Title Page
Title: Proton pump inhibitors and risk of hepatocellular carcinoma: a case-control study in Taiwan
Running head: Proton pump inhibitors and hepatocellular carcinoma
Shih-Wei Lai, MD1,2; Kuan-Fu Liao, MD and MS3,4,5; Hsueh-Chou Lai, MDand MS6,7; Cheng-Li Lin, MS8,9; Fung-Chang Sung, PhD, MPH8,9
(The first two authors contributed equally to this study.)
1School of Medicine, 3Graduate Institute of Integrated Medicine, 6School of Chinese Medicine, and 8Department of Public Health, China Medical University, Taichung, Taiwan
2Department of Family Medicine, 7Department of Internal Medicine, and
9Management Office for Health Data, China Medical University Hospital, Taichung, Taiwan
4Department of Internal Medicine, Taichung Tzu Chi General Hospital, Taichung, Taiwan
5Department of Health Care Administration, Central Taiwan University of Science and Technology, Taichung, Taiwan
Corresponding author:
Hsueh-Chou Lai, MD and MS
Department of Internal Medicine, China Medical University Hospital, No. 2, Yuh-Der Road, Taichung City, 404, Taiwan
Phone: 886-4-2205-2121 Fax: 886-4-2203-3986
E-mail: t674233@ms54.hinet.net
Word count: 750 in the main text, with 2 tables and 7 references Keywords: hepatocellular carcinoma; proton pump inhibitor
Proton pump inhibitors (PPIs) are effective drugs that are frequently used for the management of peptic ulcer and gastro-esophageal reflux disease as well as for the elimination of Helicobacter infection. However, the long-term use of PPIs has been shown to cause hypergastrinemia . Experimental and clinical studies have also shown that hypergastrinemia might correlate with the risk for gastro-intestinal cancers, with the exception of hepatocellular carcinoma (HCC) . Few studies on the association between PPIs use and HCC risk are available. To clarify this issue, we designed this population-based, case-control study by using the Taiwan National Health Insurance Program database to explore the association between PPIs use and HCC risk. The details regarding the insurance program have been reported previously . In order to secure patient privacy, all types of personal identification on files connected with the present study were scrambled using surrogate identification numbers. This study was exempt from full review by the institutional review board. Based on the International Classification of Diseases, 9th Revision-Clinical Modification (ICD-9 codes) and A-codes, cases were defined as patients who were newly diagnosed with HCC (ICD-9 codes 155, 155.0, and 155.2, and A-code A095) from 2000 to 2010 and aged 20 years or older at the diagnosis date. We defined the index date for each case as the HCC diagnosis date. For each HCC case, we randomly selected four subjects from the same dataset as control subjects, frequency matched by sex, age (within five years), and index date. Subjects with HCC or any other cancer type (ICD-9 codes 140-208 and A-codes A08x-A14x) before the index date were excluded. The co-morbidities before the index date that could be associated with HCC risk included diabetes mellitus, cirrhosis, alcoholic liver damage, nonalcoholic fatty liver disease, hepatitis B infection, hepatitis C infection, and tobacco use. All co-morbidities were diagnosed based on ICD-9 codes and A codes. To explore the effects of medications on HCC
risk, the medication history of patients taking five commercially available PPIs before the index date were analyzed, including omeprazole, pantoprazole, lansoprazole, rabeprazole, and esomeprazole. Other medications before the index date, such as histamine-2 receptor antagonists, statins, non-statin lipid-lowering drugs, metformin, and thiazolidinediones, were also included. Some of the patients who had early-undiagnosed HCC initially exhibited abdominal symptoms that might have been treated with PPIs. To minimize a possible confounding effect, cases treated with PPIs only within one year of HCC diagnosis were excluded from the analysis. Therefore, cases treated with PPIs included only those treated more than one year before the HCC diagnosis.
