Hyperglycemia and Chronic Liver Diseases on Risk of Hepatocellular Carcinoma in Chinese Patients with Type 2 Diabetes-National Cohort of Taiwan
Diabetes Study
Chia-Ing Li,§ 1,2 Hsuan-Ju Chen,§3,4 Hsueh-Chou Lai,1,5 Chiu-Shong Liu,1,2,6
Wen-Yuan Lin,2,6 Tsai-Chung Li,7,8,Cheng-Chieh Lin 1,2,6 *
1. School of Medicine, College of Medicine, China Medical University, Taichung, Taiwan
2. Department of Medical Research, China Medical University Hospital, Taichung, Taiwan
3. Management Office for Health Data, China Medical University Hospital, Taichung, Taiwan
4. School of Public Health, College of Public Health, China Medical University, Taichung, Taiwan
5. Division of Hepatogastroenterology, Department of Internal Medicine, China Medical University Hospital, Taichung, Taiwan
6. Department of Family Medicine, China Medical University Hospital, Taichung, Taiwan
7. Graduate Institute of Biostatistics, College of Management, China Medical University, Taichung, Taiwan
8. Department of Healthcare Administration, College of Health Science, Asia University, Taichung, Taiwan
* Correspondence to: Cheng-Chieh Lin & Tsai-Chung Li
China Medical University, 91 Hsueh-Shih Road, Taichung, 40421, Taiwan, Tel: 886-4-2205-2121 ext. 7629, Fax: 886-4-2207-8539, e-mail: cclin@mail.cmuh.org.tw §Authors share co-senior authorship.
Running title: Hyperglycemia and Chronic Liver Diseases on Risk of Hepatocellular Carcinoma
Novelty & Impact Statements
This is the first and largest study to examine whether glycated hemoglobin A1C
(HbA1C) and chronic liver diseases is associated with hepatocellular carcinoma
(HCC) in Chinese type 2 diabetic patients. This study found a significant linear trend
in HCC incidence with increasing HbA1c and significant HRs of HCC for patients
with a level of HbA1c ≥ 9% with alcoholic liver damage, liver cirrhosis, hepatitis B
Abstract
This study examined whether glycated hemoglobin A1C (HbA1C) and chronic liver diseases are associated with hepatocellular carcinoma (HCC) risk in type 2 diabetic patients. A retrospective cohort study consisting of 51,705 patients with type 2 diabetes aged 30 and over enrolled in the National Diabetes Care Management Program before 2004 was used in Cox proportional hazards models. HbA1C was independently associated with HCC incidence, and multivariate-adjusted hazard ratio (HR) of HCC was 1.20 (95% confidence interval, CI: 1.02-1.41) for patients with a level of HbA1c ≥ 9% compared with patients with a level of HbA1c < 7% after multivariate adjustment. We observed a significant linear trend in HCC incidence with increasing HbA1c (P for trend = 0.02, HR = 1.07, 95% CI = 1.01-1.12 for every 1% increment in HbA1c). We observed significant HRs of HCC for patients with a level of HbA1c ≥ 9% with alcoholic liver damage, liver cirrhosis, HBV, HCV, and any one of chronic liver diseases compared with patients with a level of HbA1c < 9% and no counterpart comorbidity in the entire sample (HR = 8.63, 95% CI = 1.41-52.68; HR = 5.02, 95% CI = 3.10-8.12; HR = 2.53, 95% CI = 1.10-5.85; HR = 1.79, 95% CI = 1.01-3.17; and HR = 3.59, 95% CI = 2.56-5.02, respectively). Our results suggest significant joint associations of HbA1c ≥ 9% and chronic liver diseases. Lifestyle or treatment interventions such as maintaining a satisfactory glycemic control and chronic liver diseases may reduce the burden of HCC.
Introduction
Liver cancer is the fourth leading cause of cancer mortality worldwide. In 2008, the International Agency for Research on Cancer (IARC) reported that liver cancer is the fifth most common cancer in men (523 432 cases, 7.9% of the total) and the seventh in women (226 312 cases, 3.7% of the total).1,2 The region with the highest
incidence of liver cancer is Eastern Asia. According to statistics reported by the Department of Health in Taiwan, the standardized incidence rates and age-standardized mortality in Taiwan are approximately 3-fold higher than those worldwide in men and in women. 1,2
The worldwide prevalence of diabetes is increasing, and the prevalence and incidence rates of diabetes have increased in various age, gender, and race groups. The International Diabetes Federation proposed that the causes for increased diabetes prevalence are population ageing and unhealthy lifestyle behaviors,3 including
physical inactivity, smoking, alcohol consumption, and an unhealthy diet. Recent epidemiological findings of cohort and case-control studies have indicated a link between type 2 diabetes and cancer in several organs,particularly the liver.4-8 Type 2
diabetes mellitus and many types of cancer share common risk factors such as
smoking, alcohol consumption, obesity, physical inactivity, a high calorie intake, and particularly saturated fat intake.9 Certain possible biologic mechanisms also support
the independent contribution of diabetes as a risk factor for cancer, such as plasma levels of insulin-like growth factor 1 (IGF-1) 10-13 and dysregulation of the tuberous
sclerosis (TSC) 1 /TSC2/mTOR signaling pathway by IκB kinaseβ (IKKβ).14-17
For diabetes care, glycated hemoglobin A1C (HbA1c) is the most widely used marker of long-term glycoregulation, reflecting endogenous glucose levels 120 days before measurement.18 HbA1c is at the core of hyperglycemia clinical management
and is a crucial monitoring tool in treating diabetic patients. HbA1c has been treated as an improved long-term glycemic control marker compared with fasting glucose because it can be tested in a non-fasting status and is a relatively stable marker for glucose levels. Hyperglycemia has been hypothesized to play a role in the morbidity and mortality of microvascular 19-21 and macrovascular complications 22-28 in diabetics.
Its role in the morbidity and mortality of cancer has recently been explored in the general population 29-31 and in patients with diabetes.32-38 Among those studies of
patients with diabetes, only 2 focused on liver cancer.35,36 One study adopted a
case-control study design and the other study was a cohort study with the primary aim of exploring whether low low-density lipoprotein (LDL) cholesterol and triglyceride levels and the use of insulin or statins modify the promoting effect of chronic hepatitis B virus (HBV) infection on hepatocellular carcinoma (HCC),35 instead of examining
the association between HbA1c and HCC.
To clarify the role of glucose control on the risk of HCC incidence, we conducted a large cohort study of Chinese patients with type 2 diabetes in Taiwan. We also examined the association of glucose control status, measured according to HbA1C, and chronic liver diseases, including alcoholic liver damage, nonalcoholic
fatty liver disease, liver cirrhosis, HBV, and hepatitis C virus (HCV) with HCC incidence in numerous type 2 diabetes patients aged 30 years and over, followed up for an average of 8.17 years.
