The Effect of Supplemental Glutamine on Growth Performance, Development of the Gastrointestinal Tract, and Humoral Immune Response of Broilers
S. M. Bartell and A. B. Batal1
Department of Poultry Science, University of Georgia, Athens 30602 ABSTRACT Two experiments were conducted to eval-
uate the effect of supplemental Gln on growth perfor- mance, development of the gastrointestinal tract, and hu- moral immune response of broilers. Immediately after hatch 6 replicate pens of 6 chicks were randomly assigned to 1 of 7 (experiment 1) or 5 (experiment 2) dietary treat- ments for 21 d. On d 4, 7, 14, and 21, twelve chicks per treatment (2 chicks/pen) were killed for thymus, spleen, bursa, duodenum, jejunum, ileum, bile, and blood sample collections and weights. In experiment 1, the effect of 1 or 4% Gln addition to the feed, water, or both was com- pared with a corn-soybean meal (SBM) control diet. All diets were formulated to be isocaloric and isonitrogenous.
Weight gain improved significantly (P< 0.05) when chicks were fed diets with 1% Gln as compared with chicks fed the control diet (11% average improvement). The addition of 4% Gln to the diet or water depressed (P< 0.05) growth
Key words: glutamine, gastrointestinal tract, broiler chick, immune response
2007 Poultry Science 86:1940–1947
INTRODUCTION
L-Glutamine is the most prevalent amino acid in the bloodstream, accounting for 30 to 35% of the amino acid N in the plasma and in the free amino acid pool in the body (Newsholme et al., 1985). Because Gln contains 2 ammonia groups, one from its precursor, glutamate, and the other from free ammonia in the bloodstream, Gln acts as a “nitrogen shuttle” that helps protect the body from high levels of ammonia (Labow, 2001). Thus, Gln can act as a buffer, accepting excess ammonia and then releasing it when needed to form other amino acids, amino sugars, glucose, proteins, nucleotides, glutathione, and urea (Souba, 1993; Rennie, 2001). This capacity to accept and donate N makes Gln the major vehicle for nitrogen trans- fer between tissues. Glutamine is the principal metabolic fuel for small intestine enterocytes, lymphocytes, macro- phages, and fibroblasts (Cynober, 1999; Andrews and
©2007 Poultry Science Association Inc.
Received January 2, 2007.
Accepted May 2, 2007.
1Corresponding author: batal@uga.edu
1940
performance. Based on the results from experiment 1, 1%
Gln supplementation to the diet was determined to be ample and most practical. Thus in experiment 2, diets supplemented with 1% Gln were fed for 4, 7, 14, or 21 d after which time chicks were fed the corn-SBM control diet until the experiment was terminated at 21 d. Weight gain improved significantly (P< 0.05) when chicks were fed diets supplemented with 1% Gln throughout the 21- d study. In both experiments, chicks fed diets supple- mented with 1% Gln for 21 d had higher concentrations of bile, intestinal, and sera IgA and sera IgG (P< 0.05).
Chicks fed diets with 1% Gln had heavier intestinal rela- tive weights and longer intestinal villi (P< 0.05) as com- pared with the chicks fed the corn-SBM diet. Our results indicate that the addition of 1% Gln to the diet of broiler chicks improves growth performance and may stimulate development of the gastrointestinal tract and humoral immune response.
Griffiths, 2002) and is considered an essential amino acid in some species under inflammatory conditions such as infection and injury (Newsholme, 2001). Calder (1999) reported that in culture Gln is utilized at a high rate by cells of the immune system and is required to support optimal lymphocyte proliferation and cytokine produc- tion by lymphocytes and macrophages. Glutamine is also the precursor for the net synthesis of Arg, which has been shown to increase thymus and spleen size in mice (Adjei et al., 1994), increase cytokine production, and enhance lymphocyte proliferation (Reynolds et al., 1988).
Many benefits have been observed due to Gln supple- mentation in the diet of humans and rats; however, little research has been done with swine and poultry. Yi et al.
(2001) reported that supplementing the diet with 1% Gln improved weight gain and feed efficiency (weight gain:feed intake) of turkey poults during the first week posthatch as compared with poults fed a standard corn- soybean meal (SBM) diet. Kitt et al. (2002) reported that the addition of 1% Gln to the diet improved the feed efficiency in weanling pigs. Glutamine supplementation increased intestinal villus height in poults (Yi et al., 2001) and weanling pigs (Kitt et al., 2002). Glutamine supple- mentation has been reported to stimulate gut mucosal
proliferation in rats (Inoue et al., 1993). It has also been observed that supplementing with 1.5% Gln in total par- enteral nutrition diets maintains gut integrity (Naka, 1996), which is important in preventing bacterial infec- tions, and Gln has been shown to prevent intestinal hyper- permeability and bacterial translocation in mice during an immunological challenge (Adjei et al., 1994). During stressful conditions, intestinal permeability increases allowing bacteria to enter the bloodstream, thus causing infection (Adjei et al., 1994), and Gln has also been shown to decrease the incidence of infection in surgery and trauma patients (Newsholme, 2001; Medina, 2001; An- drews and Griffiths, 2002).
To date little research has been conducted on the use of Gln supplementation in poultry diets. Therefore, 2 studies were conducted to determine the effect of Gln supplemen- tation on growth performance, development of the gas- trointestinal tract, and humoral immune response of broiler chicks.
