1-[1-Methyl-2-(4-methylphenyl)]ethenyl-1 -(pent-1-yn-3-en-4-yl)ferrocene (9)
By a pro ce dure sim i lar to that for com pound 7b, the SmI2 pro moted re ac tion of the sub sti tuted acetylferrocene 6b
(430 mg, 1.2 mmol) with propargyl bro mide (297 mg, 2.0 mmol) gave the ti tle com pound 9 (402 mg) in 88% yield. 9: Red-brown oil; IR (KBr) 1625, 1604 cm-1; 1H NMR (CDCl 3, 300 MHz) 2.22 (3H, s), 2.27 (3H, s), 2.41 (3H, s), 3.28 (1H, d, J = 2.3 Hz), 4.28 (2H, m), 4.31 (2H,m), 4.41 (2H, m), 4.46 (2H, m), 5.70 (1H, d, J = 2.3 Hz), 6.75 (1H, s), 7.22 (2H, d, J = 8.0 Hz), 7.31 (2H, d, J = 8.0 Hz); 13C NMR (CDCl3, 75 MHz) 16.9, 18.2, 21.1, 66.8 (2 ×), 66.9 (2 ×), 70.0 (2 ×), 70.5 (2 ×), 81.3 (2 ×), 82.7, 85.8, 90.2, 101.0, 123.7, 128.8 (2 ×), 128.9 (2 ×), 133.1, 135.2, 135.6, 149.4; FAB-MS m/z 380 (M+), HRMS Calcd for C25H2 4Fe: 380.1227. Found: 380.1224.
AC KNOWL EDG MENT
We thank the Na tional Sci ence Coun cil for fi nan cial sup port.
Key Words
1,1 -Diacetylferrocene; 1,1 -Dialkenylferrocenes; Samaruim diiodide.
REF ER ENCES
1. (a) Green, M. L. H.; Marder, S. R.; Thomp son, M. E.; Bandy, J. A.; Bloor, D.; Ko lin sky, P. V.; Jones, R. J. Na -ture 1987, 330, 360. (b) Ghosal, S.; Samoc, M.; Prasad, P. N.; Tufariello, J. J. J. Phys. Chem. 1990, 94, 2847. (c) Calabrese, J. C.; Cheng, L. T. J. Am. Chem. Soc. 1991, 113, 7227. (d) Bunting, H. E.; Green, M. L. H.; Marder, S. R.; Thomp son, M. E.; Bloor, D.; Ko lin sky, P. V.; Jones, R. J. Poly he dron 1992, 11, 1489. (e) Kott, K. L.; NcMahon, R. J. J. Org. Chem. 1992, 57, 3097. (f) Wright, M. E.; Toplikar, E. G. Macromolecules 1992, 25, 6050. (g) Togni, A.; Rihs, G. Organometallics 1993, 12, 3368. (h) Coe, B. J.; Jones, C. J.; McCleverty, J. A.; Bloor, D.; Cross, G. J. Organomet. Chem. 1994, 464, 225. (i) Alain, V.; Blanchard-Desce, M.; Chen, C.-T.; Marder, S. R.; Fort, A.; Barzoukas, M. Synth. Met. 1996, 81, 133. (j) Mata, J.; Uriel, S.; Peris, E.; Llusar, R.; Houbrechts, S.; Persoons, A. J. Organomet. Chem. 1998, 562, 197. (k) Herrmann, R.; Pedersen, B.; Wag ner, G.; Youn, J.-H. J. Organomet. Chem. 1998, 571, 261. (l) Balavoine, G. G. A.; Daran, J.-C.; Iftime, G.; Lacroix, P. G.; Manoury, E.; Delaire, J. A.; Maltey-Fanton, I.; Nakatani, K.; Bella, S. Di Organometallics 1999, 18, 21.