Methods
Study population
We conducted a retrospective cohort study, the Taiwan Diabetes Study, among all enrollees in the National Diabetes Care Management Program (NDCMP), which was implemented by Taiwan's Ministry of Health and Welfare since 2001. The main aim of this NDCMP program is to increase the quality of diabetes care via increasing
the frequency of monitoring, providing continuity of care, and decreasing diabetes-related complications. The Taiwan Diabetes Study is a population-based cohort study of 63 084 ethnic Chinese patients with type 2 diabetes who were enrolled in the NDCMP in Taiwan from 2002 to 2004. The date of entry into the NDCMP was defined as the index date. All patients with a clinically confirmed diagnosis of diabetes mellitus (DM) based on American Diabetes Association (ADA) criteria (International Classification of Diseases, ninth revision, Clinical Modification (ICD-9-CM) diagnosis code 250) were invited to enroll in this program. Initially, a total of 63 084 participants were included in the NDCMP from 2001-2004. We excluded people who had type 1 diabetes (ICD-9-CM; code 250.x1/x3) and gestational diabetes (n = 2108), those aged under 30 years (n = 658), a diagnosis of liver cancer at baseline (n = 194), diagnosed other cancers at baseline (n=1,544), and less than one-year of follow-up (n=931). We also excluded participants with missing data on
socio-demographic factors (n = 376), lifestyle behaviors (n = 72), blood biochemical indices (n = 6966), and less than 1 year of follow-up (n = 1005) from the analysis. Finally, 51,705 participants remained (24389 men and 27316 women) in the analysis (Figure 1). This study was approved by the Ethical Review Board of the China Medical University Hospital.
Data sources for baseline and follow-up assessments
The Taiwan National Health Insurance (NHI) program, launched in 1995, has covered approximately 99% of the 23.74 million people in Taiwan since 1999. By the end of 2010, the NHI Bureau had contracts with 100% of hospitals and 92% of clinics nationwide.39 NDCMP was also covered by NHI program. Claims data are randomly
audited by the insurance system. Expert reviews on a random sample for every 50 to 100 ambulatory and inpatient claims in each hospital and clinic are conducted
quarterly to enhance the validity of the claims data. A severe penalty is placed on every false diagnostic report by the NHI Bureau. Previous studies have also shown the high validity of the data from the NHI program.40, 41 We used the data sets for inpatient
care by admission and outpatient care visits from 2002 to 2011 in this study. Each person in Taiwan has a unique personal identification number (PIN). For security and privacy purposes, the data on patient identities were scrambled cryptographically and entered into the National Health Insurance Research Database (NHIRD). All NHI data sets can be interlinked with the scrambled PIN of each patient. The data sets consisted of information for all insured people, including demographic data, date and source of diagnosis, ambulatory care, inpatient admission, outpatient and inpatient treatment, and physicians providing services. Because of the comprehensive coverage of the NHI program, the proportion of enrollees withdrawing from the NHI program is low. Therefore, the bias caused by the loss to follow-up rate was negligible.
Enrollees at the time of entering the NDCMP underwent a comprehensive
assessment of the status of their disease and complications and a series of blood tests, urine tests, body measurements, medication, and lifestyle behaviors. All patients were followed-up regularly every 3 to 6 months. NDCMP education includes
encouragement to have regular exercise and to frequently self-monitor blood sugar levels, as well as to modify their lifestyle behavior, to provide information of foot care, subcutaneous insulin injection techniques and diabetes nutrition intake.
Following a 12-hour overnight fast, blood was drawn from an antecubital vein in the morning and sent for analysis within 4 hours post-collection.
Outcome ascertainment
The primary outcome measure was HCC, which was determined through record linkage with ambulatory and inpatient care data in the NHIRD. The time of follow-up
began with recruitment (index date) and ended with a new diagnosis of HCC, death, withdrawal from the insurance program, or the end of follow-up on December 31, 2011. The HCC incident was coded according to the ICD-9-CM. The ICD-9-CM code for HCC is 155. All HCC cases in the analysis met at least one of the following criteria: at least 3 ambulatory claims or at least one inpatient care claim. By linking the unique identification number with this computerized file, 1472 patients with HCC incidence were identified from this cohort. We attempted to minimize the effect of any existing medical conditions on baseline HbA1c by excluding HCC cases (n = 349) or any cancers (n=1,544) that occurred in the participants during the first year of follow-up.
Covariates
Data on other chronic medical conditions were collected for the 24-month period preceding cohort entry by using outpatient and inpatient claim data. Instead of 12-month period, 24-12-month period was used because some of these chronic medical conditions are not common, or individuals with these chronic conditions may not seek for health care services due to no sign or symptoms. We need to have longer time period in order not to miss their diagnosis. The histories of acute pancreatitis (ICD-9-CM code 557.0), chronic pancreatitis (ICD-9-(ICD-9-CM code 557.1), alcoholic liver damage CM code 571.0, 571.1, and 571.3), nonalcoholic fatty liver disease (ICD-9-CM code 571.8), liver cirrhosis (ICD-9-(ICD-9-CM 571.2, 571.5, and 571.6), cholelithiasis (ICD-9-CM code 574), alcohol dependence syndrome (ICD-9-CM code 303), pseudocyst of pancrease (ICD-9-CM code 577.2), jaundice (ICD-9-CM code 782.4), HBV (ICD-9-CM codes 070.2, 070.3, and V02.61), HCV (ICD-9-CM codes 070.41, 070.44, 070.51, 070.54, and V02.62), cholecystitis (ICD-9-CM codes 575.0 and 575.1), cholangitis (ICD-9-CM code 576.1), gastric ulcer (ICD-9-CM codes 531), and
duodenal ulcer (ICD-9-CM codes 532) were identified as comorbidities before the index date.