MATERIALS AND METHODS
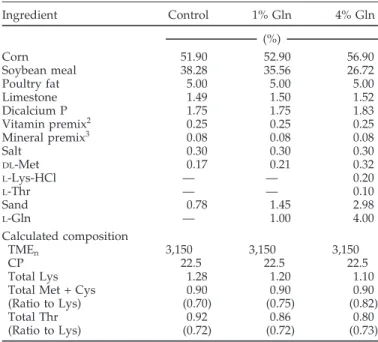
Two studies were conducted with Cobb 500 by-product male chicks obtained from a local hatchery and immedi- ately placed in Petersime battery cages (Petersime Incuba- tor Co., Gettysburg, OH) with wire-mesh floors in an environmentally controlled room. Chicks were weighed and randomly allotted to pens such that each pen of chicks had a similar initial weight distribution. Chicks were maintained on a 24-h constant lighting schedule, and the room temperature was maintained at 24 to 27°C. Chicks had ad libitum access to feed and water. The treatment diets were formulated to meet the NRC (1994) recommen- dations and were fed from 0 to 21 d of age. The experimen- tal diets were formulated to be isonitrogenous and isoca- loric with 22.5% CP, 3,150 kcal of TME/kg, a constant fat level of 5%, with sand as a filler (Table 1).L-Lysine-HCl and L-Thr had to be supplemented to the 4% Gln diet to meet the NRC (1994) amino acid recommendations because of the large change in SBM inclusion (2.72% dif- ference in SBM between control and 1% Gln diet and 8.84% difference between the 1% Gln and 4% Gln diets).
Glutamine levels in the control and treatment diets were not measured or could not be calculated as no accurate method of measuring Gln in feed ingredients has been determined, and no estimates of Gln levels in corn or SBM could be found. Group body weight and feed intakes were measured on d 4, 7, 14, and 21. Weight gain and feed efficiency (gain:feed) were calculated for each pen.
At the occurrence of mortality feed intake was adjusted based on bird days on feed.
Experiment 1
Experiment 1 was conducted to evaluate the effects of Gln supplementation, the optimal level of Gln supple- mentation (1 or 4% Gln) and the best route of administra- tion (addition to the feed, water, or both) and to determine if the positive benefits observed in humans and rats can
Table 1.Composition of the dietary treatments (as-fed basis), experi- ments 1 and 21
Ingredient Control 1% Gln 4% Gln
(%)
Corn 51.90 52.90 56.90
Soybean meal 38.28 35.56 26.72
Poultry fat 5.00 5.00 5.00
Limestone 1.49 1.50 1.52
Dicalcium P 1.75 1.75 1.83
Vitamin premix2 0.25 0.25 0.25
Mineral premix3 0.08 0.08 0.08
Salt 0.30 0.30 0.30
DL-Met 0.17 0.21 0.32
L-Lys-HCl — — 0.20
L-Thr — — 0.10
Sand 0.78 1.45 2.98
L-Gln — 1.00 4.00
Calculated composition
TMEn 3,150 3,150 3,150
CP 22.5 22.5 22.5
Total Lys 1.28 1.20 1.10
Total Met + Cys 0.90 0.90 0.90
(Ratio to Lys) (0.70) (0.75) (0.82)
Total Thr 0.92 0.86 0.80
(Ratio to Lys) (0.72) (0.72) (0.73)
1Diets were formulated to provide 22.5% CP, 3,150 kcal of TME/kg, 0.90% total sulfur amino acids, at least 1.10% total Lys and 0.80% Thr.
2Vitamin mix provided the following (per kg of diet): thiamin mononi- trate, 2.4 mg; nicotinic acid, 44 mg; riboflavin, 4.4 mg;D-Ca pantothenate, 12 mg; vitamin B12(cobalamin), 12.0g; pyridoxineⴢHCl, 4.7 mg;D- biotin, 0.11 mg; folic acid, 5.5 mg; menadione sodium bisulfite complex, 3.34 mg; choline chloride, 220 mg; cholecalciferol, 27.5g; transretinyl acetate, 1,892g; all-rac α-tocopheryl acetate, 11 mg; ethoxyquin, 125 mg.
3Trace mineral mix provided the following (per kg of diet): Mn (MnSO4ⴢH2O), 60 mg; Fe (FeSO4ⴢ7H2O), 30 mg; Zn (ZnO), 50 mg; Cu (CuSO4ⴢ5H2O), 5 mg; I (ethylene diamine dihydroiodide), 0.15 mg; Se (NaSeO3), 0.3 mg.
be viewed in poultry. Four hundred twenty chicks were randomly divided into 7 treatment groups of 6 replicates of 10 birds each. The treatment groups were as follows:
1) control, a standard corn-SBM diet, 2) corn-SBM diet supplemented with 1% Gln, 3) 1% Gln added to standard city drinking water, with the water treatments mixed every 7 d and the birds fed the control diet, 4) corn-SBM diet supplemented with 1% Gln and 1% Gln added to city drinking water, 5) corn-SBM diet supplemented with 4% Gln, 6) 4% Gln added to standard city drinking water, with the water treatments mixed every 7 d and the birds fed the control diet, and 7) corn-SBM diet supplemented with 4% Gln and 4% Gln added to city drinking water.
Due to the problem of Gln precipitating out of solution in the water treatments and low water intake, the water treatments were discontinued at d 14.
Experiment 2
Experiment 2 was conducted to determine how long the 1% Gln needed to be supplemented in the diet to achieve the improvement in growth performance, en- hancement in development of the gastrointestinal tract, and antibody concentrations observed in experiment 1.
