行政院國家科學委員會專題研究計畫 成果報告
從大豆除臭油中分離生物活性物質(第 3 年) 研究成果報告(完整版)
計 畫 類 別 : 個別型
計 畫 編 號 : NSC 95-2221-E-011-041-MY3
執 行 期 間 : 97 年 08 月 01 日至 98 年 07 月 31 日 執 行 單 位 : 國立臺灣科技大學化學工程系
計 畫 主 持 人 : 朱義旭
計畫參與人員: 博士班研究生-兼任助理人員:古拿旺 博士班研究生-兼任助理人員:申娜薇
報 告 附 件 : 出席國際會議研究心得報告及發表論文
公 開 資 訊 : 本計畫可公開查詢
中 華 民 國 98 年 09 月 11 日
行政院國家科學委員會補助專題研究計畫 ▓ 成 果 報 告
□期中進度報告 從大豆除臭油中分離生物活性物質
計畫類別:▓ 個別型計畫 □ 整合型計畫 計畫編號:NSC95-2221-E-011-041-MY3 執行期間:2006 年 8 月 1 日至 2009 年 7 月 31 日
計畫主持人:朱義旭 共同主持人:
計畫參與人員:古拿旺、申娜薇
成果報告類型(依經費核定清單規定繳交):□精簡報告 ▓完整報告
本成果報告包括以下應繳交之附件:
□赴國外出差或研習心得報告一份
□赴大陸地區出差或研習心得報告一份
▓出席國際學術會議心得報告及發表之論文各一份
□國際合作研究計畫國外研究報告書一份
處理方式:除產學合作研究計畫、提升產業技術及人才培育研究計畫、
列管計畫及下列情形者外,得立即公開查詢
□涉及專利或其他智慧財產權,▓一年□二年後可公開查詢
執行單位:台灣科技大學化工系
中 華 民 國 98 年 8 月 1 日
摘要
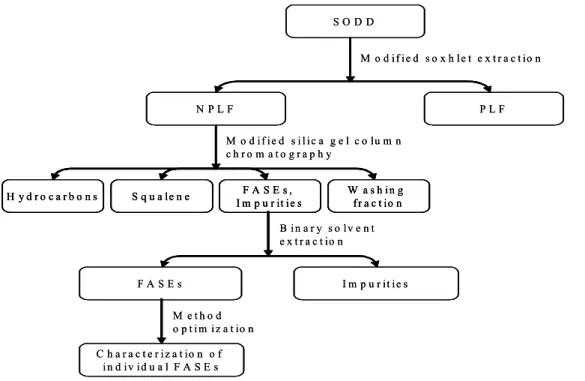
由於在大豆油精製過程之差異,台灣之大豆除臭油(SODD)通常含約 41-45%游離脂肪酸 (FFA)及 10-20%三酸甘油酯(TAG)。SODD 中尚含有相當含量之生物活性物質如生育酚、植 物固醇、固醇之脂肪酸酯(FASE)及鯊烯。本研究目的為發展一經濟可行之程序以從 SODD
中分離出鯊烯及 FASE。本研究利用修正索式萃取法及修正矽膠管柱層析法從 SODD 中分
離並純化鯊烯,再經以水-丙酮為溶劑萃取後可得高純度及高轉化率之 FASE。修正索式萃取
法可將 SODD 分離成兩部分:一為富含鯊烯及 FASE 之非極性脂質(NPLF),另一則為富含生
育酚、植物固醇、TAG 及 FFA 之極性脂質(PLF)。修正索式萃取法所得到之 NPLF 可從 SODD
中 去 除 87.99-95.68% 之 生 育 酚 、 97.26-99.76% 之 植 物 固 醇 、 76.13-82.11% 之 FFA 及 91.03-97.25%之甘油酯。利用修正索式萃取法及修正矽膠管柱層析法可從原來含 1.83%鯊烯
之SODD 中得到純度為 95.90%之鯊烯,總回收率可達 93.09%。本研究所設計之修正矽膠管
柱層析可經由調節閥當作索式萃取或管柱層析使用。修正矽膠管柱層析比傳統矽膠管柱層 析可節省大量溶劑並大幅縮短操作時間。本研究也使用水-丙酮溶劑系統從 NPLF 中得到純 度為86.74%之 FASE,總回收率達 85.32%。
關鍵詞:大豆除臭油,矽膠管柱層析法,修正索式萃取法
ABSTRACT
Depending on conditions in the refining process, soybean oil deodorizer distillate (SODD) in Taiwan typically contains about 41-45% free fatty acids (FFAs) and 10-20% triacylglycerols (TAGs). Bioactive compounds such as tocopherols, free phytosterols, fatty acid steryl esters (FASEs) and squalene also make up a significant portion of SODD. The objective of this research is to develop economically viable techniques for the separation and purification of squalene and fatty acid steryl esters from SODD. The quantitative analysis of the separation processes and the feasibility of this method to isolate the constituent compounds were investigated. A modified soxhlet extraction and modified silica gel column chromatography were employed to isolate and purify squalene from SODD. Then, FASEs in high purity and recovery could be further achieved by combination of water-acetone extraction. Here, a modified soxhlet extraction was employed for the efficient separation of FASEs and squalene into non polar lipid fraction (NPLF) and tocopherols, free phytosterols, TAGs, and FFAs into polar lipid fraction (PLF). The effectiveness of this separation was confirmed in subsequent experiments, carried out under the same conditions except with different SODD compositions. The modified soxhlet extraction could obtain NPLF which eliminating tocopherols, free phytosterols, FFAs, and acylglcerols from SODD by 87.99 – 95.68%, 97.26 – 99.76%, 76.13 – 82.11%, and 91.03 – 97.25%, respectively.
By combining a modified soxhlet extraction with a modified silica gel column chromatography, squalene with high purity (95.90%) and high recovery (93.09%) was obtained from SODD with an initial squalene content of 1.83%. The design of modified silica gel column chromatography can be used either as soxhlet extraction or column chromatography by setting the valve. Modified silica gel column chromatography possesses advantages over classical one because it significantly reduces the amount of solvent and time required to achieve the same degree of separation. In addition, Water-acetone extraction was employed sequentially in this study to obtain FASEs with purity of 86.74% and total recovery of 85.32 % from SODD.