2. (a) Pon der, B. W.; Barnhill, C. W. US Pat ent 3739004 (1973). (b) Huskins, C. W.; Van Landuyt, D. C. US Pat ent 3843426 (1974). (c) Gauiter, J. C. C.; Raynal, S. F. US Pat ent 4661608 (1987). (d) Gautier, J. C. C.; Fontanille. M. H.; Raynal, S. F. US Pat ent 4647628 (1987). (e) Miller, E. J.; Weigelt, C. A.; Serth, J. A.; Rusyid, R.; Brenner, J.; Luck, L. A.; Godlewski, M. J. Organomet. Chem. 1992, 440, 91. (f) Grevels, F.-W.; Kuran, A.; Ozkar, S.; Zora, M. J. Organomet. Chem. 1999, 587, 122. 3. In cor po ra tion of ferrocenyl alkenes in charge-transfer com plexes, por phy rins, crown ethers, see: (a) Togin, A.; Hobi, M.; Rihs, G.; Rist, G.; Albinati, A. Organo -metallics 1994, 73, 1224. (b) An drews, M. P.; Blackburn, C.; McAleer, J. F.; Pa ter, V. D. J. Chem. Soc., Chem. Commun. 1987, 1122. (c) Bur rell, A. K.; Camp bell, W.; Of fi cer, D. L. Tet ra he dron Lett. 1997, 38, 1249. For spec
-tro scopic and bind ing stud ies of ferrocene alkenes and polyenes, see: (d) Bochmann, M.; Lu, J.; Can non, R. D. J. Organomet. Chem. 1996, 518, 97. (e) Solcaniova, E.; Toma, S.; Liptaj, T. Col lect. Czech. Chem. Commun. 1986, 51, 670. (f) Liu, J.; Cas tro, R.; Abboud, K. A.; Kaifer, A. E. J. Org. Chem. 2000, 65, 6973. For use as an an a log of anticancer drug Tamoxifen, see: (g) Top, S.; Tang, J.; Vessieres, A.; Carrez, D.; Provot, C.; Jaouen, G. Chem. Commun. 1996, 955.
4. (a) Jong, S.-J.; Fang, J.-M. J. Org. Chem. 2001, 66, 3533. (b) Fang, J.-M.; Jong, S.-J. US Pat ent 6211392 B1 (April 3, 2001).
5. (a) Gi rard, P.; Namy, J.-L.; Kagan, H. B. J. Am. Chem. Soc. 1980, 102, 2693. (b) Hamann-Gaudinet, B.; Namy, J.-L.; Kagan, H. B. Tet ra he dron Lett. 1997, 38, 6585. 6. Ferrocenyl group is known to sta bi lized the car boca
-tion. For for ma tion of ferrocenyl -carbocations from the cor re spond ing al co hols and dienes, see: (a) Siglmueller, F.; Herrmann, R.; Ugi, I. Tet ra he dron 1986, 42, 5931. (b) Ortaggi, G. Gazz. Chim. Ital. 1987, 117, 75. (c) Zou, C.; Wrighton, M. S. J. Am. Chem. Soc. 1990, 112, 7578. (d) Klimova, E. I.; Klimova, T. B.; Mar ti nez, M. G.; Meleshonkova, N. N.; Ruis, L. R. Mendeleev Commun. 1997, 233.
7. In ad di tion to Ref. 1d, 1f, 1i, 1j, 2e, 3a and 3e, see: (a) Osgerby, J. M.; Pauson, P. L. J. Chem. Soc. 1961, 4604. (b) Pon der, B. W.; Kneisel, R. C.; Lewis, D. H. Org. Prep. Proc. Int. 1971, 3, 171. (c) Lewis, D. H.; Neal, M. C.; Pon der, B. W. Synth. Commun. 1972, 2, 93. For a sim i lar re ac tion us ing arsonium salts, see: (d) You, X.; Sun, H.; Peng, X.; Yue, C.; Li, C.; Wu, H. Inorg. Chim. Acta 1995, 234, 139.
8. In ad di tion to Ref. 2a, see: (a) Van Landuyt, D. C. US Pat -ent 3751441 (1973). (b) Stephens, W. D.; Wil lis, T. C.; Combs, C. S. US Pat ent 3847871 (1974). (c) Duran, M.; Konstantinovic, S.; Pavlovic, V.; Predojevic, J.; Ratkovic, Z.; Rufinska, A.; Simova, S. J. Serb. Chem. Soc. 1995, 60, 737. Chem. Abst. 124, 56207. (d) Qiu, C.; Zhang, Y. Huaxue Shiji 1992, 14, 104.
9. (a) Koenig, B.; Zieg, H.; Bubenitschek, P.; Jones, P. G. Chem. Ber. 1994, 127, 1811. (b) Qian, Y. Huagong Shikan 1998, 12, 11. (c) Naskar, D.; Das, S. K.; Giribabu, L.; Maiya, B. G.; Roy, S. Organometallics 2000, 19, 1464.