Three hundred chicks were randomly divided into 5 treat- ment groups of 6 replicates of 10 birds each. The chicks
were fed a standard corn-SBM control diet or a corn-SBM diet supplemented with 1% Gln (Table 1). The experimen- tal treatments were as follows: 1) control, a corn-SBM diet fed until 21 d of age, 2) a corn-SBM diet supplemented with 1% Gln fed for 4 d after which time the chicks were fed the control corn-SBM diet until 21 d of age, 3) a corn- SBM diet supplemented with 1% Gln fed for 7 d, then the control diet fed from d 8 to 21, 4) the corn-SBM diet supplemented with 1% Gln fed for 14 d after which time the control corn-SBM diet was fed until 21 d of age, and 5) a corn-SBM diet supplemented with 1% Gln fed for the entire 21 d experimental period.
Sampling
In experiments 1 and 2, twelve chicks per treatment (2 chicks per pen) were randomly selected on d 0, 4, 7, 14, and 21 for sampling of blood, organ weights, and intesti- nal measurements. Chicks were weighed and killed by cervical dislocation, and then the abdominal cavity was opened. The thymus, spleen, and bursa were removed and weighed. The thymus weight was determined as the 5 lobes located bilaterally on the sides of the esophagus.
For intestinal weight measurements, the small intestine was removed and divided into 3 segments: duodenum (from gizzard to entry of the bile and pancreatic ducts), jejunum (from entry of the ducts to Meckel’s diverticu- lum), and ileum (from Meckel’s diverticulum to the ileocecal junction). The ileum was flushed with 10 to 20 mL of deionized water, and the empty weight was re- corded. Because morphologic analysis of the duodenum and jejunum was to be determined, these segments were flushed with 20 mL of physiological saline solution, and the empty weight was recorded. Organ weights were expressed on a relative (g/100 g of BW) BW and an abso- lute basis. For morphologic analysis, approximately 5 cm of the middle portion of the duodenum and jejunum (the apex of the duodenum and midway between the point of entry the bile ducts and Meckel’s diverticulum of the jejunum) was excised and fixed in 10% formalin. Six cross sections of 70% ethanol-preserved segments for each duo- denal and jejunal sample were then prepared for staining with hematoxylin and eosin using standard paraffin em- bedding procedures (Uni et al., 1995). A total of 4 intact, well-oriented villi were selected in 6 replicates for each intestinal cross section (24 measurements for each intesti- nal sample with 288 measurements per treatment). Villus height was measured from the tip of the villi to the villus crypt junction. Morphological indices were determined using computer-aided light microscope (16× magnifica- tion of the objective lens) image analysis (Image-Pro Plus Version 3.0, Media Cybernetics, Silver Spring, MD).
Blood, bile, and jejunum samples were collected from 2 birds per pen (12 birds per treatment) on d 7, 14, and 21 in experiments 1 and 2. Blood was obtained by jugular venipuncture from each bird. Blood samples were centri- fuged at 1,000× g for 10 min at room temperature, and the serum fraction was frozen and stored at−20°C until analyzed. The birds were killed by cervical dislocation,
and bile and jejunal samples were obtained. Bile was aspirated from the gall bladder with a 25-gauge needle coupled to a 3-mL syringe and then stored at−20°C until IgA analysis was conducted. The jejunum, i.e., the portion of the small intestine between the opening of the pancreo- biliary ducts and the Meckel’s diverticulum, was excised from each bird. Ten centimeters of the middle portion of the jejunum was separated and stored at−20°C until prepared for analysis. At the time of analysis, the jejunal samples were thawed at room temperature, 2 g of jejunal sample was weighed, 20 mL of deionized water was added, and it was homogenized for 30 s with a mechanical homogenizer (VirTis, Gardiner, NY). An aliquot (5 mL) of the sample was centrifuged at 20,000× g for 30 min.
The supernatant was obtained and stored at−20°C until analyzed for IgA concentration.
Analysis of Ig in Serum, Bile, and Intestine
Serum samples for all treatment and age groups were analyzed for IgA and IgG at the same time to avoid varia- tion that may occur with analyses done at different times.
Serum, bile, and jejunal IgA, and serum IgG was deter- mined using a double antibody technique ELISA kit (Be- thyl Laboratories Inc., Montgomery, TX). Absorbance was measured at 450 nm. The absorbance of the control wells were adjusted to zero prior to measuring absorbance in the samples. Because absorbance units are linearly related to the logarithm of the Ig concentration (Piquer et al., 1991), we considered that the absorbance measurements obtained could be used as estimates of Ig concentrations.
Therefore, no standard curve was used to calculate Ig con- centration.
Statistical Analysis
All the data were subjected to ANOVA procedures for completely randomized designs using the GLM proce- dure of SAS (SAS Institute, 2002). Statistical significances of differences among treatment group means were deter- mined using Duncan’s multiple range test (Duncan, 1955).
Single degree of freedom orthogonal contrasts were per- formed to compare the effects of 1% Gln supplementation in the feed vs. the control diet in experiment 1 and in experiment 2 to compare the effect of Gln supplementa- tion for any length of period vs. the control. A probability level of P≤ 0.05 was used to denote statistical significance.
RESULTS Experiment 1
By d 14, BW gain significantly increased in birds that were fed diets supplemented with 1% Gln when com- pared with the control birds fed the standard corn-SBM diet (P< 0.0001; Table 2). Weight gain had increased 11%
by 21 d of age in the birds fed diets supplemented with 1% Gln compared with the birds fed the control diet.