Data on use of medications prescribed for the treatment of disease were calculated for the 12-month period preceding cohort entry. We identified subjects’ outpatient prescriptions within one-year of their enrollment to define their anti-diabetes or statin medication use. A patient was defined as a user of an anti-anti-diabetes or statin medication if his/her number of prescription days for this specific anti-diabetes or statin drug is greater than three months. Under this definition, a patient may have more than one type of anti-diabetes medication use. The anti-diabetes medications of individual patients were further classified into seven categories: no medication, metformin monotherapy or metformin plus oral anti-diabetes drug (OAD) monotherapy other than metformin or sulfonylurea (OAD-other), sulfonylurea
monotherapy or sulfonylurea plus OAD-other combination, OAD-other monotherapy or OAD-other combination, metformin plus sulfonylurea combination, insulin
monotherapy, insulin plus one or more OAD. Statistical analysis
For each patient, the baseline HbA1c measurement was determined based on data sets of electronic lab records. The study participants were grouped into 4
categories according to clinical criteria of HbA1c at baseline: <7 %, 7% to 7.9%, 8% to 8.9%, and ≥ 9%. Cox proportional hazards model was performed to evaluate association between HbA1c level and HCC incidence rates for multivariable adjustment. We calculated hazard ratios (HRs) and 95% confidence intervals (CIs) after adjusting for age and multiple variables. Among the 3 multivariate models used in this study, the first adjusted for age (continuous), sex (male, female), smoking (yes, no), alcohol consumption (yes, no), duration of diabetes (continuous), statin use, and
type of hypoglycemic drug (seven categories described in Covariates section). The second model adjusted for blood biochemical indexes including fasting plasma glucose (FPG), triglyceride (TG), high-density lipoprotein (HDL), low-density
lipoprotein (LDL), glutamic pyruvic transaminase (GPT), and body mass index (BMI) in addition to the variables in the first model. The third model additionally included comorbidities at baseline such as chronic kidney disease (CKD) (yes if the estimated glomerular filtration rate < 60 [mL/min/1.73 m2], no), acute pancreatitis (yes, no),
chronic pancreatitis (yes, no), acute hepatitis (yes, no), alcoholic liver damage (yes, no), nonalcoholic fatty liver disease (yes, no), liver cirrhosis (yes, no), cholelithiasis (yes, no), alcohol dependence syndrome (yes, no), pseudocyst of pancrease (yes, no), jaundice (yes, no), jaundice (yes, no), HBV (yes, no), HCV (yes, no), cholecystitis (yes, no), cholangitis (yes, no), gastric ulcer (yes, no), and duodenal ulcer (yes, no). We verified the proportional hazards assumption according to the graph of the log (−log(survival)) versus the log of the survival time graph by adjusting for all remaining covariates and by testing the statistical significance of a covariate that allowed HbA1c to have a time varying effect. We observed no statistically significant violation. To explore the joint effect of HbA1c and each chronic liver disease, three dummy variables were created. Using individuals HbA1c <9% and without chronic liver disease as reference group, these three dummy variables measured the effects of HbA1c >9% only, chronic liver disease only, and combined HbA1c >9% and chronic liver disease. The interactions of HbA1c with age, sex, and other significant variables at baseline including alcoholic liver damage, nonalcoholic fatty liver disease, liver cirrhosis, hepatitis B virus infection, hepatitis C virus infection, and any one of these chronic liver diseases (CLD) were further examined by adding their product terms into the full model and the likelihood ratio test was used to test its significance. In
addition, relative excess risk due to interaction (RERI), proportion attributable to interaction (AP), and synergy index (S index) were calculated to assess whether the interaction was on an additive scale. PERI or AP=0 means no interaction; PERI or AP>0 means positive interaction; and PERI or AP<0 means negative interaction. S index=1 means no interaction; S index>1 means positive interaction; and S index<1 means negative interaction.
We conducted sensitivity analyses to examine the consistency of our findings. The main multivariable-adjusted analyses were repeated by excluding participants with stroke (ICD-9-CM codes 430 – 438), hypoglycemia (ICD-9-CM codes 251), cancers other than liver cancer (ICD-9-CM codes 140 – 154 and 156 – 208), HBV, and HCV separately and together. We subsequently excluded patients with
intrahepatic bile duct cancer (ICD-9-CM code 1551). All analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC). The 2-sided level of
significance was set at .05. Results
Among the 50441 patients with type 2 diabetes, the incidence rate of HCC was 2.65 per 1000 person-years (3.46 and 1.94 per 1000 person-years for men and women, respectively). Their mean age was 60.59 years (standard deviation [SD], 11.29 years) with a mean follow-up period of 8.17 years (1.90 years). The prevalence of HbA1c ( ≥ 9%) was 31.71% in men and 33.63% in women.
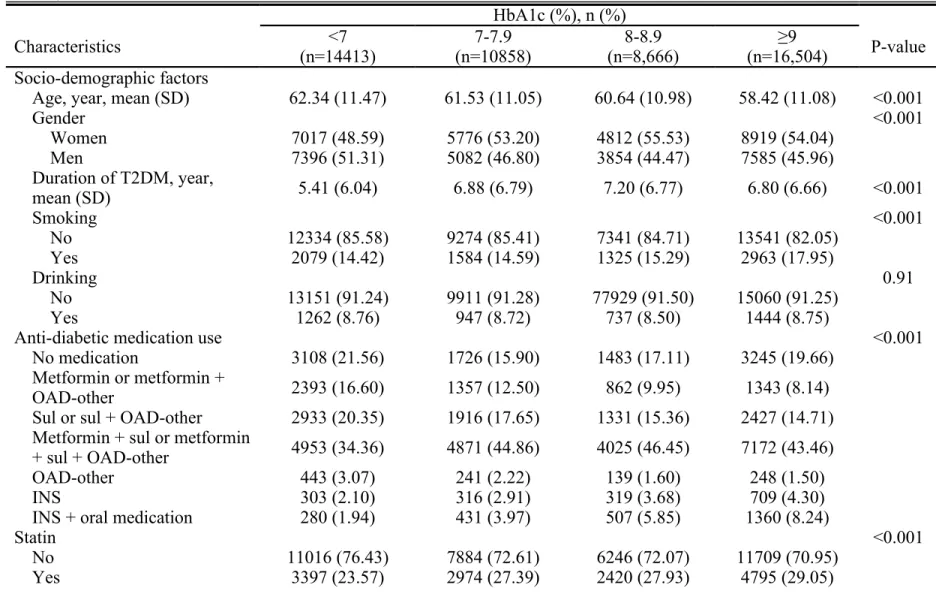
Table 1 shows the baseline characteristics of patients with type 2 diabetes in the NDCMP according to clinical criteria cut-off points of HbA1c. Patients with HbA1c ≥ 9% were associated with lower mean age, lower prevalence of gender male, use of combined metformin plus sulfonylurea or metformin plus sulfonylurea plus OAD-other combination, obesity (BMI ≥ 27 kg/m2), cholelithiasis, cholangitis, higher mean
medication, high triglycerides (≥ 150 mg/dL), high FPG (≥110 mg/dL), high HDL ( <40 mg/dL in males; < 50 mg/dL in females), high LDL (≥ 100 mg/dL), high GPT (≥ 40 U/L), and alcoholic dependence syndrome.
