99
Recruitment and Hatching Dates of Grey Mullet (Mugil cephalus L.)
Juveniles in the Tanshui Estuary of Northwest Taiwan
Chin-Wei Chang1,2, Wann-Nian Tzeng1,* and Ying-Chou Lee2
1Institute of Zoology, College of Science, National Taiwan University, Taipei, Taiwan 106, R.O.C.
2Institute of Fisheries Science, College of Science, National Taiwan University, Taipei, Taiwan 106, R.O.C.
(Accepted December 20, 1999)
Chin-Wei Chang, Wann-Nian Tzeng and Ying-Chou Lee (2000) Recruitment and hatching dates of grey mullet (Mugil cephalus L.) juveniles in the Tanshui estuary of northwest Taiwan. Zoological Studies 39(2): 99-106. Ages, growth, and hatching dates of grey mullet Mugil cephalus L. juveniles in the Tanshui estuary of northwest-ern Taiwan were examined, using growth increments in otoliths of fish collected in November 1995 to March 1996 and November 1996 to March 1997. Growth increments are deposited daily, and were used to determine the ages. Juveniles were composed of multiple cohorts, and their sizes ranged between 17 and 39 mm in total length, corresponding to ages of 29 to 67 d after hatching. The growth rate during the estuarine residency was 0.45 mm d-1. Hatching dates back-calculated from daily ages were from October to February, which range earlier and later than the spawning period from November to January of a previously known migratory population. This suggests that grey mullet juveniles in the estuary may come from both a resident population and a migratory population, or that there are earlier and later migratory spawning populations.
Key words: Mugil cephalus L., Otolith, Daily age, Hatching date, Tanshui estuary.
*To whom correspondence and reprint requests should be addressed. Tel: 886-2-23639570. Fax: 886-2-23636837. E-mail: wnt@ccms. ntu.edu.tw
G
rey mullet, Mugil cephalus L., is a coastal mi-gratory fish important for food and roe. It is widely cultured in Taiwan. Artificial breeding of grey mullet has been practiced since the 1980s, but most of the juveniles for aquaculture are still obtained from wild stocks in estuaries because they are abundant and cheap (Kuo et al. 1973, Liao 1981). Accordingly, un-derstanding the recruitment dynamics of juveniles in estuaries is important for rational management of grey mullet stocks.Tung (1981) found that the grey mullet lives in coastal waters of mainland China, becomes mature at ages of 3-4 yr, and migrates to the coastal waters of southwestern Taiwan for spawning during the pe-riod from December to January. However, juveniles in estuaries of Taiwan appear from November to March, which is earlier than the known spawning season. Therefore, it has been speculated that grey mullet juveniles in estuaries of Taiwan might be re-cruited from both resident and migratory spawning populations (Liu 1986 1991). The resident
popula-tion might spawn from October to December, while the migratory population does so from late Decem-ber to late January (Chen and Su 1986). However, these speculations have not been validated.
Many studies have been conducted on resource assessment and fishery oceanography (Tung 1969 1981, Chen 1982, Huang and Su 1986), fishery biol-ogy (Tung 1981, Chen and Su 1986, Liu 1986, Su and Kawasaki 1995), and morphology and taxonomy (Chen et al. 1989, Liu and Shen 1991) of the migra-tory population of grey mullet. However, studies on early life history and recruitment dynamics of grey mullet in estuaries are fragmentary and insufficient to validate the above speculations (Tang 1975, Lee and Kuo 1990, Chang 1997).
Daily growth increment of otoliths was discov-ered by Pannella (1971), and has been widely used to determine daily age and birth date (Campana and Neilson 1985, Tzeng et al. 1998), growth rates (Volk et al. 1984, Tzeng 1990), recruitment patterns (Healey 1982, Wang and Tzeng 1999), and habitat
transitions of juvenile fishes (Campana 1984, Jones 1992, Tzeng and Tsai 1994, Cheng and Tzeng 1996).
The objectives of this study were to determine the ages, and growth and hatching dates of grey mul-let juveniles in the Tanshui estuary of Taiwan, and to understand their early life history and recruitment dynamics.