Although the cumulative (0 to 21 d) weight gain of birds
Table 2.Effect of Gln supplementation on BW gain (g/chick) of broilers, experiment 1 Days of age
Treatment1 0 to 4 4 to 7 7 to 14 14 to 21 0 to 212
Corn-SBM 46a 63a 237b 360ab 706ab
1% Gln in feed 49a 61a 261a 400a 771a
1% Gln in water 40bc 57b 232bc —3 —
1% Gln in feed + water 38c 57b 237b — —
4% Gln in feed 45ab 53b 215c 321b 634b
4% Gln in water 29d 40c 196d — —
4% Gln in feed + water 30d 38c 174e — —
Pooled SEM 1.8 2.1 6.8 22.2 26.8
P-value 0.0001 0.0001 0.0001 0.03 0.0001
a–eMeans within columns having the same superscript do not differ significantly (P< 0.05).
1Means represent 6 pens per treatment; 10 chicks per pen (60 chicks per treatment).
2Using single degree of freedom contrast the control and 1% Gln in the feed are significantly different (P<
0.05) at 21 d of age.
3Results are not shown for the water treatments after 14 d as these treatments were terminated at d 14.
fed diets supplemented with 1% Gln was not statistically different from birds fed the control diet, it is numerically greater, and when a single degree of freedom orthogonal contrast was performed they were significantly different (P< 0.0001). Due to the problem of Gln precipitating out of solution in the water treatments and the low water intake observed in these groups, the water treatments were discontinued at d 14; therefore, data for these treat- ments are not displayed in the tables after 14 d of age.
Body weight gain was significantly depressed in birds fed diets supplemented with the 4% level of Gln (P< 0.0001).
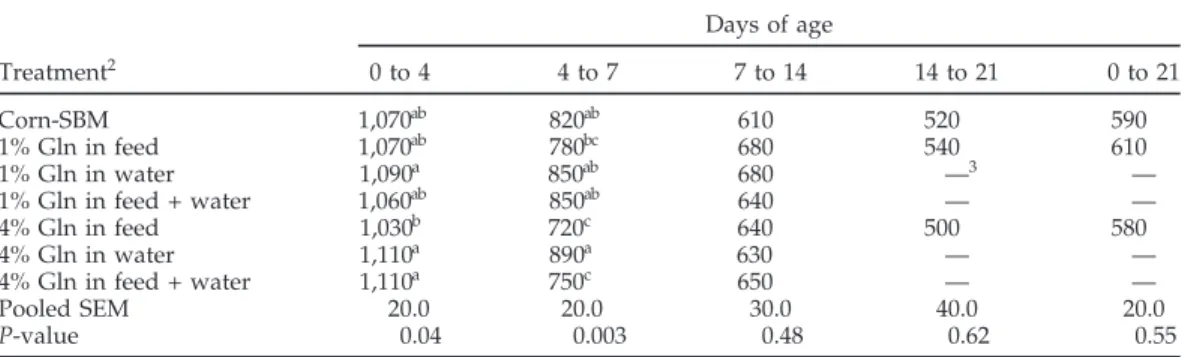
Glutamine supplemented at the 1 or 4% level in the feed, water, or both did not consistently affect feed effi- ciency (Table 3). The thymus (0.51 vs. 0.35 g) and spleen (0.13 vs. 0.08 g) relative weights of broilers were signifi- cantly heavier when 1% Gln was supplemented in the feed and water compared with the birds fed the control corn-SBM diet (P< 0.05; data not shown). The duodenum (1.56 to 1.66 g vs. 1.30 g) and jejunum (2.41 to 2.45 g vs.
2.07 g) relative weights were significantly heavier with the addition of 1 and 4% Gln supplementation in the feed, water, or both compared with the control birds (P< 0.05;
data not shown). Glutamine supplementation in the feed,
Table 3.Effect of Gln supplementation on the feed efficiency1of broilers, experiment 1 Days of age
Treatment2 0 to 4 4 to 7 7 to 14 14 to 21 0 to 21
Corn-SBM 1,070ab 820ab 610 520 590
1% Gln in feed 1,070ab 780bc 680 540 610
1% Gln in water 1,090a 850ab 680 —3 —
1% Gln in feed + water 1,060ab 850ab 640 — —
4% Gln in feed 1,030b 720c 640 500 580
4% Gln in water 1,110a 890a 630 — —
4% Gln in feed + water 1,110a 750c 650 — —
Pooled SEM 20.0 20.0 30.0 40.0 20.0
P-value 0.04 0.003 0.48 0.62 0.55
a–cMeans within columns having the same superscript do not differ significantly (P< 0.05).
1Gain:feed = weight gain (g)/feed intake (kg).
2Means represent 6 pens per treatment; 10 chicks per pen (60 chicks per treatment).
3Results are not shown after 14 d for the water treatments as these treatments were terminated at 14 d of age.
water, or both did not affect the chick’s bursa weight.