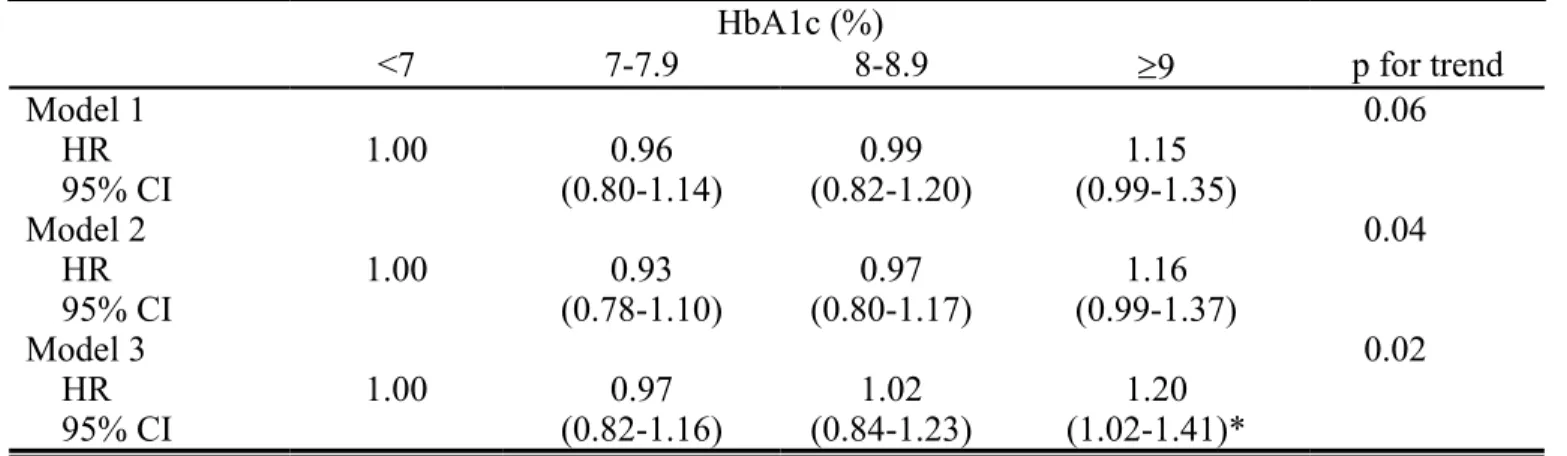
Figure 2 presents the Kaplan-Meier cumulative risk for HCC within subgroups defined by HbA1c level. Patients with HbA1c >9% faced a higher risk (log-rank test P <0.001, Figure 2). Table 2 shows the adjusted HR of HCC according to clinical criteria of baseline HbA1c in patients with type 2 diabetes. Compared with patients with a level of HbA1c < 7.0%, patients with a level of HbA1c ≥ 9.0% were
significantly associated with a risk of HCC (HR = 1.20, 95% CI = 1.02-1.41) when adjusting for age, sex, duration of type 2 diabetes, smoking, drinking, type of diabetes treatment, TG, FPG, HDL, LDL, GPT, BMI, and comorbidities. After multivariate adjustment, the risk of HCC increased as the levels of HbA1c increased (P for trend: 0.02, HR = 1.07, 95% CI=1.01-1.12 for every 1% increment in HbA1c).
Table 3 shows sensitivity analyses excluding participants with stroke, hypoglycemia, HBV, and HCV separately and together or excluding cases of
intrahepatic bile duct cancer. After excluding participants with stroke, hypoglycemia, HBV, and HCV separately and together, the association between levels of HbA1c and HCC remained similar. After excluding participants with intrahepatic bile duct cancer, the association between levels of HbA1c and HCC risk again remained similar.
Figure 3 shows the adjusted HR of HCC for the joint effects of HbA1c ≥ 9% and alcoholic liver damage, nonalcoholic fatty liver disease, liver cirrhosis, HBV, HCV, and any one of these CLD for the entire sample, and stratified by insulin and statin use. Due to limited number of patients with statin use, we cannot estimate the joint effect of HbA1c ≥ 9% with these chronic liver diseases in statin users. We observed significant HRs of HCC for patients with a level of HbA1c ≥ 9% with
alcoholic liver damage, liver cirrhosis, HBV, HCV, and any one of CLD compared with patients with a level of HbA1c < 9% and no counterpart comorbidity in the entire sample (HR = 8.63, 95% CI = 1.41-52.68; HR = 5.02, 95% CI = 3.10-8.12; HR = 2.53, 95% CI = 1.10-5.85; HR = 1.79, 95% CI = 1.01-3.17; and HR = 3.59, 95% CI = 2.56-5.02, respectively). No significant interactions between HbA1c level and
alcoholic liver damage, nonalcoholic fatty liver disease, liver cirrhosis, HBC, HCV, and any one of CLD were observed (all p for interaction terms >0.05). After
stratification according to insulin or statin use, similar significant joint effects of HbA1c ≥ 9% with alcoholic liver damage, liver cirrhosis, HBV and HCV were observed except for HbA1c ≥ 9% with alcoholic liver damage in non-insulin users and HbA1c ≥ 9% with HBV in insulin users. The primary effects of HbA1c ≥ 9% were all statistically significant, had narrow 95% CIs, and remained similar in entire sample, non-insulin users, and non-statin users. The other risk factors exerting a significant primary effect were liver cirrhosis, HBV, HCV, and CLD either in entire sample, or stratified by insulin use and statin use except for HBV in insulin users. We detected significant interaction of HbA1c with any one of CLD in non-insulin users (p<0.05). PERI, AP, and S-index were -0.98, -0.29, and 0.70, respectively, indicating that there exists a negative interaction.
Discussion
We evaluated the association between the level of HbA1c and HCC risk in a large patient cohort with type 2 diabetes who were enrolled in the NDCMP in Taiwan. This retrospective cohort study included 51705 patients with type 2 diabetes aged 30 years and over at baseline. We observed a significant association between the level of HbA1c and the risk of HCC. Our sensitivity analysis showed that the association between the level of HbA1c and HCC risk in the entire sample was similar to those
from the analysis excluding patients with stroke, hypoglycemia, other cancers, HBV, and HCV at baseline, and by excluding incidence cases of intrahepatic bile duct cancer. The consistent findings from our sensitivity analysis indicated that our study results were robust. After further examining the joint effects of the level of HbA1c and chronic conditions, we observed significant joint associations of the level of HbA1c ≥ 9% and chronic liver diseases such as alcoholic liver disease, HBV infection, and HCV infection with HCC risk.