Keywords: soybean oil deodorizer distillate, modified soxhlet extraction, modified silica gel column chromatography
TABLE OF CONTENTS
Page
CHINESE ABSTRACT I
ENGLISH ABSTRACT II
TABLE OF CONTENTS III
LIST OF FIGURES V
LIST OF TABLES VI
CHAPTER
1 INTRODUCTION 1
1.1 Soybean Oil Deodorization Distillate 1
1.2 Free Fatty Acids 3
1.3 Squalene 4
1.4 Tocopherols / Tocotrienols 5
1.5 Phytosterols / Phytostanols 6
2 LITERATURE REVIEWS 9
2.1 Isolation of Bioactive Compounds from Deodorizer Distillate
9
2.1.1 Chemical Process 9
2.1.2 Enzymatic Process 10
2.1.3 Physical Process 12
2.2 Bioactive Compounds Analysis in Deodorizer Distillate 13
2.2.1 Indirect Analysis 14
2.2.2 Direct Analysis 14
2.3 Research Aims 15
3 MATERIALS AND METHODS 17
3.1 Materials 17
3.2 Equipments 17
3.3 Analysis 18
3.3.1 Determination of FFAs contents in SODD 18
3.3.2 Analysis by TLC and HT-GC 18
3.3.3 Analysis by GCMS 19
3.3.4 Analysis of FTIR 19
3.4 Methodology 20
3.4.1 Modified Soxhlet Extraction 20 3.4.2 Silica Gel Column Chromatography 21 3.4.2.1 Classical Silica Gel Column Chromatography 21 3.4.2.2 Modified Silica Gel Column Chromatography 21
3.4.3 Binary Solvent Extraction 22
3.4.3.1 Repeated Solvent Extraction 22 3.4.3.2 Combination of Solvent Extraction 23
4 RESULTS AND DISCUSSION 25
4.1 Modified Soxhlet Extraction 25
4.1.1 Effects of Silica Gel to SODD Mass Ratio 26 4.1.2 Effects of Extraction Temperature 27
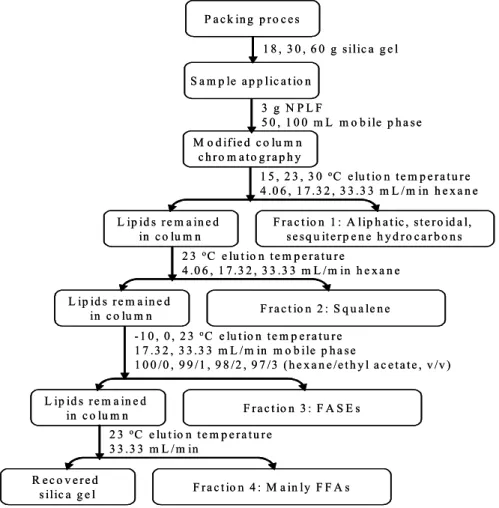
4.2 Silica Gel Column Chromatography 31 4.2.1 Classical Silica Gel Column Chromatography 31 4.2.2 Modified Silica Gel Column Chromatography 31 4.2.2.1 Isolation of Hydrocarbons 32 4.2.2.2 Isolation and Purification of Squalene 34
4.2.2.3 Isolation of FASEs 35
4.3 Binary Solvent Extraction 37
4.3.1 Repeated Solvent Extraction 38 4.3.2 Combination of Solvent Extraction 40
5 CONCLUSIONS 43
REFERENCES 44
LIST OF FIGURES
FIGURE NAME Page
1-1 Structures of squalane and squalene 4
1-2 Structures and methyl positions of the 8 natural E vitamins. 6 1-3 Nomenclature of phytosterols. (example: β-sitosterols =
stigmast-5-en-3b-ol = 24R- ethylcholest-5-en-3β-ol)
7
1-4 Classification of phytosterols based on the number of methyl groups on 4-carbon and based on common forms
7
3-1 A schematic drawing of the MSE 18
3-2 A schematic drawing of the MSGCC. Insert: Enlargement of the chamber and three-way valve. (A) Vapor region, (B) Mobile phase region, and (C) Packing region.
19
3-3 Flow chart for the separation of FASEs and squalene from SOD 20 3-4 Flow chart showing the extraction of NPLF from SODD 22 3-5 Flow chart showing the separation of squalene and FASEs from
NPLF of SODD.
23
3-6 Flow chart showing the sequence water-acetone extraction on the 3rd fraction of NPLF
24
4-1 Typical results of the HT-GC and the TLC analyses. (A) The GC chromatograms of SODD sample I, NPLF and PLF. Insert:
Enlargement of the region of tocopherols and free phytosterols.
(B) The analysis of the NPLF and the PLF by TLC developed in pure hexane.
25
4-2 HTGC analysis of fractions obtained from MSGCC of NPLF of SODD sample III. The GC chromatograms of the NPLF (A), the 1st fraction (B), the 2nd fraction (C), the 3rd fraction (D), and the washing fraction (E) eluted from the MSGCC.
32
4-3 TLC analyses of fractions obtained from MSGCC of NPLF developed in pure n-hexane. (A) NPLF of SODD sample III, (B) the 1st fraction, (C) the 2nd fraction, (D) the 3rd fraction, (E) the washing fraction.
33
4-4 HT-GC chromatograms. (A) SODD sample IV, (B) NPLF, (C) the 3rd fraction from MSGCC, (D) the precipitation fraction from solvent extraction (water:acetone = 20:80, v/v), and (E) the supernatant fraction from solvent extraction
42
LIST OF TABLES
TABLE NAME Page 1-1 Typical compositions of vegetable oil deodorizer distillates 2
1-2 Overview of melting points of some fatty acids 4 2-1 Taiwan vegetable oil Imports and Production 15
3-1 Compositionsof SODD used in this study 17
4-1 The effects of silica gel to SODD mass ratio on the composition of NPLF at an extraction temperature of 65 oC
26
4-2 The effects of extraction temperature on the composition of NPLF at silica gel to SODD mass ratio of 3
28
4-3 A comparison of fractionation of SODD obtained by MSE and molecular distillation
29
4-4 Compositions of NPLF obtained from SODD sample I, II, III, and IV.