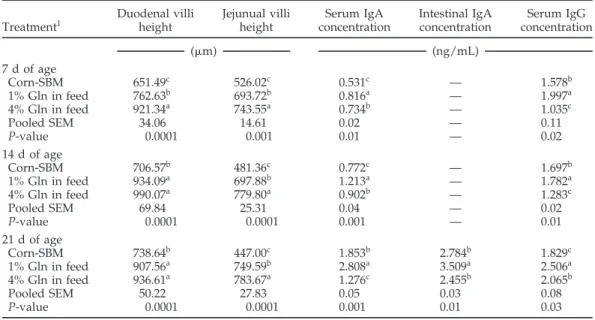
Villi length in the duodenum and jejunum (Table 4) were significantly longer in the birds fed diets supplemented with Gln, with the birds fed the diet with 4% Gln having the longest villi (P< 0.0001).
The birds fed diets supplemented with 1% Gln had significantly higher IgA concentrations in the serum (Ta- ble 4) and bile (data not shown) than the controls birds (P< 0.05). By d 21, the birds fed diets supplemented with 4% Gln had significantly lower IgA concentrations in the serum (P< 0.001) compared with birds fed the control diet and birds fed diets supplemented with 1% Gln. The d-21 IgA intestinal concentrations were significantly higher in the birds fed the 1% Gln supplemented diet than the birds fed the control corn-SBM diet (P< 0.01).
The birds fed diets supplemented with 1% Gln had sig- nificantly higher IgG concentrations in the serum by 21 d of age than the birds fed the control diet (P< 0.03).
Experiment 2
There was no significant benefit from 1% Gln supple- mentation in the feed on chick performance until d 14
Table 4.Effect of Gln supplementation on villus height and humoral immune response of broilers, experiment 1 Duodenal villi Jejunual villi Serum IgA Intestinal IgA Serum IgG
Treatment1 height height concentration concentration concentration
(m) (ng/mL)
7 d of age
Corn-SBM 651.49c 526.02c 0.531c — 1.578b
1% Gln in feed 762.63b 693.72b 0.816a — 1.997a
4% Gln in feed 921.34a 743.55a 0.734b — 1.035c
Pooled SEM 34.06 14.61 0.02 — 0.11
P-value 0.0001 0.001 0.01 — 0.02
14 d of age
Corn-SBM 706.57b 481.36c 0.772c — 1.697b
1% Gln in feed 934.09a 697.88b 1.213a — 1.782a
4% Gln in feed 990.07a 779.80a 0.902b — 1.283c
Pooled SEM 69.84 25.31 0.04 — 0.02
P-value 0.0001 0.0001 0.001 — 0.01
21 d of age
Corn-SBM 738.64b 447.00c 1.853b 2.784b 1.829c
1% Gln in feed 907.56a 749.59b 2.808a 3.509a 2.506a
4% Gln in feed 936.61a 783.67a 1.276c 2.455b 2.065b
Pooled SEM 50.22 27.83 0.05 0.03 0.08
P-value 0.0001 0.0001 0.001 0.01 0.03
a–cMeans within columns and age period having the same superscript do not differ significantly (P< 0.05).
1Means represent 6 pens per treatment; 10 chicks per pen (60 chicks per treatment).
(Table 5). Overall, birds fed diets supplemented with 1%
Gln for at least 14 d had significantly better BW gain compared with the birds fed the control diet, with the birds fed 1% Gln for 21 d having the largest gain (P<
0.02). The BW gain difference was 10.9% between the birds fed the control diet and the birds fed diets supple- mented with 1% Gln for 21 d. There was no improvement in feed efficiency due to the addition of Gln (Table 6).
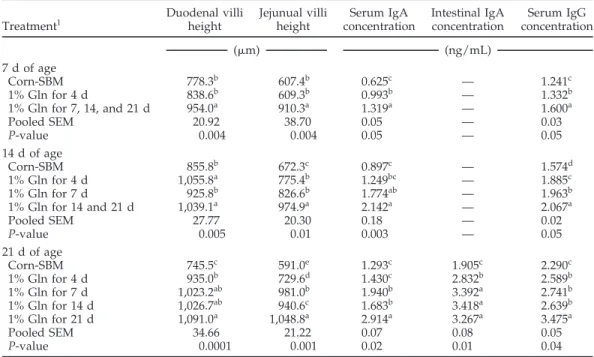
The birds fed diets supplemented with 1% Gln for 21 d had significantly heavier duodenum (1.37 vs. 1.06 g) and jejunum (2.05 vs. 1.83 g) relative weights as compared with the birds fed the control diet (P < 0.05; data not shown). There was no impact on the thymus, spleen, and bursa weights due to the addition of Gln. The supplemen- tation of Gln in the diet of broilers for any length of time yielded significantly longer villi in the duodenum and jejunum (Table 7) compared with the villi length of the control birds (P < 0.0001). The birds fed diets supple- mented with 1% Gln for 7 d or more had significantly higher IgA concentrations in the serum and bile (data not
Table 5.Effect of Gln supplementation fed for varying lengths of time on BW gain (g/chick) of broilers, experiment 2
Days of age
Treatment1 0 to 4 4 to 7 7 to 142 14 to 21 0 to 21
Corn-SBM 45 80 265 350b 739b
1% Gln for 4 d 47 74 275 346b 742b
1% Gln for 7 d 52 81 276 367ab 775ab
1% Gln for 14 d 51 79 275 386ab 791a
1% Gln for 21 d 51 78 275 401a 805a
Pooled SEM 2.8 2.0 6.3 12.8 15.3
P-value 0.29 0.16 0.71 0.03 0.02
a,bMeans within columns having the same superscript do not differ significantly (P< 0.05).
1Means represent 6 pens per treatment; 10 chicks per pen (60 chicks per treatment).