MATERIALS AND METHODS Sampling design
Juveniles of grey mullet were collected every 2 wk from Gongshytyan Creek, a tributary to the Tanshui estuary, for 2 fishing seasons, November 1995-March 1996 and November 1996-March 1997 (Fig. 1). They were collected by a beach seine net with a mesh size of 1 mm. The net is traditional fish-ing gear used to collect mullet juveniles in the inter-tidal zone. The structure of the net is similar to that described by Liao (1981).
At the time of collection, sea surface tempera-ture (SST) at the sampling site was also measured. Catch data of grey mullet juveniles along the coast of Taiwan during the fishing seasons of 1967-1996 were obtained from the Taiwan Fishery Yearbook and were analyzed to understand this species’ re-cruitment dynamics (Anon 1967-1997).
Validation of daily growth increment
Radtke (1984) indicates that growth increments in otoliths of laboratory-reared juveniles of grey mul-let are deposited daily, and thus, the 1st increment is formed on the 1st day after hatching. To validate the periodicity of growth increments, 35 wild-caught grey mullet juveniles were immersed in tetracycline (TC) at a concentration of 600 mg l-1 for 24 h (Chang 1997). Five fish were sacrificed every 5 d during the 35-d rearing period to examine the new increments deposited in otoliths beyond the TC mark.
Sagittae, the largest ones of 3 pairs of otoliths of grey mullet juveniles were removed and mounted with cold acrylic resin (L. R. White resin, London Resin Company) and ground with polishing paper (Metaserv grinder-polisher, Buehler) until their pri-mordium was revealed. Growth increments were ex-amined under both transmitted and fluorescence light microscopes. A fluorescence microscope (Optiphot, Nikon) with incident ultraviolet light from a 50-W mercury lamp was used to detect the fluores-cent TC-mark where TC reacted with calcium and a
yellowish band appeared on the otoliths, while trans-mitted visible light was used to count growth increments. Excitation wavelength of the incident ul-traviolet light was limited by a band-pass filter (400-440 nm) and a long-pass barrier filter (470 nm) (Tzeng and Yu 1989). Also, the microstructure of the growth increment was further examined under a scanning electron microscope (SEM) after the oto-liths were etched with 5% ethylenediaminete-traacetate (EDTA) (Tzeng 1990).
Data analysis
To understand population structure and recruit-ment dynamics of grey mullet juveniles in the estuary, the monthly length frequencies were ana-lyzed by Bhattacharya’s polymodal analysis of the FAO-ICLARM Stock Assessment Tools (Gayanilo et al. 1994).
Otoliths of 50 juveniles from each season were randomly selected and used for examining daily growth increments. However, only 79 otoliths (37 for season 1995 and 42 for 1996) were readable. Growth increments in each otolith were read 3 times, and the mean value was used to represent the daily age. The relationship between total length and daily age was calculated by linear regression. The slope of the regression represents the growth rate of juve-niles during estuarine residency.
Meanwhile, the mean growth rate (GR, mm d-1) of juveniles was calculated from total length (TL, mm) and daily age (t, d) using the following equation:
Fig. 1. Sampling site («) of grey mullet juveniles in Gongshytyan Creek, a tributary to the Tanshui estuary in northwest Taiwan.
... (1)
GR was used to estimate the daily age of other fish without counting otolith growth increment as follows:
... (2)
The hatching dates for all specimens were esti-mated by back-calculation from sampling dates and daily ages estimated from equation (2). A linear re-gression between sampling and hatching dates was estimated. The intercept of the regression repre-sents the time in days required for the juveniles to arrive at the estuary. The slope represents the rela-tive speed by which juveniles arrived at the estuary.
The difference in mean growth rates between the 2 seasons of 1995 and 1996 was tested with
t-test. Differences in the length-age relationship and
the hatching-sampling date relationship between the 2 seasons were tested by analysis of covariance (ANCOVA).