30
4-5 Characteristics of silica gel used in MSE 30
4-6 Effect of (silica gel)/NPLF on the impurity (squalene) content and recovery in the 1st fraction
34
4-7 Effect of (silica gel)/NPLF on the purity and recovery of squalene in the 2nd fraction
34
4-8 Effect of mobile phase flow rate on the purity and recovery of squalene in the 2nd fraction
35
4-9 Effect of mobile phase polarity on composition and recovery of 3rd fraction
36
4-10 Effect of flow rate on the purity and recovery of FASEs in the 3th fraction
37
4-11 Comparison between modified and classical column cromagraphy on the separation and purification of squalene and FASEs from NPLF of SODD sample III
38
4-12 The fractionation of NPLF obtained by MSGCC with an NPLF of low squalene and FASEs contents
39
4-13 The fractionation of NPLF of SODD sample IV obtained by MSGCC
40
4-14 Comparison of results obtained by solvent extraction and distillation operated on the 3rd fraction of NPLF (wt.%)
41
4-15 Enrichment of FASEs employing a sequence of separation steps using SODD sample C as the starting material
41
CHAPTER 1
INTRODUCTION
1.1 SOYBEAN OIL DEODORIZATION DISTILLATE
Practically all commercial vegetable oils produced worldwide are refined oils (pure triacylglycerols, TAGs). Crude vegetable oils contain impurities or by-product such as gums (phospatides), waxes, metals, free fatty acids (FFAs), colored particles and substances, diacylglyecrols (DAGs), monoacylglycerols (MAGs), odoriferous components (aldehydes and ketones), pesticides, herbicides, and polycyclic aromatic hydrocarbons (PAHs). They can negatively affect the taste and smell of oils.
Refining can be done by chemical refining or physical refining (Figure 1-1). The physical process is technically more costly. However, it requires only three processing steps, whereas the conventional process may need up to four. The main advantage of physical refining is its environmental friendliness. Effluent is drastically reduced, FFAs are recovered as such without soap splitting and refining loss is lower. The disadvantage is that for some oils more careful degumming is required (Bockisch, 1998; Poku, 2002; Teoh, 2002).
In physical refining, FFAs are vaporized during the deodorization process. Consequently the distillate contains mainly FFAs (>70%) with only small amounts of unsaponiables (5-10 %) and is usually sold as a source of technical fatty acids (Verleyen et al., 2001). In chemical refining, FFAs are neutralized by caustic solution and washed out of the oil before deodorization. The deodorizer distillate from chemical refining thus has a lower FFAs content (30-50%) and a higher level of unsaponifiables ranging between 25 and 33% (De Greyt et al., 2000).
Hydrocarbons are the least polar compounds of the unsaponifiable matter of vegetable oils.
The presence of these compounds was detected in the 1940s, when Jasperson and Jones encountered in the deodorization distillates from several vegetable oils, large amounts of terpenic hydrocarbons accompanied by smaller amounts of n-alkanes. Crude vegetable oils contain elements of the n-alkane series from C10 to C35, the odd numbered elements being the most abundant. Other hydrocarbons were also detected in low concentration. Among them are n-alkenes, sesquiterpenenic (farnesene), terpenic (kaurene), carotenes, low-molecular-mass aromatics (from benzene to tetramethylbenzene, including styrene), and polycyclic aromatic hydrocarbon (mainly those of low molecular mass).
In refined vegetable oil, new hydrocarbons are formed as consequences of reactions occurring during the refining process such as bleaching with acidic earth and deodorization at high temperature. These hydrocarbons include steroidal hydrocarbons from the dehydration of phytosterols, terpenic hydrocarbons from terpenic alcohols, and other compounds deriving from squalene isomerization (Moreda et al., 2001). Each Δ5-phytosterols give rise to three sterene isomers (steroidal skeleton) with the two double bonds at 3,5 -; 2,4 – and 2,5 – positions, the first one being the most abundant. Among these steroidal hydrocarbons, the stigmadienes are the most abundant in all refined vegetables oil since they derive from β-sitosterol by dehydration. In another study, Goh et al. (1985) reported that squalene, sesquiterpene, diterpene, paraffinic
hydrocarbon and other volatile degradation or oxidized products are removed and concentrated in palm fatty acid distillate (PFAD) during the refining of palm oil.
The concerted effort to recover deodorizer distillate began in earnest in the late 1960s for two reasons: (a) Increasing demand for phytosterols and tocopherols (natural vitamin E) by the pharmaceutical companies. (b) The refiner’s interest in cleaning up their messy soap stock skimming operations. Unfortunately, the value of distillate seems to follow the general economy closely and, as a result, has fluctuated quite violently. When the tocopherols and phytosterols market is strong, disposal of distillate is profitable. However, at other times it must be sold as a soap stock or can be a disposal problem. Some countries permit the use of distillates as an animal feed extender (Borher, 2002), but in others it is prohibited because of the distillation and concentration of pesticides in the deodorization process (Zehnder, 1995).
Soybean oil is the most consumed oil in the world, representing 30% of the consumption in the worldwide market in 2006. Projections for the major vegetable oils see an increase in 2006 to global total of 114.96 million metric tons (MMT) compared with the estimate of 110.49 MMT for the year 2005. Palm oil at a forecasted 34.68 MMT is expected to continue to lead soybean oil at 34.22 MMT (Inform, 2006). Soybean oil deodorizer distillate (SODD) is obtained as byproduct of a deodorization step during the production of soybean oil. SODD represents about 3% of refined oil or 0.6 % of soybean seed as feed in the refining process (Bohra, 2002).
During the deodorization of soybean oil, substances that usually give a bad taste and/or foul odor (aldehydes and ketones) are mostly removed via steam-stripping distillation. Also removed in this steam-stripping distillation are tocopherols, phytosterols, FFAs, TAGs, DAGs, MAGs, FASEs and hydrocarbons (Helme, 1984; Erickson and Wiedermann, 1989; Aparicio et al., 2000;
Verleyen et al., 2001). The removal of tocopherols and phytosterols during deodorization step (operation at 260oC) is about 40 to 50%.
Typical compositions of vegetable oil deodorizer distillates are shown in Table 1-1. SODD, CODD, SuODD, RODD, OODD and PFAD stand for soybean, corn, sunflower, rapeseed, olive oil deodorizer distillate and palm fatty acid distillate, respectively.