2Using single degree of freedom contrast the control vs. 1% Gln fed for any length of time is significantly different (P< 0.05) at 14 d of age.
shown) than the control birds (P< 0.05). Although the chicks fed diets supplemented with Gln had higher IgA concentrations, when Gln supplementation was discon- tinued, the concentrations began to decline to those of the control birds. The d-21 intestinal IgA concentrations were higher in the birds fed diets supplemented with 1%
Gln for any length of time compared with that of the control birds (P< 0.01). The birds fed diets supplemented with 1% Gln had significantly higher IgG concentrations in the serum compared with the control birds (P< 0.04).
DISCUSSION
Significant improvements in body weight gain were observed when 1% Gln was supplemented in the feed for 21 d as compared with the birds fed the corn-SBM diet (an average 11% increase). This finding was surprising because improvements in weight gain had not been re- ported in swine (Kitt et al., 2002) or other species due to Gln supplementation. Yi et al. (2001) did report an
Table 6.Effect of Gln supplementation fed for various lengths of time on the feed efficiency1 of broilers, experiment 2
Days of age
Treatment2 0 to 4 4 to 7 7 to 14 14 to 21 0 to 21
Corn-SBM 880 860 590b 600 630
1% Gln for 4 d 890 830 640ab 590 630
1% Gln for 7 d 890 870 700a 570 650
1% Gln for 14 d 910 870 670a 610 670
1% Gln for 21 d 890 860 630ab 620 650
Pooled SEM 20.0 20.0 20.0 20.0 20.0
P-value 0.68 0.21 0.01 0.66 0.32
a,bMeans within columns having the same superscript do not differ significantly (P< 0.05).
1Gain:feed = weight gain (g)/feed intake (kg).
2Means represent 6 pens per treatment; 10 chicks per pen (60 chicks per treatment).
improvement in BW gain in turkey poults fed diets sup- plemented with 1% Gln, but it was only noted for the first week of age. An improvement in feed efficiency was not observed; however, improvements in feed efficiency had been noted in swine (Kitt et al., 2002) and turkey poults (Yi et al., 2001) when they were fed a diet supple- mented with 1% Gln. The weight depression observed in chicks fed diets supplemented with 4% Gln may indicate a toxic effect when supplemented at 4% in the feed. How- ever, the reduction in weight gain of the birds fed the diet supplemented with 4% Gln may also be due to the large decrease in SBM in the 4% Gln diet (26.7% SBM) vs. the control diet (38.3% SBM). Although the diets were formulated to meet or exceed the NRC (1994) total amino acid recommendations, the control diet and the diet with only 1% Gln had much higher levels of all the indispens- able amino acids (except for the total sulfur amino acids) than the levels in the 4% Gln diet, which may be the main
Table 7.Effect of Gln supplementation fed for various lengths of time on villous height and humoral immune response of broilers, experiment 2
Duodenal villi Jejunual villi Serum IgA Intestinal IgA Serum IgG
Treatment1 height height concentration concentration concentration
(m) (ng/mL)
7 d of age
Corn-SBM 778.3b 607.4b 0.625c — 1.241c
1% Gln for 4 d 838.6b 609.3b 0.993b — 1.332b
1% Gln for 7, 14, and 21 d 954.0a 910.3a 1.319a — 1.600a
Pooled SEM 20.92 38.70 0.05 — 0.03
P-value 0.004 0.004 0.05 — 0.05
14 d of age
Corn-SBM 855.8b 672.3c 0.897c — 1.574d
1% Gln for 4 d 1,055.8a 775.4b 1.249bc — 1.885c
1% Gln for 7 d 925.8b 826.6b 1.774ab — 1.963b
1% Gln for 14 and 21 d 1,039.1a 974.9a 2.142a — 2.067a
Pooled SEM 27.77 20.30 0.18 — 0.02
P-value 0.005 0.01 0.003 — 0.05
21 d of age
Corn-SBM 745.5c 591.0e 1.293c 1.905c 2.290c
1% Gln for 4 d 935.0b 729.6d 1.430c 2.832b 2.589b
1% Gln for 7 d 1,023.2ab 981.0b 1.940b 3.392a 2.741b
1% Gln for 14 d 1,026.7ab 940.6c 1.683b 3.418a 2.639b
1% Gln for 21 d 1,091.0a 1,048.8a 2.914a 3.267a 3.475a
Pooled SEM 34.66 21.22 0.07 0.08 0.05
P-value 0.0001 0.001 0.02 0.01 0.04
a–eMeans within columns and age period having the same superscript do not differ significantly (P< 0.05).
1Means represent 6 pens per treatment; 10 chicks per pen (60 chicks per treatment).
reason for the lower growth performance of broilers fed the diet supplemented with 4% Gln. The weight depres- sion observed in chicks fed diets supplemented with 4%
Gln may also indicate the high levels of Gln have an effect and depress feed intake. Because there was no difference in feed efficiency but an effect on weight gain, it is clear that there was a decrease in feed intake.