RESULTS Length frequency distribution
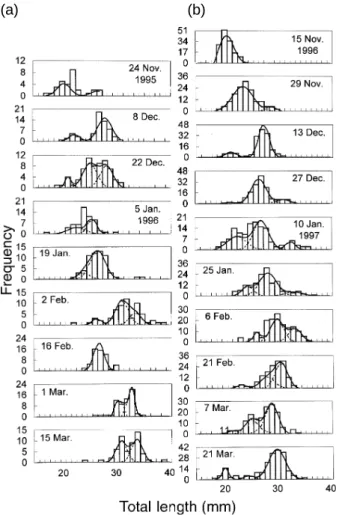
Totally 2041 juvenile grey mullets were collected from the estuary in November 1995–March 1996 and November 1996–March 1997. Total length of the ju-veniles ranged between 17.0 and 39.0 mm, corre-sponding to ages of 29 to 67 d after hatching. One to 3 length modes were present in each sampling date. The smallest mode was 20.4 mm TL and the largest one 34.3 mm (Table 1; Fig. 2). They indicate that juveniles in the estuary were composed of different cohorts.
Daily growth increment
A daily growth increment in otoliths constitutes an incremental zone and a discontinuous zone. The
Table 1. Sampling dates, numbers and total length
of grey mullet juveniles in 1995 and 1996 seasons in the Tanshui estuary
Sampling Sample Total length (mm)
date size Range Mode
1995 Season 24 Nov. 1995 30 17.0-27.0 20.7 8 Dec. 62 20.0-30.5 22.7 28.5 22 Dec. 67 18.5-31.0 21.5 25.6 28.7 5 Jan. 1996 58 16.5-30.0 23.0 25.9 19 Jan. 61 21.5-35.0 24.4 27.0 2 Feb. 61 20.0-37.0 26.7 31.5 34.1 16 Feb. 55 23.5-30.0 27.1 1 Mar. 61 26.0-34.5 30.9 33.2 15 Mar. 56 24.0-39.0 31.3 34.3 1996 Season 15 Nov. 1996 143 18.5-24.0 20.9 29 Nov. 145 19.0-29.5 23.9 29.3 13 Dec. 150 19.0-31.0 21.5 27.8 27 Dec. 152 20.0-32.5 21.5 26.9 31.7 10 Jan. 1997 136 19.0-36.0 22.9 27.6 33.1 25 Jan. 153 22.0-37.0 24.8 28.6 33.5 6 Feb. 146 23.0-35.5 26.6 30.3 33.7 21 Feb. 149 22.0-33.0 23.5 28.4 31.1 7 Mar. 149 20.0-33.5 22.0 25.6 29.3 21 Mar. 207 18.0-35.0 20.4 24.8 30.2 Total 2,041 17.0-39.0
Fig. 2. Length frequency distribution of grey mullet juveniles in the 1995 (a) and 1996 (b) recruitment seasons. (Dotted lines, the frequency of each normal component separated by Bhatta-charya’s method; solid lines, total frequency of all normal components). (a) (b) ∑ = = n i i i n 1 t TL 1 GR GR TL t=
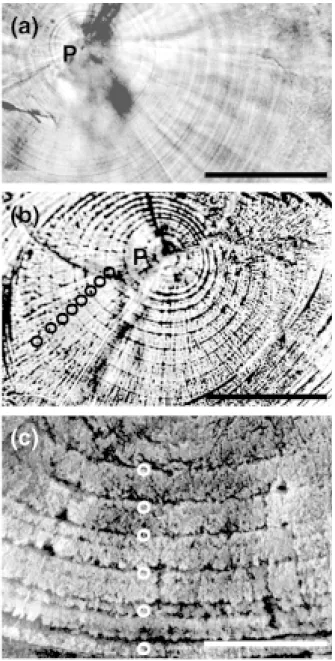
incremental zone is a light band and the discontinu-ous zone a dark band under transmitted visible light (Fig. 3a). The discontinuous zone becomes deeper and more discernible after EDTA etching (Fig. 3b, c). The relationship between new growth incre-ments (Y) beyond the TC mark and number of days after the marking (X) was calculated as follows:
Y = –1.58 + 0.98 X ... (3)
(r = 0.99, df = 33, p < 0.001).