Table 1-1 Typical compositions of vegetable oil deodorizer distillates
Compounds SODD a SODD b SODD c SODD d SODD e SODD f
Squalene 1.28 3.10 2.62 4.00
Tocopherols 16.48 49.70 10.40 8.97 12.74 10.00
Free phytosterols 17.06 18.80 10.30 11.39 2.00
FASEs 2.59 12.80 2.00
MAGs 1.24 4.10
DAGs 2.70 3.50
TAGs 5.13 9.50
FFAs 33.00 2.70 30.10 57.80 23.62 82.00
Others m 20.52 19.30
Continued Table 1-1
Compounds SODD g SODD h OODD i SuODD j PFAD k
Squalene 1.90 28.00
Tocopherols 10.20 15.00 6.00 1.00 l
Free phytosterols 11.20 20.00 4.60 5.10 0.30
FASEs 0.50
MAGs 10.50
DAGs 13.20
TAGs 20.00 16.30 28.50
FFAs 45.00 34.20 45.00 40.00
Others m
a Verleyen et al., 2001 g Fizet, 1996
b Gast et al., 2005 h Copeland and Belcher, 2004
c Hirota et al., 2003 i Bondioli et al., 1993
d Martins et al., 2006 j Moreira and Baltanas, 2004
e Ramamurthi and McCurdy, 1993 k Jacobs, 2005
f Mendes et al., 2002, 2005 l Tocotrienols
m Hydrocarbons, aldehydes, ketones, pesticides, herbicides, breakdown product of tocopherols and phytosterols
1.2 FREE FATTY ACIDS (FFAs)
FFAs in SODD have low quality due to harsh conditions in the processing steps which resulted in reactions such as oxidation, cis-trans conversion etc (Mendes et al., 2002). These fatty acids have limited use such as for the production of biodiesel and as fluidizing agents for lecithin.
Trans fatty acids (TFAs) are mono- or polyunsaturated fatty acids with one or more double bonds in the trans configuration. In crude vegetable oils, double bonds are nearly always in the cis configuration with the hydrogen atoms sterically located on the same side of the double bond . Because of the low activation energy (125 kJ/mol), TFAs are relatively easily formed at elevated temperatures (e.g. during deodorization or hydrogenation).
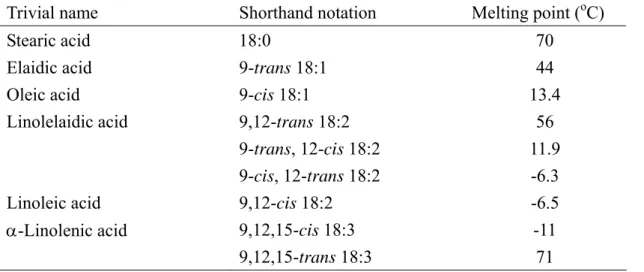
TFAs have structures comparable to saturated fatty acids so they have higher melting point than the corresponding cis isomer, as shown in Table 1-2 (De Greyt et al., 1999). Interest in TFAs is due to the epidemiological evidence that linked TFAs intake with higher plasma total and low-density-lipoprotein-cholesterol concentrations and increased mortality from coronary heart disease (Mensink and Katan, 1990; Willet et al., 1993; Judd et al., 1994). Although there is controversy over the possible health effect of TFAs, most health organizations advice not to increase current average intakes.
In general, three different dietary sources of TFAs can be distinguished: ruminant fats, partially hydrogenated fats, and refined oils. Ruminant fats are the only natural source of TFAs with vaccenic acid (11-trans 18:1) as the predominant one. On the other hand, partially hydrogenated fats are known as major dietary source of TFAs. Besides saturation of double bonds, partial hydrogenation is also characterized by the positional and geometrical isomerization of double bonds, resulting in large amounts of trans 18:1 isomers. Refined oils contain only a limited amount of TFAs, mainly trans 18:2 and 18:3 isomers formed during deodorization.
Table 1-2 Overviews of melting points of some fatty acids
Trivial name Shorthand notation Melting point (oC)
Stearic acid 18:0 70
Elaidic acid 9-trans 18:1 44
Oleic acid 9-cis 18:1 13.4
Linolelaidic acid 9,12-trans 18:2 56
9-trans, 12-cis 18:2 11.9
9-cis, 12-trans 18:2 -6.3
Linoleic acid 9,12-cis 18:2 -6.5
α-Linolenic acid 9,12,15-cis 18:3 -11
9,12,15-trans 18:3 71
TFAs formation during deodorization was expressed by “Degree of Isomerization (DI)”. DI of a given fatty acids expresses the percentage present in trans configuration compared to the initial content (cis + trans) in crude oil. Depending on process conditions, 3 to 24 % of α-linolenic acid will be converted into trans configuration. The DI of linoleic acid is clearly lower. Even under the most extreme process conditions, at most 2% of linoleic acid will be isomerized. In general, the rate of cis/trans-isomerization of α-linolenic acid (C18:3) is respectively about 10 and 100 times higher than that of linoleic (C18:2) and oleic acid (C18:1). In practice, this means that TFAs formation during refining will be inevitably higher in oils rich in α-linolenic acid such as soybean and rapeseed oil (De Greyt et al., 1999).
1.3 SQUALENE / SQUALANE
Small quantities of squalene are found in sebaceous secretions, so it is a natural product.
Squalene (Figure 1-1) has applications in cosmetic preparation and in the biosynthesis of cholesterol (Moreda et al., 2001). Natural squalene can be found in many tissues, notably the livers of sharks and other fishes. Due to environmental concerns including the protection of marine life, the extraction of squalene from SODD is of great interest. Squalane is prepared by the hydrogenation of squalene and is fully saturated and is not subject to autoxidation. Both squalene and squalene can be metabolized and have a good record in toxicology studies (Christian, 1982). Yarkoni and Rapp (1980) had used squalene as well as hexadecane and other lipids, with mycobacterium cell wall, to augment non-specific immunity against tumors.
1 2
3 4
5 6
7 8
9 10
11 12
13 14
15 16
17 18
19 20
21 22
23 24
25 26 27
28 29 30
1 2
3 4
5 6
7 8
9 10
11 12
13 14
15 16
17 18
19 20
21 22
23 24
25 26 27
28 29 30
Squalane
Squalene
Figure 1-1 Structures of squalane and squalene
Squalene and squalane were found to be equally effective and squalene was preferred because of its greater stability. Squalane is a free-flowing oil and has been used in pharmaceuticals and as skin lubricant, as ingredient in suppositories, and as a vehicle for lipophilic drugs. Squalene in Syntex Adjuvant Formulation conforms to National Formulary guidelines and is used at a final concentration of 5% (w/v). Microfluidized squalene or squalene emulsions are efficient adjuvant, eliciting both humoral and cellular immune responses. Squalane or squalene emulsions have been administered in human cancer vaccines, with mild side effects and evidence of efficacy, in terms of both immune response and antitumor activity (Allison, 1999).