The findings regarding the relationship between HCC or all-cancer risk and HbA1c in patients with type 2 diabetes are controversial. Two studies did not detect a significant association between HbA1c and a risk of all cancers incidence32 or
mortality 33. A nationwide population-based prospective cohort study in Sweden
reported no association between the level of HbA1c and a risk of all cancers incidence in patients with type 2 diabetes.32 The European Prospective Investigation into Cancer
and Nutrition (n = 4,345) showed that each 1% increase in HbA1c level was non-significantly associated with a risk of all cancers mortality in patients with diabetes (HR = 1.05, 95% CI = 0.95-1.17).33 Three studies observed a positive association
between the level of HbA1c and liver cancer incidence,35, 36, 38 one study observed a
negative association between HbA1c and cancer incidence,34 and one study reported
no association between HbA1c and cancer incidence.37 Donadon et al. determined that
diabetic patients (n = 145) have a significant 26%-50% increase in liver cancer incidence for each 1% increase in HbA1c level compared with liver cirrhosis patients .36 Yang et al. recently showed that diabetic patients with a level of HbA1c ≥7% had
an increased risk of liver cancer incidence compared with diabetic patients with a level of HbA1c < 7% (HR = 8.73, 95% CI: 1.75-43.60) (n = 1,319).35 Similarly,
sampling subjects according to insulin use status (n=971 for insulin users and n=1935 for non-insulin users), HbA1c was reported to be associated with liver cancer
incidence (per percentage HR=1.26, 1.03-1.55).38 By contrast, a retrospective
case-control study indicated that diabetic patients with a level of HbA1c < 8% compared with those with a level of HbA1c ≥ 8% had a significantly higher probability of cancer development (adjusted odds ratio, OR = 3.59, 95% CI: 1.45-8.91).34 The
case-control studies had potential selection and recall bias and were limited by a small sample size. The only cohort study detecting the effect of HbA1c level on liver cancer primarily focused on examining the effect modification of low LDL cholesterol and low triglyceride levels and the use of insulin or statins on the association between chronic HBV infection and liver cancer incidence.35 Our study specifically
investigating the association between HbA1c level and HCC risk has a suitable design, focusing the research effort on the primary objective and creating a strong basis for interpreting the study results. Therefore, our study provides credible findings and shows the joint effect of the HbA1c level and chronic liver diseases on HCC risk in Chinese patients with type 2 diabetes.
HbA1c has been associated with increased risks of liver-related diseases and all cancers in the general population 31, 42, 43 and patients with type 2 diabetes. 35,36, 38
Previous studies have consistently reported the association of HCC with other comorbidities, particularly for DM patients with cirrhosis, HBV, and HCV.44 Our
findings provide further evidence that a high level of HbA1c and chronic liver disease increase the risk of HCC in Chinese people with type 2 diabetes. The biologic
mechanisms by which diabetes may cause substantial liver cancer remain unclear. Experimental studies have indicated that high glucose levels or insulin resistance compose the etiology for developing type 2 diabetes. Recent studies have indicated
that excess obesity is linked to hyperinsulinemia, insulin resistance, cytokine production, and promoting hepatic fibrogenesis, causing fat accumulation in
hepatocytes, oxidative stress, and progressive liver damage such as nonalcoholic fatty liver disease.45- 47 A cross-sectional study indicated J-shaped associations between
HbA1c level and elevated liver enzymes and hepatic steatosis.42 The severe form of
nonalcoholic fatty liver disease (nonalcoholic steatohepatitis) was linked to liver cancer.48 Thus, the relationship between HbA1c and the risk of HCC is biologically
plausible.
Two additional possible biologic mechanisms independently contribute to diabetes as a risk factor for cancer. First, recent studies have shown that dysregulation of the TSC1/TSC2/mTOR signaling pathway by IκB kinaseβ (IKKβ) is a common molecular switch for both cancer pathogenesis and diet- and obesity-induced insulin resistance, and links obesity-derived chronic inflammation with insulin resistance and cancer pathogenesis.14, 16, 17, 45 Second, increased serum or plasma levels of the IGF-1
may also promote tumor cell growth. Epidemiologic or experimental evidence has shown the association between IGF-1 and colorectal, prostate, and breast cancers.10-13
This study has several merits. First, we used a cohort with a large sample size to investigate whether HbA1c levels are associated with HCC risk in Chinese patients with type 2 diabetes who were enrolled in the NDCMP. Second, this study is a retrospective cohort study, in which information was collected before the subsequent diagnosis of HCC, preventing the recall bias inherent in case-control studies in which exposure was collected before HCC diagnosis. Finally, we adjusted for several variables, including lifestyle behaviors, anti-diabetes medication use, biomarkers such as FPG, HDL, LDL, TG, and GPT, and comorbidity, which allowed us to minimize the effect of potential confounders.
However, this study has several limitations. First, we did not have HbA1c and drug use information in the follow-up period. For lack of HbA1c measurements in the follow-up period, we could not determine changes in HbA1c over time or whether an association existed between changes in HbA1c level and HCC risk. As for lack of drug use information in the follow-up period, the effect of HbA1c might not be accurately estimated. Thus, it may have potential impact on the selection of the cutoff point of HbA1c>=9% and on measures of tested interaction. Second, the study
population was exclusively Chinese patients with type 2 diabetes in Taiwan; therefore, future studies should examine the effect of HbA1c level on HCC risk in patients with type 2 diabetes in diverse regions. Third, the possibility of residual confounding cannot be ruled out in this study. This may result in a spurious association between HbA1c levels and HCC risk. In addition, measurement errors could be possible because of the large amount of data collected during clinical practice. Fourth, there is potential misclassification error due to undiagnosed or misdiagnosed liver cancer cases. However, this proportion would be small because the NHI programme regularly conducts expert reviews of patients’ charts to randomly confirm the validity of claims from all hospitals. In order to enhance the true positive rates of HCC, we defined HCC cases as those who had at least 3 ambulatory claims or at least one inpatient care claim of the ICD-9-CM code for HCC. Error may arise from miscoding and misclassification. This kind of misclassification error would result in underestimation of the effect if the association between HbA1c and HCC exists, indicating that the true effect would be stronger, a lesser threat to the validity of our finding. In addition, due to limited number of diagnoses can be coded in ambulatory or inpatient claim data, patients with number of diseases more than the number of diagnoses that can be coded in the administrative claim data would be less
likely to be included in our study. Thus, our study sample may not be representative of the entire population with type 2 diabetes in term of number of comorbidity. If the incidence rates of HCC were similar across HbA1c levels, the estimate of HR would be valid. Finally, there was potential selection bias might exist due to the differential characteristics between patients with type 2 diabetes who enrolled in NDCMP and those who did not. To assess this possibility, we examined the age and sex
distributions between our study subjects and the entire population with type 2 diabetes using NHIRD dataset, and similar distributions were found (mean age for our study subjects and entire population: 60.6 vs 62.1 years; sex distribution: 52% vs 51% for females). The non-differential distributions in age and sex indicate this kind of selection error might be random, thus, the biased results in the effect may be toward the null, a lesser threat to validity.