The intercept, 1.58, indicates that deposition of growth increments was delayed 1.58 d after TC marking. The slope 0.98 is not significantly different from 1 (p > 0.05), indicating that the growth incre-ment was deposited daily at a constant rate (Fig. 4), so that, growth increments in otoliths can be used to determine age in days.
Growth during estuarine residency
Relationships between total length (Y) and age (X) of grey mullet juveniles in the 1995 and 1996 sea-sons were fitted with a linear regression equation (Fig. 5). The equations for both seasons are signifi-cant (p < 0.01), and residuals of these 2 equations are all randomly distributed, indicating that the fit-ness of the equations is good. The r-values of these 2 regression equations are 0.84 and 0.79 for the 1995 and 1996 seasons, respectively.
ANCOVA indicates that the regression lines do not significantly differ between the 2 seasons (p > 0.05). Therefore, the equations of both seasons were combined as follow:
Y = 5.88 + 0.45 X ... (4)
(r = 0.81, df = 78, p < 0.001).
The slope, at 0.45 mm d-1, is the growth rate of the juveniles during estuarine residency.
Hatching date
Mean growth rates of grey mullet juveniles in the 1995 and 1996 seasons were calculated to be 0.57 ± 0.05 (n = 37) and 0.59 ± 0.06 mm d-1 (n = 42), re-spectively, which are not significantly different (t-test,
Fig. 3. Microstructure of daily growth increments in polished otoliths of grey mullet juveniles photographed with a transmitted light microscope (a, b) and SEM (c). (Otoliths in (b) and (c) were etched with EDTA; circles, discontinuous zones; P, primordium; scale bars, 50 µm for (a) and (b), 15 µm for (c)).
Fig. 4. Relationship between new growth increments in otoliths and number of days after TC immersion of grey mullet juveniles.
p > 0.05). The mean growth rates of both seasons
were pooled and the combined growth rate is 0.58 ± 0.06 mm d-1 (n = 79). This rate was used to estimate the daily age of juveniles without counting otolith growth increments, and to back-calculate hatching dates of all juveniles used in this study.
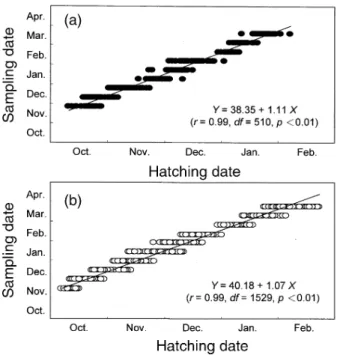
The hatching dates of grey mullet juveniles were from October to January for the 1995 season (Fig. 6a) and from October to February for the 1996 son (Fig. 6b). These indicate that the spawning sea-son of the fish is quite long, extending for 4 to 5 mo. The duration of hatching dates in 1996 is approxi-mately 1 mo longer than that in 1995, due to the pres-ence of small juveniles in the late 1996 fishing sea-son (Fig. 2) caused by a delay in the lowest SST period (Fig. 7). Distributions of hatching dates on sampling dates overlapped, indicating that the juve-nile populations were constituted of multiple cohorts on each sampling date. The same phenomenon was also observed in the length frequency distribu-tion with multiple modes (Fig. 2).
Regressive relationships between sampling and hatching days of grey mullet juveniles in both sea-sons are significant (p < 0.01). The r-values of the regression equations reach 0.99 in both seasons
(Fig. 6).
The slopes of both equations in figure 6 are sig-nificantly larger than 1 (ANCOVA, p < 0.01), and the regression equations are significantly different be-tween the 1995 and 1996 seasons (p < 0.01). These indicate that late hatching juveniles entered the estu-ary late. Meanwhile, recruitment was delayed in 1995 as compared to 1996. The intercept indicates that juveniles entered the estuary at an age of approxi-mately 38-40 d after hatching.