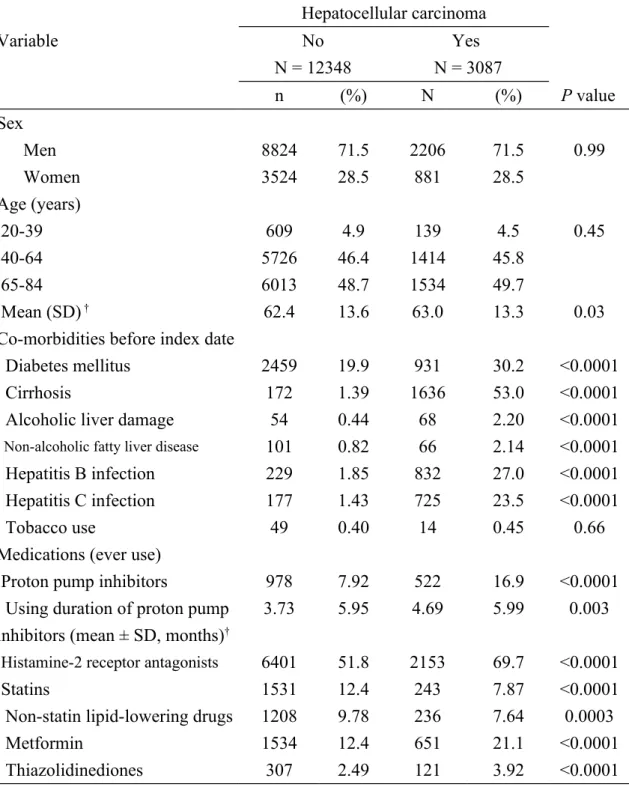
A total of 3087 cases with HCC and 12348 subjects without HCC as controls were included. Table 1 shows the data on the HCC cases and controls. The cases show an increased possibility of acquiring diabetes mellitus, cirrhosis, alcoholic liver damage, non-alcoholic fatty liver disease, hepatitis B infection, and hepatitis C infection, PPIs, histamine-2 receptor antagonists, metformin, and thiazolidinediones intake. The cases had longer mean duration of exposure to PPIs than the controls (4.69 vs. 3.73 months,
P = 0.003). After multi-confounder adjustment, multivariate logistic regression
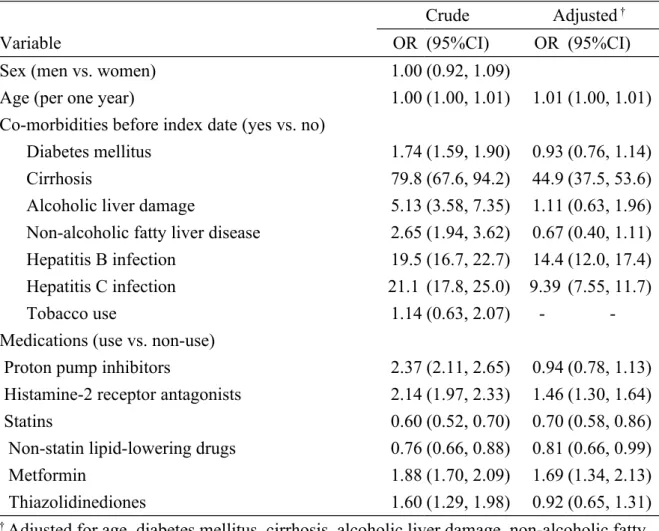
analysis demonstrated the overall OR of HCC as 0.94 (95% CI 0.78, 1.13) for the PPIs group, when compared with the PPIs non-use group. Significant factors
associated with HCC include age (OR 1.01, 95% CI 1.00, 1.01), cirrhosis (OR 44.9, 95% CI 37.5, 53.6), hepatitis B infection (OR 14.4, 95% CI 12.0, 17.4), hepatitis C infection (OR 9.39, 95% CI 7.55, 11.7), histamine-2 receptor antagonists use (OR 1.46, 95% CI 1.30, 1.64), and metformin use (OR 1.69, 95% CI 1.34, 2.13). Statins (OR 0.70, 95% CI 0.58, 0.86) and non-statin lipid-lowering drugs intake (OR 0.81, 95% CI 0.66, 0.99) were negatively associated with HCC risk (Table 2).
This study showed no significant association could be detected between PPIs use and HCC risk. To minimize the protopathic bias caused by existing chronic liver diseases, we also explored the interaction effects between chronic liver diseases and PPI use in relation to HCC. As a reference for subjects with chronic liver diseases who did not use PPIs, the OR of HCC was 0.92 (95% CI 0.73, 1.17) for patients with chronic liver diseases who used PPIs (data not shown in the table). Some study limitations should be addressed. Data on serum gastrin was not provided due to the inherent limitation of the dataset. Thus, a link among PPIs use, gastrin level, and HCC cannot be established.
Funding
This study was partly supported by grants from Taiwan Department of Health Clinical Trial and Research Center of Excellence (DOH101-TD-B-111-004), the Cancer Research Center of Excellence (DOH 101-TD-C-111-005), and the National Science Council (NSC 100-2621-M-039-001). The funding agencies did not influence the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgements
The authors would like to thank the National Health Research Institute in Taiwan for providing the insurance claims data.
Conflict of Interest Statement
REFERENCES
[1] Ligumsky M, Lysy J, Siguencia G, Friedlander Y. Effect of long-term, continuous versus alternate-day omeprazole therapy on serum gastrin in patients treated for reflux esophagitis. J Clin Gastroenterol. 2001;33:32-5.