In summary, our study shows a positive association between HbA1c level and HCC risk in Chinese patients with type 2 diabetes, indicating that a level of HbA1c ≥ 9% is a predictor of HCC risk. In addition, significant joint associations of the level of HbA1c ≥ 9% and chronic liver diseases such as alcoholic liver damage, hepatitis B virus infection, and hepatitis C virus infection with HCC risk. HCC incidence is rapidly increasing worldwide, and public health interventions are required to halt this epidemic. Lifestyle or treatment interventions such as maintaining a satisfactory glycemic control and chronic liver diseases may reduce the burden of HCC. Conflict of interest statement
None declared. Acknowledgement
This study was supported primarily by the Bureau of National Health Insurance (DOH94-NH-1007), the Ministry of Science and Technology of Taiwan (National Science Council) (NSC 101-2314-B-039 -017 -MY3 & NSC 102-2314-B-039 -005 –
MY2), Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW103-TDU-B-212-113002) and Health and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (MOHW103-TD-B-111-03, Taiwan).
References
1. International Agency for Research on Cancer. GLOBOCAN 2008. Available at: http://globocan.iarc.fr/. Accessed August 1, 2014.
2. Registry TC. Age-standardized incidence of liver and intrahepatic bile duct cancer of the long-time trend. Available at:
http://cph.ntu.edu.tw/uploadimages/Year_Digestive.xls. Accessed August 1, 2014.
3. International Diabetes Federation. About diabetes-risk factor. Available at: http://www.idf.org/about-diabetes/risk-factors. Accessed August 1, 2014.
4. El-Serag HB, Tran T, Everhart JE. Diabetes Increases the Risk of Chronic Liver Disease and Hepatocellular Carcinoma. Gastroenterology. 2004;126:460-8. 5. Fujino Y, Mizoue T, Tokui N, Yoshimura T. Prospective study of diabetes mellitus and liver cancer in Japan. Diabetes Metab Res Rev. 2001;17:374-9. 6. Keitaro Tanaka, Ichiro Tsuji, Akiko Tamakoshi, Keitaro Matsuo, Kenji Wakai,
Chisato Nagata, Tetsuya Mizoue, Manami Inoue, Shoichiro Tsugane, Shizuka Sasazuki. Diabetes Mellitus and Liver Cancer Risk: An Evaluation Based on a Systematic Review of Epidemiologic Evidence among the Japanese Population. Jpn J Clin Oncol. 2014. doi:10.1093/jjco/hyu108.
7. Printz C. Diabetes associated with increased risk of liver cancer. Cancer. 2014;120:1288.
8. Chiang CH, Lee LT, Hung SH,Lin WY, Hung HF, Yang WS, Sung PK, Huang KC. Opposite association between diabetes, dyslipidemia, and hepatocellular carcinoma mortality in the middle-aged and elderly. Hepatology. 2014;59:2207-15.
9. World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR). Food, Nutrition and the Prevention of Cancer: A Global Perspective. Washington, DC, WCRF/AICR, 1997.
10. Grimberg A, Cohen P. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J Cell Physiol 2000;183:1-9. 11. Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P,
Hennekens CH, Pollak M. Plasma insulin-like growth factor-1 and prostate cancer risk: a prospective study. Science 1998; 279:563-6.
12. Bohlke K, Cramer DW, Trichopoulos D, Mantzoros CS. Insulin-like growth factor-1 in relation to premenopausal ductal carcinoma in situ of the breast. Epidemiology 1998;9:570-3.
cancer pathogenesis? Growth Horm IGF Res. 2000;10:297-305.
14. Lee DF, Kuo HP, Chen CT, Wei Y, Chou CK, Hung JY, Yen CJ, Hung MC. IKKbeta suppression of TSC1 function links the mTOR pathway with insulin resistance. Int J Mol Med. 2008;22:633-8.
15. Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004;118:285-96.
16. Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R, Hung MC. IKappa B kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 2004;117:225-37. 17. Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, Sun HL, Li LY, Ping B,
Huang WC, He X, Hung JY, et al. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell 2007;130:440-55.
18. Rahbar S. The discovery of glycated hemoglobin: a major event in the study of nonenzymatic chemistry in biological systems. Ann N Y Acad Sci. 2005;1043:9-19.
19. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53.
20. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med.
1993;329:977-86.
21. Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care 2000;23(suppl 2):B21-B29.
22. Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, Golden SH. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141:421-31.
23. Adler AI, Stevens RJ, Neil A, Stratton IM, Boulton AJ, Holman RR. UKPDS 59: hyperglycemia and other potentially modifiable risk factors for peripheral vascular disease in type 2 diabetes. Diabetes Care. 2002;25:894-9.
24. Kothari V, Stevens RJ, Adler AI, Stratton IM, Manley SE, Neil HA, Holman RR. UKPDS 60: risk of stroke in type 2 diabetes estimated by the UK Prospective Diabetes Study risk engine. Stroke. 2002;33:1776-81.
Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405-12.
26. Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, Holman RR. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ. 1998;316:823-8.
27. Adler AI, Neil HA, Manley SE, Holman RR, Turner RC. Hyperglycemia and hyperinsulinemia at diagnosis of diabetes and their association with subsequent cardiovascular disease in the United Kingdom prospective diabetes study (UKPDS 47). Am Heart J. 1999;138:S353-S359.
28. Stevens RJ, Coleman RL, Adler AI, Stratton IM, Matthews DR, Holman RR. Risk factors formyocardial infarction case fatality and stroke case fatality in type 2 diabetes: UKPDS 66. Diabetes Care 2004;27:201-7.
29. Joshu CE, Prizment AE, Dluzniewski PJ, Menke A, Folsom AR, Coresh J, Yeh HC, Brancati FL, Platz EA, Selvin E. Glycated hemoglobin and cancer incidence and mortality in the Atherosclerosis in Communities (ARIC) Study, 1990-2006. Int J Cancer. 2012;131(7):1667-77.
30. Silbernagel G, Grammer TB, Winkelmann BR, Boehm BO, März W. Glycated hemoglobin predicts all-cause, cardiovascular, and cancer mortality in people without a history of diabetes undergoing coronary angiography. Diabetes care 2011;34:1355-61.
31. Saydah S, Tao M, Imperatore G, Gregg E. GHb level and subsequent mortality among adults in the U.S. Diabetes care. 2009;32:1440-6.