1.4 TOCOPHEROLS / TOCOTRIENOLS
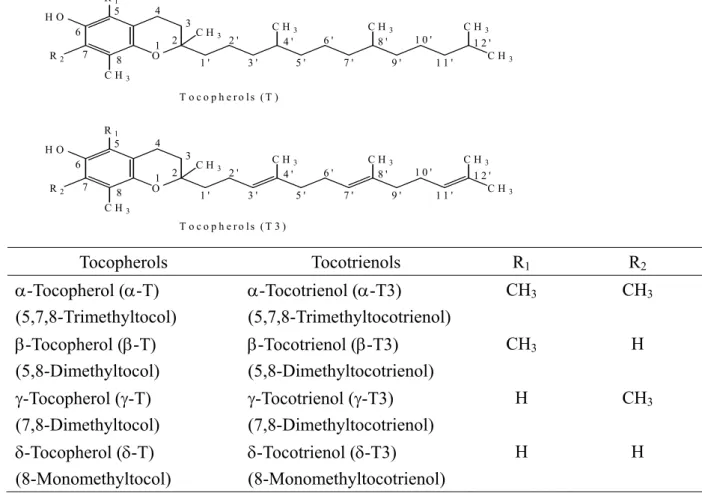
Tocopherols and tocotrienols (known as tocochromanols) are organic compounds that are found in plant material. These compounds are important because they retard the oxidation and spoilage of plant matter. Tocochromanols are also components of vitamin E and posses similar general structural features. Tocochromanols generally have an aromatic chromanol head and 16-carbon hydrocarbon tail. The number and position of methyl substituents in the chromanol nucleus give rise to the α−,β−,γ− and δ−homologues. Saturation of the hydrocarbon chain differentiates tocopherols from tocotrienols with an unsaturated chain as shown in Figure 1-2 (IUPAC-IUB recommendations of 1982).
Each form has its own biological activity, which is the measure of potency or functional use in the body (Traber and Packer, 1995). Such activities include platelet aggregation and antioxidant functions (Niki et al., 1989; Fukuzawa et al., 1989). Alpha tocopherol is the most active form of vitamin E in humans (d-α-tocopherol, d-β-tocopherol, d-γ-tocopherol, d-δ-tocopherol, and d-α-tocotrienol showing, respectively, 1.49, 0.75, 0.30, 0.15 and 0.45 units of activity (Machlin, 1984)). It is also a powerful biological antioxidant (Traber, 1999; Farrell and Roberts, 1994). Non-α-tocopherol can be converted into α-tocopherol by well-known techniques, such as methylation (Gast et al., 2005). Vitamin E in supplements is usually sold as alpha-tocopheryl acetate, a form that protects its ability to function as an antioxidant. The synthetic form is labeled “d,l” while the natural form is labeled “d”. The synthetic form is only half as active as the natural form (Burton et al., 1998; U.S. Department of Agriculture, 2004). A mixture of α-, γ- and δ- isomers containing 60 wt% tocopherols is widely utilized as an additive to various kinds of foods including fats and oils.
Antioxidants such as vitamin E act to protect cells against the effect of free radicals, which are potentially damaging by-products of energy metabolism. Vitamin E has also been shown to play a role in immune function, in DNA repair, and other metabolic processes (Traber, 1999;
Farrell and Roberts, 1994).
High serum cholesterol levels are implicated in numerous diseases and disorders. A decrease in low density lipoproteins (LDLs) and/or an increase in the ratio of high density lipoproteins (HDLs) to LDLs will lower the risk of heart disease and retard the progression of the abovementioned diseases and disorders. Tocopherols and tocotrienols are two classes of compounds that are known to have a beneficial effect on the level of cholesterol in the
bloodstream (Jacobs, 2005).
1 2 3 4 5 6
7 8 O
H O
R1
R2
C H3 1 '
2 ' 3 '
4 ' 5 '
6 ' 7 '
8 ' 9 '
1 0 ' 1 1 '
1 2 ' C H3
C H3 C H3 C H3 C H3
1 2 3 5 4
6
7 8 O
H O
R1
R2
C H3
1 ' 2 '
3 ' 4 '
5 ' 6 '
7 ' 8 '
9 ' 1 0 '
1 1 ' 1 2 '
C H3
C H3 C H3 C H3 C H3
T o c o p h e r o l s ( T )
T o c o p h e r o l s ( T 3 )
Tocopherols Tocotrienols R1 R2
α-Tocopherol (α-T) (5,7,8-Trimethyltocol)
α-Tocotrienol (α-T3) (5,7,8-Trimethyltocotrienol)
CH3 CH3
β-Tocopherol (β-T) (5,8-Dimethyltocol)
β-Tocotrienol (β-T3) (5,8-Dimethyltocotrienol)
CH3 H
γ-Tocopherol (γ-T) (7,8-Dimethyltocol)
γ-Tocotrienol (γ-T3) (7,8-Dimethyltocotrienol)
H CH3
δ-Tocopherol (δ-T) (8-Monomethyltocol)
δ-Tocotrienol (δ-T3) (8-Monomethyltocotrienol)
H H
Figure 1-2 Structures and methyl positions of the eight natural E vitamins. The chemical abstract name for tocopherol is 2-methyl-2-(4’,8’,12’-trimethyltridecyl)-6-chromanol and for tocotrienol is 2-methyl-2-(4’,8’,12’-trimethyltrideca-3’,7’,11’-trienyl)-6-chromanol.
In many foods, α- and γ- tocopherols account for most of the vitamin E activity. The primary form of vitamin E in dietary and animal feed supplements is α-tocopherol. However, γ-tocopherol is the major form present in SODD. It has been discovered that the individual members in tocopherols exhibit different biological properties from one another and play different roles in cells. Numerous studies recently have promoted the benefits of γ-tocopherols for human diet.
Gamma tocopherol may be involved in the pathogenesis of prostate cancer (Helzlsouer et al., 2000), and is a superior trapping agent for electrophilic species, like nitrogen oxides or NO (Christen et al., 1997) or peroxynitrite (Bartok et al., 2005).
1.5 PHYTOSTEROLS / PHYTOSTANOLS
Phytosterols (plant sterols) are members of the “triterpene” family which includes more than 100 different phytosterols and more than 4000 other types of triterpene. Phytostanols are a fully-saturated subgroup of phytosterols. Phytostanols occur in trace levels in many plant species and they occur in high levels in tissues of only a few cereal species. Common dietary sources of phytostanols are corn, wheat, rye, and rice. Phytosterols can be converted to phytostanols by
chemical hydrogenation.
Phytosterols nomenclature is confusing. The two main nomenclature (Figure 1-3) currently utilized follow the IUPAC-IUB recommendations of 1976 and 1989. Phytosterols by their common names and the IUPAC-IUB recommendations of 1976 will be used in this study. A convenient way to describe and catalog phytosterols is to divide them based on the number of methyl groups on carbon-4 and based on common forms (Figure 1-4).