Relationship between catch and SST
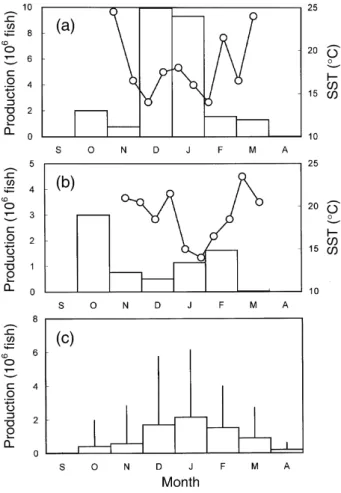
Monthly catch of grey mullet juveniles along the coast of Taiwan in the 1995 season reached a peak in December when SST decreased to the lowest level (Fig. 7a). However, monthly catch in the 1996 season does not correspond to that in 1995. The period of lowest SST in 1996 was delayed and thus, there was no peak catch in December or January (Fig. 7b). The change in the monthly catch of 1995 is similar to that of the 30-yr mean monthly catch during the period from 1967 to 1996 (Fig. 7c).
DISCUSSION AND CONCLUSIONS
Grey mullet juveniles entered the Tanshui estu-ary from November to March at sizes ranging between
Fig. 5. Relationship between total length and daily age of grey mullet juveniles in the 1995 (a) and 1996 (b) seasons.
Fig. 6. Relationship between sampling and hatching date of grey mullet juveniles in the 1995 (a) and 1996 (b) seasons.
17 and 39 mm, and ages of between 29 and 67 d after hatching. The sizes of juveniles in the estuary found in this study are similar to those of other stud-ies (Tung 1981, Lee 1992, Tzeng 1995, Tzeng et al. 1995). The mean growth rate of 0.58 ± 0.06 mm d-1 is fairly close to 0.55-0.75 mm d-1 of laboratory-reared specimens (Kuo et al. 1973, Nash and Shehadeh 1980, Tung 1981).
The length frequency distribution indicates that grey mullet juveniles on each sampling date were constituted of multiple cohorts. Lee and Kuo (1990) noted that there were 5 groups of grey mullet juve-niles recruited to the west coast of Taiwan. Tung (1960) also found that there were approximately 4 groups of grey mullet that migrated to the coastal waters of southwestern Taiwan for spawning. Accordingly, multiple spawners may have contrib-uted to the multiple cohorts of grey mullet juveniles in
the estuary.
The regressive relationship between sampling and hatching dates indicates that recruitment of grey mullet juveniles in the 1995 season was delayed as compared to that of 1996 (Fig. 6). This might be due to the population density (catch) being higher in 1995 than in 1996 (Fig. 7). The higher density might have led to slower growth and subsequently to delayed re-cruitment in 1995. On the other hand, the duration of low SST in the 1995 season was longer than that in 1996. The low SST prevailing in the winter indicates that the cold China Coastal Current was strong. As grey mullet spawners migrate with the cold China Coastal Current to Taiwan, the longer duration of low SST in the 1995 season was probably favorable for spawning and consequently for recruitment of juve-niles to the Tanshui estuary.
Eggs of grey mullet hatch 34-60 h after fertiliza-tion at a water temperature of 22-24 °C (Kuo et al. 1973, Tung 1981). In other words, hatching dates are only 2-3 d from the dates of spawning. According to the back-calculated hatching dates, the grey mul-let juveniles in the Tanshui estuary should have spawned between October and February. Maturing grey mullets did not migrate to the coastal waters of southwestern Taiwan until December to January (Tung 1981). This suggests that there should be other spawning populations sustaining the early and late recruitments of grey mullet juveniles in the stud-ied area. Liu (1986) suggested that there is a resi-dent population sustaining the early recruits. An-other possibility is that the onset of spawning migra-tion of mullet in southwestern Taiwan might be earlier than that mentioned above. According to the peak fishing season of the spawners which migrated with the China Coastal Current to the coastal waters of southwestern Taiwan, the main spawning season was estimated to be approximately around the winter solstice (December 22 ± 10 d). In fact, a portion of spawners may spawn earlier than this peak fishing season (Tung 1981).