The birds fed diets supplemented with Gln had signifi- cantly longer intestinal villi than the intestinal villi of birds fed the control corn-SBM diet. If the intestinal villi height can be increased early in the chick’s life, then the chick may be able to utilize nutrients more efficiently earlier in life and thus have improved growth perfor- mance. Lilja (1983) reported that avian species with a high growth rate capacity were characterized by a rapid early development of the digestive organs and liver. Birds with faster growth rates were reported by Nitsan et al. (1991) to secrete high levels of digestive enzymes, implying that
initial growth is only limited by the early development of the digestive organs. By reducing the time for develop- ment of the digestive organs, growth improvements could be achieved. Increased villi height has been proposed to increase performance by improving nutrient absorption (Coates et al., 1954; Izat et al., 1989). The increase in villi height that was observed might indicate that the birds fed diets supplemented with 1% Gln might have had greater nutrient absorption and utilization because in- creases in villi height result in more surface area for nutri- ent utilization. The increase in surface area might also explain the significantly heavier intestinal relative weights (P< 0.05) and improved weight gain that were observed due to Gln supplementation. Even through the birds fed diets supplemented with 4% Gln had increased villi height and actually had the longest villi in compari- son with the controls or the 1% Gln, they had the lowest growth performance. This may be due an imbalance in amino acids in the 4% Gln diet, or it could also suggest that in fact increased villi height does not necessarily lead to increased nutrient utilization and then increased per- formance.
Higher IgA concentrations in the serum, bile, and intes- tines observed in the birds fed diets supplemented with Gln support evidence reported by Burke et al. (1989) that rats fed diets supplemented with Gln maintained higher serum IgA levels than the other treatment groups that were not fed diets with Gln supplementation. The diges- tive mucosa is continuously exposed to dietary, bacterial, viral, and parasitic antigens (Strobel, 1986). Specific pro- tection against these antigens is achieved mainly by the secretion of IgA, which is synthesized in the gut-associ- ated lymphoid tissue (Piquer et al., 1991). The increase in IgA concentrations has been related to the increase in the number of lymphoid cells observed in the gallbladder (Leslie et al., 1976) of chickens and small intestine (Piquer, 1990) of turkeys. This suggests that the effect of Gln on the preservation of gut mass may include intestinal lymphoid tissue as well. The IgA functions primarily by preventing the attachment of bacterial to the mucosal cell (Burke et al., 1989). The barrier function of the gut epithelium depends on the presence of IgA, and until IgA is present, the hatchling is more susceptible to oral pathogens (Sell, 1991). The role of the gut as a barrier is to prevent the spread of intraluminal bacteria in systemic organs and tissues. This may indicate that the birds fed diets supple- mented with 1% Gln had better gut barrier function be- cause the birds had higher IgA concentrations in the intes- tines and thus may be more resistant to infection. How- ever, these statements must be further studied and evaluated.
Glutamine supplementation has been shown to in- crease the proportion of CD4+ (T-helper):CD8+ (T cyto- toxic/suppressor) cells (Kew et al., 1999; Yeh, 2001), which suggests that the supplementation of Gln stimu- lates the proliferation of CD4+ (T-helper) cells in prefer- ence to CD8+ cells. The IgG expression is T-helper cell dependent (Singh, 1996) and is indicative of T-helper cell response (Mathers and Cuff, 2004). Because IgG levels
did increase in birds fed diets supplemented with Gln, this may indicate that Gln is important for the synthesis of the IgG antibodies or perhaps required for thymus- derived (T)-cell helper function and response. The data we compared here indicated that alterations of total IgG production induced by dietary Gln in chicks without an antigenic challenge might reflect the potential of specific antibody IgG production when chicks are challenged with an antigen. However, further investigations are required.
Immune tissue development is the basis of immune functionality. The supplementation of Gln in diets fed to chicks significantly promoted the growth of the spleen and thymus (in experiment 1) but had no effect on the bursa weight. The increase in immune tissue weight re- sulting from Gln supplementation correlated with the functionality of thymus and spleen in terms of IgA and IgG production. The results of this experiment give in- sights into a potential dietary method to modulate chicken immune responses toward improving chicken performance under a given condition. For example, the inflammatory response is the first line of defense against novel pathogens, but cells and mediators of the inflam- matory responses have been implicated in the pathology of many poultry diseases, including coccidiosis (Trout and Lillehoj, 1993). Modification of antibody production and activity by dietary Gln supplementation may provide an avenue to strengthen the chicks immunity and protec- tion against various pathogens. However, long-term ef- fects of immunomodulation induced by Gln supplemen- tation on the resistance of chickens to commercially rele- vant infectious challenges and chick performance remain to be investigated.
REFERENCES
Adjei, A. A., Y. Matsumoto, T. Oku, Y. Hiroi, and S. Yamamoto.
1994. Dietary arginine and glutamine combination improves survival in septic mice. Nutr. Res. 14:1591–1599.
Andrews, F. J., and R. D. Griffiths. 2002. Glutamine: Essential for immune nutrition in the critically ill. Br. J. Nutr. S1:3–8.
Burke, D. J., J. C. Alverdy, E. Aoys, and G. S. Moss. 1989. Gluta- mine-supplemented total parenteral nutrition improves gut immune function. Arch. Surg. 124:1396–1399.
Calder, P. C. 1999. Glutamine and immune function (abstract).
Page 5 in Proc. Int. Congr. Amino Acids (Germany). Springer- Verlag, Vienna, Austria.
Coates, M. E., M. K. Davies, and S. K. Kon. 1954. The effect of antibiotics on the intestine of the chick. Br. J. Nutr. 9:110–119.
Cynober, L. A. 1999. Glutamine metabolism in stressed patients (abstract). Page 5 in Proc. Int. Congr. Amino Acids (Ger- many). Springer-Verlag, Vienna, Austria.
Duncan, D. B. 1955. Multiple range and multiple F tests. Biomet- rics 11:1–42.