1 2
3
4 5
6 7
9 8 1 0
1 1 1 2
1 3 1 4 15
1 6 1 7
H O
1 8
1 9
2 0
2 1 2 2
2 3 2 4 2 5 2 6 2 7
2 8 2 9
IU P A C -IU B 1 9 7 6
3 1 3 0
1 2
3
4 5
6 7 9 8 1 0
1 1 1 2
1 3 1 4 1 5
1 6 1 7
H O
1 8
1 9
2 0
2 1 2 2
2 3 2 4 2 5 2 6 2 7 2 41 2 42 IU P A C -IU B 1 9 8 9
2 9 2 8
Figure 1-3 Nomenclature of phytosterols. (Example: β-sitosterols = stigmast-5-en-3b-ol = 24R- ethylcholest-5-en-3β-ol)
Phytosterols
Based on the number of methyl groups on carbon-4
Conjugated Phytosterols Free Phytosterols Based on common forms
Fatty acid steryl esters (FASEs)
Hydroxycinnamate steryl esters (HSEs)
Steryl glycosides (SGs)
Acylated steryl glycoside (ASGs) 4,4-Dimethyl phytosterols
4-Monomethyl phytosterols
4-Desmethyl phytosterols Phytosterols
Based on the number of methyl groups on carbon-4
Conjugated Phytosterols Free Phytosterols Based on common forms
Fatty acid steryl esters (FASEs)
Hydroxycinnamate steryl esters (HSEs)
Steryl glycosides (SGs)
Acylated steryl glycoside (ASGs) 4,4-Dimethyl phytosterols
4-Monomethyl phytosterols
4-Desmethyl phytosterols
Figure 1-4 Classification of phytosterols based on the number of methyl groups on 4-carbon and based on common forms.
The composition of free phytosterols in SODD is frequently a mixture of sitosterol, campesterol, stigmasterol, and other minor constituent of brassicasterol. Also, FASEs have been
usually characterized following the ester bond cleavage of the aliphatic and steryl ester by saponification. This approach gives useful information about their composition but prevents the recognition of the individual steryl ester and thus valuable information is lost.
Phytosterols and phytostanols are effective in lowering low density lipoprotein serum cholesterol (LDL-C), as long as they are formulated and delivered in a “bioavailable” physical state. The effect of plant sterols is highly dependent on their physical state and that hypercholesterolemic subjects consuming 1.5 or 3.0 g/day of free plant sterols in a
“microcrystalline” (unesterified) form experienced a 7.5-11.6% reduction in LDL-C levels.
Stigmasterol is also used in manufacturing progesterone and corticoids. Sitosterol is used to produce estrogens, contraceptives, diuretics, and male hormones (Moreau et al., 2002).
The mechanism of action behind the cholesterol lowering effects of phytosterols and phytostanols is not fully understood (Wester, 2000; Ostlung, 2002), but several theories have been proposed (Piironen, 2000; Pollak and Kritchevsky, 1981). One theory suggests that cholesterol in intestine, already marginally soluble, is precipitated into a non-absorbable state in the presence of added phytosterols and phytostanols. A second theory is based upon the fact that cholesterol must enter bile-salt and phospholipid-containing “mixed micelles” in order to pass through intestinal cells and to be absorbed into the bloodstream. Cholesterol is only marginally soluble in these micelles and it is displaced by phytosterols (and phytostanols), preventing its absorption. Unlike cholesterols, phytosterols and to a greater extent, phytostanols are very poorly absorbed and the small amount that is absorbed is actively re-excreted in bile. This results in low serum levels of these sterol molecules.
FASEs are more effective at lowering serum cholesterol levels than free phytosterols. In addition, steryl esters are simpler and cheaper to synthesize than stanyl esters because no hydrogenation is required. This effective physiological activity has led to the development of several functional foods, such as salad oils and dressings with added sterols and margarine blended with steryl esters. In particular, because FASEs and oils (TAGs) completely dissolve each other, a lot of attention is being focused on the addition of FASEs in oil-related foods (Hirota et al., 2003).
CHAPTER 2
LITERATURE REVIEWS
2.1 ISOLATION OF BIOACTIVE COMPOUNDS FROM DEODORIZER DISTILLATE Since bioactive compounds are minor components in deodorizer distillate, enrichment is vital before they can be fractionated and separated into individual compound. Numerous methods have been proposed for treating deodorizer distillate to isolate one or more components. Tocopherols and free phytosterols are among the compounds of nutritional interest that are frequently purified from deodorizer distillate. These methods are protected by patent and technical details about them are scarce. Most of these methods are complicate and expensive. The isolation of squalene, FASEs, tocopherols and free phytosterols from deodorizer distillate in high yield and purity remains a challenge.
Deodorizer distillate typically contains high level of FFAs and TAGs and efficient removal of them is crucial for the enrichment of bioactive compounds. Difficulties associated with separating tocopherols and/or free phytosterols from deodorizer distillate are that they have similar boiling points because they have similar molecular weight. Tocopherols are prone to degradation if either high-temperature (Helme, 1984; Copeland and Belcher, 2004; Fizet, 1996) or alkaline condition is involved (Lietz and Henry, 1997).
During esterification of FFAs in deodorizer distillate, the addition of strong acid greatly accelerates the reaction, which also applies to the undesired esterification of the tocopherols (Fizet, 1996). Tocopherols levels decreased about 11%, 25%, 38% and 61% for ½ h processing during deodorization step at 240, 260, 280 and 300oC, respectively (Helme, 1984).
One difficulty associated with separating squalene and/or FASEs from deodorizer distillate are that their polarities are roughly the same and FASEs are prone to degradation if either high temperature (Verleyen et al., 2001), acid (Fizet, 1996) or alkaline condition is involved (Moreau et al., 2002). Furthermore, it has been reported that prolonged exposure to alkaline conditions resulted in significant losses of squalene (Moreda et al., 2001).
Tocopherols have been purified from deodorizer distillate in industrial scale by a combination of molecular distillation, ethanol fractionation, chemical alcoholysis, and ion-exchange chromatography. Extractive isolation of tocopherols by cold ethanol did not produce high yield and purity. The recoveries of tocopherols are less than 75 %, because a part of tocopherols coprecipitates with free phytosterols (Shimada et al., 2000).
There are several methods for removing FFAs and acylglycerols: hydrolysis, esterification, transesterification, distillation, crystallization, adsorption, and liquid-liquid extraction. The first three methods are either chemical or enzymatic process, and the next three are physical process.