32. Miao Jonasson J, Cederholm J, Eliasson B, Zethelius B, Eeg-Olofsson K, Gudbjörnsdottir S. HbA1C and cancer risk in patients with type 2 diabetes--a nationwide population-based prospective cohort study in Sweden. PloS one 2012;7:e38784.
33. Sluik D, Boeing H, Montonen J, Kaaks R, Lukanova A, Sandbaek A, Overvad K, Arriola L, Ardanaz E, Saieva C, Grioni S, Tumino R, et al. HbA1c measured in stored erythrocytes is positively linearly associated with mortality in individuals with diabetes mellitus. PloS one. 2012;7:e38877.
34. Dabrowski M. Glycated hemoglobin, diabetes treatment and cancer risk in type 2 diabetes. A case-control study. Ann Agric Environ Med. 2013;20:116-21.
35. Yang X, Wang Y, Luk AO, So WY, Ma RC, Kong AP, Xu G, Chan JC. Enhancers and attenuators of risk associations of chronic hepatitis B virus infection with hepatocellular carcinoma in type 2 diabetes. Endocr Relat Cancer. 2013;20:161-71.
36. Donadon V, Balbi M, Valent F, Avogaro A. Glycated hemoglobin and antidiabetic strategies as risk factors for hepatocellular carcinoma. World J Gastroenterol. 2010;16:3025-32.
37. de Beer JC, Liebenberg L. Does cancer risk increase with HbA1c, independent of diabetes? BJC. 2014;110:2361-8.
38. Yang X, Ko GT, So WY, Ma RC, Yu LW, Kong AP, Zhao H, Chow CC, Tong PC, Chan JC. Associations of hyperglycemia and insulin usage with the risk of cancer in type 2 diabetes: the Hong Kong diabetes registry. Diabetes.
2010;59:1254-60.
39. Department of Health, Executive Yuan. The National Health Insurance Statistics, 2010. Available at: http://www.nhi.gov.tw/English/webdata/webdata.aspx? menu=11&menu_id=296&WD_ID=296&webdata_id=4010. Accessed August 1, 2014.
40. Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan.
Pharmacoepidemiol Drug Saf. 2011;20:236-42.
41. Kang JH, Chen YH, Lin HC. Comorbidity profiles among patients with
ankylosing spondylitis: a nationwide population-based study. Ann Rheum Dis. 2010;69:1165-8.
42. Christman AL, Lazo M, Clark JM, Selvin E. Low glycated hemoglobin and liver disease in the U.S. population. Diabetes care 2011;34:2548-50.
43. Ma H, Xu C, Xu L, Yu C, Miao M, Li Y. Independent association of HbA1c and nonalcoholic fatty liver disease in an elderly Chinese population. BMC
Gastroenterol. 2013;13:3.
44. Lai SW, Chen PC, Liao KF, Muo CH, Lin CC, Sung FC. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol. 2012;107:46-52. 45. Parekh S, Anania FA. Abnormal lipid and glucose metabolism in obesity:
implications for nonalcoholic fatty liver disease. Gastroenterology. 2007;132:2191-207.
46. Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(2 Suppl 1):S99-S112.
47. Yang X, So WY, Ma RC, Kong AP, Xu G, Chan JC. Diabetes and cancer: the mechanistic implications of epidemiological analyses from the Hong Kong Diabetes Registry. Diabetes/metabolism research and reviews. 2012;28:379-387. 48. White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver
disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342-59.
Figure Legends:
Figure 1. Flowchart of recruitment procedures for the current study.
Figure 2. Kaplan-Meier cumulative risk for HCC within subgroups defined by HbA1c level.
Figure 3. The adjusted HR of HCC for the effects of HbA1c ≥ 9% and alcoholic liver damage, nonalcoholic fatty liver disease, liver cirrhosis, hepatitis B virus infection, hepatitis C virus infection, and any one of these chronic liver diseases for the entire sample, and stratified by insulin use and statin use. ALD: alcoholic liver damage; NAFLD: nonalcoholic fatty liver disease; LC: liver cirrhosis; HBV: hepatitis B virus infection; HCV: hepatitis C virus infection; CLD: chronic liver diseases.
Table 1. Baseline characteristics of patients with type 2 diabetes in the NDCMP according to clinical criteria of glycated hemoglobin A1C (continued)
HbA1c (%), n (%)
Characteristics (n=14413)<7 (n=10858)7-7.9 (n=8,666)8-8.9 (n=16,504)≥9 P-value Socio-demographic factors
Age, year, mean (SD) 62.34 (11.47) 61.53 (11.05) 60.64 (10.98) 58.42 (11.08) <0.001
Gender <0.001 Women 7017 (48.59) 5776 (53.20) 4812 (55.53) 8919 (54.04) Men 7396 (51.31) 5082 (46.80) 3854 (44.47) 7585 (45.96) Duration of T2DM, year, mean (SD) 5.41 (6.04) 6.88 (6.79) 7.20 (6.77) 6.80 (6.66) <0.001 Smoking <0.001 No 12334 (85.58) 9274 (85.41) 7341 (84.71) 13541 (82.05) Yes 2079 (14.42) 1584 (14.59) 1325 (15.29) 2963 (17.95) Drinking 0.91 No 13151 (91.24) 9911 (91.28) 77929 (91.50) 15060 (91.25) Yes 1262 (8.76) 947 (8.72) 737 (8.50) 1444 (8.75)
Anti-diabetic medication use <0.001
No medication 3108 (21.56) 1726 (15.90) 1483 (17.11) 3245 (19.66) Metformin or metformin +
OAD-other 2393 (16.60) 1357 (12.50) 862 (9.95) 1343 (8.14)
Sul or sul + OAD-other 2933 (20.35) 1916 (17.65) 1331 (15.36) 2427 (14.71) Metformin + sul or metformin
+ sul + OAD-other 4953 (34.36) 4871 (44.86) 4025 (46.45) 7172 (43.46)
OAD-other 443 (3.07) 241 (2.22) 139 (1.60) 248 (1.50)
INS 303 (2.10) 316 (2.91) 319 (3.68) 709 (4.30)