[2] Jensen RT. Consequences of long-term proton pump blockade: insights from studies of patients with gastrinomas. Basic Clin Pharmacol Toxicol. 2006;98:4-19. [3] Smith JP, Fantaskey AP, Liu G, Zagon IS. Identification of gastrin as a growth peptide in human pancreatic cancer. Am J Physiol. 1995;268:R135-41.
[4] Thorburn CM, Friedman GD, Dickinson CJ, Vogelman JH, Orentreich N, Parsonnet J. Gastrin and colorectal cancer: a prospective study. Gastroenterology. 1998;115:275-80.
[5] Waldum HL, Gustafsson B, Fossmark R, Qvigstad G. Antiulcer drugs and gastric cancer. Dig Dis Sci. 2005;50 Suppl 1:S39-44.
[6] Lai SW, Chen PC, Liao KF, Muo CH, Lin CC, Sung FC. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol. 2012;107:46-52.
[7] Liao KF, Lai SW, Li CI, Chen WC. Diabetes mellitus correlates with increased risk of pancreatic cancer: a population-based cohort study in Taiwan. J Gastroenterol Hepatol. 2012;27:709-13.
Table 1. Basic data of hepatocellular carcinoma cases and control subjects Hepatocellular carcinoma Variable No N = 12348 Yes N = 3087 n (%) N (%) P value Sex Men 8824 71.5 2206 71.5 0.99 Women 3524 28.5 881 28.5 Age (years) 20-39 609 4.9 139 4.5 0.45 40-64 5726 46.4 1414 45.8 65-84 6013 48.7 1534 49.7 Mean (SD) † 62.4 13.6 63.0 13.3 0.03
Co-morbidities before index date
Diabetes mellitus 2459 19.9 931 30.2 <0.0001
Cirrhosis 172 1.39 1636 53.0 <0.0001
Alcoholic liver damage 54 0.44 68 2.20 <0.0001
Non-alcoholic fatty liver disease 101 0.82 66 2.14 <0.0001
Hepatitis B infection 229 1.85 832 27.0 <0.0001
Hepatitis C infection 177 1.43 725 23.5 <0.0001
Tobacco use 49 0.40 14 0.45 0.66
Medications (ever use)
Proton pump inhibitors 978 7.92 522 16.9 <0.0001
Using duration of proton pump inhibitors (mean ± SD, months)†
3.73 5.95 4.69 5.99 0.003
Histamine-2 receptor antagonists 6401 51.8 2153 69.7 <0.0001
Statins 1531 12.4 243 7.87 <0.0001
Non-statin lipid-lowering drugs 1208 9.78 236 7.64 0.0003
Metformin 1534 12.4 651 21.1 <0.0001
Thiazolidinediones 307 2.49 121 3.92 <0.0001
Data are presented as the number of subjects (%).
Chi-square test and †t-test comparing subjects with and without hepatocellular carcinoma
Table 2. Odds ratios and 95% confidence interval of hepatocellular carcinoma associated with PPIs and covariates
Crude Adjusted †
Variable OR (95%CI) OR (95%CI)
Sex (men vs. women) 1.00 (0.92, 1.09)
Age (per one year) 1.00 (1.00, 1.01) 1.01 (1.00, 1.01)
Co-morbidities before index date (yes vs. no)
Diabetes mellitus 1.74 (1.59, 1.90) 0.93 (0.76, 1.14)
Cirrhosis 79.8 (67.6, 94.2) 44.9 (37.5, 53.6)
Alcoholic liver damage 5.13 (3.58, 7.35) 1.11 (0.63, 1.96) Non-alcoholic fatty liver disease 2.65 (1.94, 3.62) 0.67 (0.40, 1.11) Hepatitis B infection 19.5 (16.7, 22.7) 14.4 (12.0, 17.4) Hepatitis C infection 21.1 (17.8, 25.0) 9.39 (7.55, 11.7)
Tobacco use 1.14 (0.63, 2.07) -
-Medications (use vs. non-use)
Proton pump inhibitors 2.37 (2.11, 2.65) 0.94 (0.78, 1.13) Histamine-2 receptor antagonists 2.14 (1.97, 2.33) 1.46 (1.30, 1.64)
Statins 0.60 (0.52, 0.70) 0.70 (0.58, 0.86)
Non-statin lipid-lowering drugs 0.76 (0.66, 0.88) 0.81 (0.66, 0.99)
Metformin 1.88 (1.70, 2.09) 1.69 (1.34, 2.13)
Thiazolidinediones 1.60 (1.29, 1.98) 0.92 (0.65, 1.31)
† Adjusted for age, diabetes mellitus, cirrhosis, alcoholic liver damage, non-alcoholic fatty liver disease, hepatitis B infection, hepatitis C infection, histamine-2 receptor antagonists, statins, non-statin lipid-lowering drugs, metformin, and thiazolidinediones