2.1.1 Chemical Process
In many of these methods, a first essential step involves subjecting deodorizer distillate to an esterification or saponification reaction. For example, in an early work (Hickman, 1944);
deodorizer distillate was subjected to high vacuum, unobstructed path distillation. A distillate
fraction containing tocopherols was treated with lime soda to saponify FFAs, followed by the extraction of unsaponifiable fraction (tocopherols and free phytosterols) with ethyl ether, in which the saponification products are insoluble. The extract is then washed and concentrated by removing solvent in a rotary evaporator, and then the residual oil dissolved in five times methyl alcohol and cooled to crystallize free phytosterols. Free phytosterols were removed by filtration, leaving a tocopherols fraction. The fatty acid soaps formed can be acidulated and converted into FFAs. The total recovery of tocopherols exceeded 50% and 25% purity.
Brown and Smith (1964) isolated free phytosterols and tocopherols from deodorizer distillate by esterifying FFAs with a monohydric alcohol under strongly acidic condition for about 1-2 h.
Free phytosterols and tocopherols were then fractionally extracted from the esterification product mixture with a combination of polar and nonpolar solvents.
Fizet (1996) esterified free phytosterols from deodorizer distillate with FFAs at 180oC for 2.5 h or 250oC for 1.5 h and then the resulting mixture was distilled at 120-150oC and 0.1 mbar to obtain a residue containing tocopherols and FASEs and a distillate containing fatty acids. This residue was then distillated at 200-220oC and 0.1 mbar to obtain a residue containing FASEs and a distillate containing tocopherols. Free phytosterols were obtained by an acid-catalyzed trans-esterification of FASEs with methanol. Some tocopherols in the distillate were transformed into fatty acid tocopherol esters. Tocopherols were obtained by subjecting distillate from the 2nd distillation to an ion exchange chromatography, especially over a strongly basic resin.
Jacobs (2005) proposed a method for recovering tocotrienols from PFAD (contained 1%
tocopherols and 0.3% free phytosterols) by stripping FFAs (0.5-1.5 Torr, 180-240oC, and 0.5-1.5 min) to form a first stripped product. Short path distillation of the first stripped product gave a first distillate. Short path stripping fatty acids from the first distillate yielded a second stripped product. Saponifying the second stripped product resulted in a saponified product. A second short path distillation of the saponified product generated a second distillate. Solvent wintering (via filtration) of the second distillate gave a stripped filtrate. A third short path distillation of the stripped filtrate recovered the desired tocotrienols (50 % purity).
In another work (Martin et al., 2006), acylglycerols in SODD were converted into FFAs and glycerol through saponification at 65oC followed by acidulation and then the treated deodorized distillate was submitted to five stages of molecular distillation. Tocopherols (34.14 % purity) were enriched 5.8 times and MAGs (32.61 %) were the main impurity in the final product.
Bondioli et al. (1993) described a supercritical carbon dioxide process for recovering squalene from OODD. FFAs and fatty acid methyl and ethyl esters in OODD were converted into their corresponding triacylglycerides. The pre-treated OODD was then extracted with supercritical carbon dioxide to yield a highly enriched squalene fraction in high purity (~90%) and high yield (~90%).
2.1.2 Enzymatic Process
Ramamurthi and McCurdy (1993) concentrated free phytosterols and tocopherols from canola deodorizer distillate (contained 1% tocopherols and 1.58% free phytosterols) and SODD (contained 12.74% tocopherols and 11.39% free phytosterols). FFAs were converted into FAMEs
by employing non-specific SP 382 lipase. Free phytosterols and tocopherols were then concentrated in the residue fraction by distillation. The conversion of FAMEs was 96%, while a small amount of FFAs was left in the residue. Free phytosterols and tocopherols were enriched 1.5 times and 1.7 times, respectively. The total recoveries of free physterols and tocopherols were 17.56% and 21.48%, respectively.
Ghosh and Bhattacharyya (1996) proposed a method for the purification of tocopherols and free phytosterols from SuODD. Hydrolysis by Candida cylindracea lipase was applied to convert acylglycerols into fatty acids and glycerol. Mucor miehei lipase was then applied to the hydrolyzed deodorizer distillate to esterify fatty acids into their corresponding butyl ester. Butyl esters were then separated from the esterified deodorizer distillate via fractional distillation at 180-230oC for 45 min. A second fraction containing tocopherols and free phytosterols was obtained at 230-260oC for 15 min. The total recovery of tocopherols is about 70.2% with 30.1 wt% purity. The total recovery of free phytosterols is about 41.9% with 36.4 wt % purity.
Purification of tocopherols from SODD was carried out by Shimada et al. (2000). SODD was distilled using molecular distillation at 250oC and 0.02 mmHg and the resulting distillate was used as a starting material. Candida rugosa or Pseudomonas sp lipase was then applied to the mixture to efficiently esterify free phytosterols into their corresponding esters and concurrently MAGs were hydrolyzed at 35oC for 24 h. FASEs were recovered as residue from the reaction mixture via molecular distillation at 250oC and 0.2 mmHg. However, the last molecular distillation failed to separate FFAs and tocopherols. A second esterification of free phytosterols was applied at 35oC for 24 h, followed by another four-stage molecular distillation (160oC and 0.2 mmHg, 200oC and 0.2 mmHg, 230oC and 0.04 mmHg, and 255oC and 0.03 mmHg) yielded tocopherols with purity and recovery of about 65.3 wt% and 54.6%, respectively.
Hirota et al. (2003) isolated naturally occurring FASEs from SODD. SODD was distilled using molecular distillation at 250oC and 0.02 mmHg and the resulting residue was rich in DAGs and TAGs. Lipolysis was then conducted to selectively hydrolyze DAGs and TAGs at 35oC for 24 h, resulting in a mixture from which fatty acid steryl esters were readily purified using two-stage molecular distillation (180oC and 0.2 mmHg, and 250oC and 0.02 mmHg). The recovery and purity of FASEs were about 87.7% and 97.3 wt%, respectively
Watanabe et al. (2004) purified tocopherols and free phytosterols as their esters from SODD.