INS + oral medication 280 (1.94) 431 (3.97) 507 (5.85) 1360 (8.24)
Statin <0.001
No 11016 (76.43) 7884 (72.61) 6246 (72.07) 11709 (70.95)
BMI (Taiwan) <0.001 <18.5 180 (1.25) 118 (1.06) 102 (1.18) 335 (2.03) 18.5-23.9 4687 (32.52) 3459 (31.86) 2789 (32.18) 6099 (36.95) 24-26.9 5047 (35.02) 3719 (34.25) 2983 (34.42) 5316 (32.21) ≥27 4499 (31.21) 3565 (32.83) 2792 (32.22) 4754 (28.81) Mean (SD) 25.66 (3.73) 25.78 (3.68) 25.77 (3.77) 25.28 (3.86) <0.001
Blood biochemical indexes
TG (mmol/L) <0.001 <1.69 8921 (61.90) 6197 (57.07) 4633 (53.46) 8209 (49.74) ≥1.69 5492 (38.10) 4667 (42.93) 4033 (46.54) 8295 (50.26) FPG (mg/dL) <0.001 <110 3132 (21.73) 1074 (9.89) 531 (6.13) 585 (3.54) ≥110 11281 (78.27) 9784 (90.11) 8135 (93.87) 15919 (96.46) HDL (mmol/L) <0.001 ≥0.45(male); 0.57(female) 7040 (48.84) 5042 (46.44) 4000 (46.16) 7828 (47.43) <0.45(male); 0.57(female) 733 (51.16) 5816 (53.56) 4666 (53.84) 8676 (52.57) LDL (mmol/L) <0.001 <1.13 4827 (33.49) 3206 (29.53) 2447 (28.24) 4329 (26.23) ≥1.13 9586 (66.51) 7652 (70.47) 6219 (71.76) 12175 (73.77) SGPT (u/l) <0.001 <40 11869 (82.35) 8548 (78.73) 6673 (77.00) 12805 (77.59) ≥40 2544 (17.65) 2310 (21.27) 1993 (23.00) 3699 (22.41) Comorbidity Acute hepatitis 0.42 Yes 20 (0.14) 8 (0.07) 8 (0.09) 16 (0.10)
Alcoholic liver damage 0.34
Yes 10 (0.07) 8 (0.07) 9 (0.10) 21 (0.13)
Nonalcoholic fatty liver 0.03
Yes 142 (0.99) 145 (1.34) 106 (1.22) 169 (1.02)
Liver cirrhosis <0.001
Yes 123 (0.85) 51 (0.47) 37 (0.43) 102 (0.62)
Yes 156 (1.08) 91 (0.84) 82 (0.95) 108 (0.65)
Alcohol dependence syndrome 0.13
Yes 11 (0.08) 9 (0.08) 4 (0.05) 22 (0.13) Jaundice 0.62 Yes 5 (0.03) 4 (0.04) 5 (0.06) 4 (0.02) Hepatitis B 0.23 Yes 102 (0.71) 71 (0.65) 54 (0.62) 87 (0.53) Hepatitis C 0.61 Yes 79 (0.55) 72 (0.66) 47 (0.54) 100 (0.61) Cholecystitis 0.37 Yes 27 (0.19) 13 (0.12) 11 (0.13) 20 (0.12) Cholangitis 0.08 Yes 17 (0.12) 11 (0.10) 11 (0.13) 7 (0.04) Gastric ulcer 0.99 Yes 272 (1.89) 202 (1.86) 160 (1.85) 308 (1.87) Duodenal ulcer 0.41 Yes 174 (1.21) 144 (1.33) 92 (1.06) 197 (1.19)
Chronic kidney disease <0.001
Yes 3135 (21.75) 2056 (18.94) 1456 (16.80) 2248 (13.62)
Abbreviations: BMI, body mass index; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; INS, insulin; LDL, low-density lipoprotein; OAD, oral anti-diabetes drug; SD, standard deviation; SUL, Sulfonylurea; SGPT, serum glutamic pyruvic transaminase; TG, triglyceride
Table 2. Hazard ratios (HRs) of hepatocellular carcinoma according to clinical criteria of baseline glycated hemoglobin A1C in patients with type 2 diabetes enrolled in the NDCMP
HbA1c (%) <7 7-7.9 8-8.9 9 p for trend Model 1 0.06 HR 1.00 0.96 0.99 1.15 95% CI (0.80-1.14) (0.82-1.20) (0.99-1.35) Model 2 0.04 HR 1.00 0.93 0.97 1.16 95% CI (0.78-1.10) (0.80-1.17) (0.99-1.37) Model 3 0.02 HR 1.00 0.97 1.02 1.20 95% CI (0.82-1.16) (0.84-1.23) (1.02-1.41)*
Model 1 adjusted for age, sex, duration of type 2 diabetes, smoking, drinking, type of anti-diabetic medication use, and statin use.
Model 2 additionally adjusted for body mass index, triglyceride, fasting plasma glucose, high-density lipoprotein, low-density lipoprotein, and glutamic pyruvic transaminase.
Model 3 additionally adjusted for acute hepatitis, alcoholic liver damage, nonalcoholic fatty liver, liver cirrhosis, cholelithiasis, alcohol dependence syndrome, jaundice, hepatitis B, hepatitis C, cholecystitis, cholangitis, gastric ulcer, duodenal ulcer, and chronic kidney disease
Table 3. Sensitivity analyses for the association between the levels of HbA1c and hepatocellular carcinoma in patients with type 2 diabetes enrolled in the National Diabetes Care Management Program, Taiwan
Model 1 Model 2 Model 3 Model 4 Model 5
HbA1c (%)
<7.0 1.00 (reference) 1.00 (reference) 1.00 (reference) 1.00 (reference) 1.00 (reference) 7.0-7.9 0.97 (0.81-1.17) 0.98 (0.82-1.17) 0.98 (0.82-1.17) 0.98 (0.82-1.19) 0.97 (0.82-1.16) 8.0-8.9 1.01 (0.83-1.23) 1.02 (0.84-1.24) 0.97 (0.80-1.18) 0.96 (0.78-1.18) 1.02 (0.85-1.24) 9.0 1.21 (1.03-1.43)* 1.21 (1.03-1.43)* 1.21 (1.03-1.43)* 1.23 (1.04-1.46)* 1.19 (1.02-1.40)* Model 1 excludes patients with stroke (n=2,676); Model 2 excludes patients with hypoglycemia (n=203); Model 3excludes patients with hepatitis B or hepatitis C (n=597); Model 4 excludes patients with stroke, hypoglycemia, hepatitis B or hepatitis C (n=3400); Model 5 excludes patients with incidence cases of intrahepatic bile ducts cancer (ICD-9-CM code: 1551) (n=14); Adjustment for age, sex, duration of T2DM, smoking, drinking, type of diabetes treatment, statin use, body mass index, triglyceride, fasting plasma glucose, high-density lipoprotein, low-density lipoprotein, glutamic pyruvic transaminase, acute hepatitis, alcoholic liver damage, nonalcoholic fatty liver, liver cirrhosis,
cholelithiasis, alcohol dependence syndrome, jaundice, hepatitis B (except model 3 and 4), hepatitis C (except model 3 and 4), cholecystitis, cholangitis, gastric ulcer, duodenal ulcer, and chronic kidney disease.