In conclusion, grey mullet juveniles in the Tanshui estuary are composed of multiple cohorts. They reached the estuary at the age of approxi-mately 40 d after hatching. The hatching dates were estimated to be from October to February, earlier and later than the spawning period of a previously studied migratory population. The juveniles re-cruited especially early and late in the estuary may come from a resident population or other migratory spawning populations. The inter-annual change in estuarine recruitment may be influenced by both population density and environmental factors, par-ticularly the prevalence of coastal currents.
Fig. 7. Monthly change in the production of grey mullet juveniles and sea surface temperature (SST, open circles) in the outlet of Gongshytyan Creek in the 1995 (a) and 1996 (b) seasons, and the average of 1967-1996 combined (c). (Vertical lines, standard deviations).
Acknowledgments: The authors are grateful to Mr.
CS Lu for fish collection, Mr. HP Chuang for assis-tance in taking SEM photographs, Dr. TF Tsai for re-viewing the manuscript, and 2 anonymous referees for helpful comments.
REFERENCES
Anon. 1967-1997. Fisheries yearbook of Taiwan area (1967-1997). Taipei: Taiwan Fisheries Bureau, Department of Ag-riculture and Forestry, Provincial Government of Taiwan. Campana SE. 1984. Interactive effects of age and
environmen-tal modifiers on the production of daily growth increments in otoliths of plainfin midshipman, Porichthys notatus. Fish. Bull. 82: 165-177.
Campana SE, JD Neilson. 1985. Microstructure of fish otoliths. Can. J. Fish. Aquat. Sci. 44: 1014-1032.
Chang CW. 1997. Daily age and growth of juvenile grey mullets Mugil cephalus L. in the Tanshui estuary as revealed from otolith microstructure. Master’s thesis, National Taiwan Univ. (Taipei). 84 pp.
Chen WY. 1982. Catches of the mullet (Mugil cephalus L.) and the climatic factors of the coastal waters of Taiwan. J. Fish. Soc. Taiwan 9: 48-54.
Chen WY, WC Su. 1986. Reproductive biology of the grey mullets, Mugil cephalus L. of Taiwan. In WC Su, ed. Study on the resource of grey mullet in Taiwan, 1983-1985. Kaohsiung: Kaohsiung Branch, Taiwan Fisheries Research Institute, pp. 73-80.
Chen WY, WC Su, KT Shao, CP Lin. 1989. Morphometric stud-ies of the grey mullet (Mugil cephalus L.) from the waters around Taiwan. J. Fish. Soc. Taiwan 16: 153-163. Cheng PW, WN Tzeng. 1996. Timing of metamorphosis and
es-tuaries arrival across the dispersal range of the Japanese eel Anguilla japonica. Mar. Ecol. Prog. Ser. 131: 87-96. Gayanilo FC Jr., P Sparre, D Pauly. 1994. The FAO-ICLARM
stock assessment tools (FISAT) user’s guide. FAO Com-puterized Information Series (Fisheries). 6: 186 pp. Healey MC. 1982. Timing and relative intensity of size-selective
mortality of juvenile chum salmon (Oncorhynchus keta) during early sea life. Can. J. Fish. Aquat. Sci. 39: 952-957. Huang CS, WC Su. 1986. Analysis on the fishing condition of grey mullet in Taiwan, 1984-1985. In WC Su, ed. Study on the resource of grey mullet in Taiwan, 1983-1985. Kaohsiung: Kaohsiung Branch, TFRI, pp. 35-48.
Jones CM. 1992. Development and application of the otolith in-crement technique. In DK Stevenson, SE Campana, eds. Otolith microstructure examination and analysis. Can. Spec. Publ. Fish. Aquat. Sci. 117: 1-11.
Kuo CM, ZH Shehadeh, KK Milisen. 1973. A preliminary report on the development, growth and survival of laboratory reared larvae of the grey mullet, Mugil cephalus L. J. Fish Biol. 5: 459-470.
Lee CY, CL Kuo. 1990. Kinds, local names and seasonal ap-pearance of the fingerlings of grey mullet, Mugil cephalus L., in coastal waters of west Taiwan. China Fish. Month. 448: 13-18.
Lee SC. 1992. Fish fauna and abundance of some dominant species in the estuary of Tanshui, northwestern Taiwan. J. Fish. Soc. Taiwan 19: 263-271.