SODD was distilled using molecular distillation at 240oC and 0.02 mmHg and the resulting distillate rich in FFAs, free physterols and MAGs. A two-step in situ reaction catalyzed by Candida rugosa lipase was then applied to the distillate. In the first step, the esterification of free phytosterols with FFAs and the hydrolysis of acylglycerols were carried out at 30oC for 16 h. In the second step, the esterification of FFAs with methanol was conducted 30oC for 24 h. FASEs were not affected by the reaction. FAMEs were separated from tocopherols (and fatty acid steryl asters) via molecular distillation at 160oC and 0.2 mmHg. Tocopherols were separated from fatty acid steryl asters via molecular distillation at 240oC and 0.02 mmHg. The total recovery of free phytosterols as their corresponding esters is about 86.3% with 97.2 wt % purity. The resulting tocopherol-rich distillate was subjected to a two-step in situ reaction. In the first step, esterification of free phytosterols with FFAs and hydrolysis of acylglycerols were carried out at
30oC for 16 h. In the second step, esterification of FFAs with methanol was conducted 30oC for 6 h. Tocopherols were readily purified from the product of the last reaction by using a three-stage molecular distillation (160oC and 0.2 mmHg, 175oC and 0.2 mmHg, and 240oC and 0.02 mmHg).
The total recovery of tocopherols is about 84.26% with 76.4 wt% purity.
Nagao et al. (2005) obtained purified tocopherols and free phytosterols as their esters from SODD. SODD was distilled using molecular distillation at 240oC and 0.02 mmHg and the resulting tocopherols, free physterol, FFAs and MAGs–rich distillate was used as a starting material in a two-step in situ reaction catalyzed by Candida rugosa lipase. In the first step, esterification of free phytosterols with FFAs and hydrolysis of acylglycerols were carried out at 40oC at 20 mmHg for 24 h with dehydration. In the second step, esterification of FFAs with methanol was conducted 30oC for 8 h without dehydration. Oil layer was recovered by standing the reaction mixture and the oil layer separated was dehydrated at 80oC/5 mmHg for 30 min.
FAMEs were separated from tocopherols and FASEs via a two-stage molecular distillation (160oC and 0.2 mmHg, and 175oC and 0.2 mmHg). Tocopherols were then separated from FASEs via molecular distillation at 230oC and 0.02 mmHg. Tocopherols with a purity of 72.3 wt % were recovered in the distillate. The total recovery of the tocopherols is 87.6%. The FASE-rich residue was subjected to another molecular distillation at 240oC and 0.02 mmHg to recover FASEs with a purity of 97%. The total recovery of free phytosterols as their corresponding esters is about 97%.
2.1.3 Physical Process
Copeland and Belcher (2004) recovered fatty acids, tocopherols and free phytosterols from a deodorizer distillate by contacting deodorizer distillate into a scrubber. The scrubber had at least two condensing zones and operated at a pressure of less than about 10 mmHg. Deodorizer distillate (contained 12% tocopherols and 14% free phytosterols) was contacted into first condensing zone (166 - 232oC, 2.5 – 4 mmHg), thereby producing a first condensate stream with relatively high concentration of tocopherols (25%) and free phytosterols (30%). The remaining uncondensed vaporized distillate flowed to a second condensing zone, producing a second condensate stream with high concentration of FFAs.
By employing molecular distillation at 160oC, 7.5x10-4 mmHg and 10.4 g/min of feed flow rate, it was possible to obtain a product that contains 6.4% FFAs and 18.3% tocopherols from a feed that contains 57.8% of FFA and 8.97% tocopherols. A FFAs elimination of 96.16% and a tocopherols recovery of 81.23% were achieved (Martins et al. 2005).
Lee et al. (1991), Mendes et al. (2002, 2005), Nagesha et al. (2003), Chang et al. (2000) and Gast et al. (2005) recovered tocopherols from SODD using supercritical CO2 extraction.
Supercritical fluid extraction is an alternative technique to conventional processes (such as molecular distillation), especially for treating and processing temperature sensitive components.
Starting with a feed that contains 48.3% tocopherols, Gast et al. (2005) were able to obtain tocopherols with a purity of 94.4 % in the bottom by supercritical CO2 extraction at 23 MPa and 353 K, with a solvent-to-feed ration of 110 and a reflux ratio of 4.6. Squalene was completely recovered in the top phase.
Adsorption is competitive with distillation for bulk separation when relative volatility is less than about 1.25 (Ruthven 1984). In the vegetable oil refining industry, adsorption is involved in bleaching or decolourization of oils using activated earth or clays as adsorbent. Study of selective adsorption of undesirable components from unrefined oils could lead to increased efficiency of refining methods, a better understanding of bleaching procedures, as well as a better control of the physcochemical / functional properties of refined oils. Chu et al. (2004) separated vitamin E from palm fatty acid distillate using silica in batch adsorption. It was found that Redlich-Peterson and Langmuir isotherm models described the equilibrium data reasonably well and thermodynamic parameters of the adsorption further confirmed the exothermic nature of the adsorption process. The overall Gibbs free energy change during the adsorption process was negative for the experimental range of temperatures (35 – 50oC), corresponding to spontaneous adsorption. Two main adsorption mechanisms were involved during vitamin E adsorption, namely external mass transfer and intraparticle diffusion. It was noted that increasing the initial vitamin E concentration resulted in a decrease in kf (external mass transfer coefficient) values, but an increase in kid (rate constant of intraparticle diffusion).
None of the above methods for isolating one or more components from a deodorizer distillate has proved satisfactory. Methods employing an esterification or saponification step introduce processing complexity and require later processing steps that often involve the use of strong mineral acids in order to convert FASEs into free sterols and FFAs, or soaps into FFAs. Mineral acids can be dangerous in handling and can induce discoloration or other degradation of distillate components (Copeland and Belcher, 2004).
The main disadvantage of using enzyme is the cost due to its stability and reusability.
However, denaturization of bioactive compounds can be suppressed (Moreira and Baltanas, 2004).
Methods employing extractive steps are expensive and risk contamination by residual solvent.
Molecular distillation is by far the preferred method to isolate both thermo sensitive and high-molecular-weight compounds from deodorizer distillate. Molecular distillation requires operation under a high temperature and a high vacuum with expensive equipment.
The development of new separation techniques has gained increasing importance in chemical, food, and pharmaceutical industries, due to the imposed environmental regulations and the necessity of minimizing energy requirement.
2.2 BIOACTIVE COMPOUNDS ANALYSIS IN DEODORIZER DISTILLATE
Several techniques are available to provide a detailed analysis of one or more compounds in deodorizer distillates. Tocopherols and free phytosterols are among the lipids of nutritional interest that are frequently determined in deodorizer distillate. The two species are commonly analyzed by GC as well as by HPLC. A advantage of HPLC compared to GC is that derivatization and silylation is not necessary. However, the application of HPLC is limited by the lack of separation of tocopherols and free phytosterols under similar conditions. If data on both are required it is possible to determine them in the same analysis by GC under similar conditions (Slover et al., 1983). Therefore, GC is the most applied technique in the analysis of deodorizer distillate. A convenient way to describe and catalog analytical procedures that have been