Inoue, Y., J. P. Grant, and P. J. Snyder. 1993. Effect of glutamine- supplemented intravenous nutrition on survival after E. coli induced peritonitis. J. Parenter. Enteral. Nutr. 17:41–46.
Izat, A. L., R. A. Thomas, and M. H. Adams. 1989. Effects of dietary antibiotic treatment on yield of commercial broilers.
Poult. Sci. 68:651–655.
Kew, S., S. M. Wells, P. Yaqoob, F. A. Wallace, E. A. Miles, and P. C. Calder. 1999. Dietary glutamine enhances murine T- lymphocyte responsiveness. J. Nutr. 129:1521–1531.
Kitt, S. J., P. S. Miller, A. J. Lewis, and R. L. Fischer. 2002. Efffects of glutamine on growth performance and small intestine
villus height in weanling pigs. Pages 29–32 in Nebraska Swine Rep. Univ. Nebraska, Lincoln.
Labow, B. L. 2001. Mechanisms governing the expression of the enzymes of glutamine metabolism-glutaminase and gluta- mine synthetase. J. Nutr. 131:2467–2474.
Leslie, G. A., R. P. Stankus, and L. N. Martin. 1976. Secretory immunological system of the fowl. V. The gallbladder: An integral part of the secretory immunological system of the fowl. Int. Arch. Allergy Appl. Immunol. 51:175–185.
Lilja, C. 1983. A comparative study of postnatal growth and organ development in some species of birds. Growth 47:317–339.
Mathers, A. R., and C. F. Cuff. 2004. Role of interleukin 4 (IL- 4) and IL-10 in serum IgG antibody responses following mucosal or systemic reovirus infection. J. Virol. 78:3352–3360.
Medina, M. A. 2001. Glutamine and cancer. J. Nutr. 131:2539–
2542.
Naka, S. 1996. Alanyl-glutamine-supplemented total parenteral nutrition improves survival and protein metabolism in rat protracted bacterial peritonitis model. J. Parenter. Enteral Nutr. 20:417–423.
Newsholme, P. 2001. Why isL-glutamine metabolism important to cells of the immune system in health post-immune, sur- gery, or infection? J. Nutr. 131:2515–2522.
Newsholme, E. A., B. Crabtree, and M. S. Ardawi. 1985. Gluta- mine metabolism in lymphocytes: Its biochemical, physiolog- ical and clinical importance. Q. J. Exp. Physiol. 70:473–489.
Nitsan, Z. G., B. Avraham, Z. Zoref, and I. Nir. 1991. Organ growth and digestive enzymes levels to fifteen days of age in lines of chickens differing in body weight. Poult. Sci.
70:2040–2048.
NRC. 1994. Nutrient Requirements of Poultry. 9th rev. ed. Natl.
Acad. Press, Washington, DC.
Piquer, F. J. 1990. Post-hatching changes in the immunoglobulin A concentration in the small intestine of turkeys. MS thesis.
Iowa State Univ., Ames.
Piquer, F. J., J. L. Sell, H. A. Al-Batshan, E. G. Mallarino, M. F.
Soto-Salanova, and C. R. Angel. 1991. Post-hatching changes in the immunoglobulin A concentration in the jejunum and bile of turkeys. Poult. Sci. 70:2476–2483.
Rennie, M. J. 2001. Interaction between glutamine availability and metabolism of glycogen, tricarboxylic acid cycle interme- diates and glutathione. J. Nutr. 131:2488–2490.
Reynolds, J. V., J. M. Daly, and S. Zhang. 1988. Immunomodula- tory mechanism of arginine. Surg. 104:141–151.
SAS Institute. 2002. SAS STAT User’s Guide Release 9.13. SAS Inst. Inc., Cary, NC.
Sell, J. L. 1991. Development of the intestinal tract of young turkeys and responses to an enteric disorder. Pages 99–102 in Proc. Calif. Anim. Nutr. Conf., Fresno, CA. California Grain and Feed Assoc., Sacramento, CA.
Singh, R. R. 1996. Neonatal peptide exposure can prime T cells and upon subsequent immunization, induce their immune deviation: Implications for antibody vs. T cell-mediated auto- immunity. J. Exp. Med. 183:1613–1621.
Souba, W. W. 1993. Glutamine and cancer. Ann. Surg.
218:715–728.
Strobel, S. 1986. Allergenicity of feeds and gastrointestinal im- munoregulation in man and experimental animals. Hum.
Nutr. Appl. Nutr. 40A(Suppl. 1):45–54.
Trout, J. M., and H. S. Lillehoj. 1993. Evidence of a role of intestinal CD8+ lymphocytes and macrophages in transport of Eimeria acervulina sporozoites. J. Parisitol. 79:790–792.
Uni, Z., Y. Noy, and D. Sklan. 1995. Posthatch changes in mor- phology and function of the small intestines in heavy- and light-strain chicks. Poult. Sci. 74:1622–1629.
Yeh, S. L. 2001. Effects of glutamine-supplemented total parental nutrition on cytokine production and T cell proliferation. J.
Parenter. Enteral Nutr. 25:269–274.
Yi, G. F., G. L. Allee, J. W. Frank, J. D. Spencer, and K. J.
Touchette. 2001. Impact of glutamine, menhaden fish meal, and spray-dried plasma on the growth and intestinal mor- phology of broilers. Poult. Sci. 80(Suppl. 1):201. (Abstr.)