Liao IC. 1981. Cultivation methods. In OH Oren, ed. Aquaculture
of grey mullets. IBP 26. Cambridge, UK: Cambridge Univ. Press, pp. 361-390.
Liu CH. 1986. Survey of the spawning grounds of grey mullet. In WC Su, ed. Study on the resource of grey mullet in Taiwan, 1983-1985. Kaohsiung: Kaohsiung Branch, TFRI, pp. 63-72.
Liu CH. 1991. Biology of the mugilid fish (family Mugilidae). Ph.D. dissertation, National Taiwan Univ. (Taipei). 203 pp. Liu CH, SC Shen. 1991. A revision of the mugilid fishes from
Taiwan. Bull. Inst. Zool., Acad. Sin. 30: 273-288. Nash EC, ZH Shehadeh. 1980. Review of breeding and
propaga-tion techniques for grey mullet, Mugil cephalus L. ICLARM Stud. Rev. 3: 1-40.
Pannella G. 1971. Fish otolith: daily growth layers and periodical patterns. Science 173: 1124-1127.
Radtke TL. 1984. Formation and structural composition of larval striped mullet otoliths. Trans. Am. Fish. Soc. 113: 192-196. Su WC, T Kawasaki. 1995. Characteristics of the life history of grey mullet from Taiwanese waters. Fish. Sci. 61: 377-381. Tang YA. 1975. Collection, handling and distribution of grey
mul-let fingerlings in Taiwan. Aquaculture 5: 81-84.
Tung IH. 1960. Surveys on migration and fishing conditions of mullet. China Fish. Month. 95: 2-14.
Tung IH. 1969. Time series analysis of the production of some important marine crustacean and fish fry. Rep. Inst. Fish. Biol. Minist. Econ. Aff., Natl. Taiwan Univ. 2: 28-44. Tung IH. 1981. On the fishery biology of gray mullet, Mugil
cephalus L., in Taiwan. Rep. Inst. Fish. Biol. Minist. Econ. Aff., Natl. Taiwan Univ. 3: 38-102.
Tzeng WN. 1990. Relationship between growth rate and age at recruitment of Anguilla japonica elvers in a Taiwan estuary as inferred from otolith growth increments. Mar. Biol. 107: 75-81.
Tzeng WN. 1995. Recruitment of larval and juvenile fishes to the Gong-Shy-Tyan River estuary of Taiwan: relative abun-dance, species composition, and seasonality. In NB Armantrout, RJ Wolotira Jr., eds. Condition of the world’s aquatic habitats. New Delhi: Oxford and IBH Publ., pp. 360-385.
Tzeng WN, JJ Hsiao, HP Shen, YT Chern, YT Wang, JY Wu. 1995. Feeding habit of the Japanese eel, Anguilla japonica, in the streams of northern Taiwan. J. Fish. Soc. Taiwan 22: 279-302.
Tzeng WN, YC Tsai. 1994. Changes in otolith microchemistry of the Japanese eel, Anguilla japonica, during its migration from the ocean to the rivers of Taiwan. J. Fish Biol. 45: 671-683.
Tzeng WN, CE Wu, YT Wang. 1998. Age of Pacific tarpon, Megalops cyprinoides, at estuarine arrival and growth dur-ing metamorphosis. Zool. Stud. 37: 177-183.
Tzeng WN, SY Yu. 1989. Validation of daily growth increments in otoliths of milkfish larvae by oxytetracycline labeling. Trans. Am. Fish. Soc. 118: 168-174.
Volk EC, RC Wissmar, CA Simenstad, DM Egger. 1984. Rela-tionship between otolith microstructure and the growth of juvenile chum salmon (Oncorhynchus keta) under different prey rations. Can. J. Fish. Aquat. Sci. 41: 126-133. Wang YT, WN Tzeng. 1999. Differences in growth rates among
cohorts of Encrasicholina punctifer and Engraulis japonicus larvae in the coastal waters off Tanshui River estuary, Tai-wan as indicated by otolith microstructure analysis. J. Fish Biol. 54: 1002-1016.