Stress urinary incontinence following vaginal trauma involves remodeling of urethral connective tissue in female mice
Running Title: Stress urinary incontinence following vaginal trauma
Address reprint requests and correspondence to: Dr. Huey-Yi Chen, Department of Obstetrics and Gynecology, China Medical University Hospital, No. 2 Yu-Der Road, Taichung 404, Taiwan; Tel: -886-4-22052121, ext 2063; Fax: -886-4 -22033295; E-mail address: d888208@ms45.hinet.net
The authors do not have any conflicts of interest.
Condensation
Stress urinary incontinence following vaginal trauma involves over-expression of
LOX and decreased syntheses of the extracellular matrix components or
increased proteolysis in the urethra.
Huey-Yi Chen1, Yu-Ning Lin 2, Yung-Hsiang Chen 3, Wen-Chi Chen4
1Department of Obstetrics and Gynecology, Sex Hormone Research Center, China Medical University Hospital, Graduate Institute of Integrated Medicine, China Medical University, Taichung, Taiwan
2College of Life Science, National Chung Hsing University, Taichung, Taiwan
3Graduate Institute of Integrated Medicine, China Medical University, Taichung, Taiwan
4Department of Urology, China Medical University Hospital, Graduate Institute of Integrated Medicine, China Medical University, Taichung, Taiwan
Abstract
Objective: The molecular mechanisms underlying stress urinary incontinence (SUI)
are not clear. This study was conducted to evaluate molecular alterations in the
urethras of mice with experimentally induced SUI.
Study design: Eighteen virgin female mice were equally distributed into three groups
as follows: 2 groups undergoing vaginal distension (VD) for 1 h with 3 mm and 8 mm dilators each, and a non-instrumented control group. Changes in leak point pressure (LPP), morphology, lysyl oxidase (LOX) expression and the metabolism of urethral
connective tissue were assessed.
Results: The LPP was significantly decreased in the 3 mm and 8 mm VD groups as
compared with that in the control group. Collagen and elastin expression in the urethra was significantly decreased in the 8-mm VD group as compared with that in
the control group, while LOX expression was significantly enhanced.
Conclusions: SUI following vaginal trauma involves over-expression of LOX and
decreased syntheses of the extracellular matrix components or increased proteolysis in
the urethra.
Keywords: Stress urinary incontinence; Vaginal distension; Leak point pressure;
Collagen; Elastin; Lysyl oxidase
Introduction
Stress urinary incontinence (SUI) is a common disease that is defined as the involuntary leakage of urine under vesical stress conditions such as coughing and
sneezing [1]. The primary etiological factor of SUI is vaginal delivery [2], usually due
to a combined muscular, nerve and connective tissue injury [3].
Although progress has been made in the treatment of SUI [4], our understanding of the molecular mechanisms underlying the condition is poor. Because of the limited availability of human tissue for study, animal models are an important adjunct in improving our understanding of SUI [5]. Over the last decade, animal models of SUI have increasingly been used to understand the pathogenesis of the condition [6].
Vaginal distension (VD) [7] and pudendal nerve transection [8] have been used for inducing SUI in rats, as evidenced by lowered leak point pressures (LPP) on
urodynamic testing.
Birth trauma from vaginal delivery may include denervation damage, ischemia and mechanical injuries to the muscular, nerve and connective components of the lower urinary tract tissues [3, 9-12]. Recent studies have indicated that in SUI- affected periurethral connective tissues, the metabolism of collagen and elastin is altered [13-15]. Lysyl oxidase (LOX), an extracellular matrix (ECM) remodeling enzyme, is required for the oxidative deamination of lysine residues in collagen and elastin molecules required for fiber cross-linking. Thus, LOX controls both the structure and the tensile strength of ECM, and is subsequently involved in the preservation of tissue integrity [16]. Mutant mice lacking lysyl oxidase-like-1
(LOXL1) have been demonstrated to develop complex and severe pelvic floor
disorders [17-19].
The use of mice in various lines of translational research has made available transgenic and knockout technologies for conducting mechanistic studies of varied target diseases. The C57BL/6 mouse, for example, has been widely used for genetic manipulation in previous studies concerning urinary and pelvic disorders [17-19].
Based on the relevant literatures described above, we hypothesized that in a mouse model of SUI: (1) vaginal trauma might induce LOX expression and (2) the metabolism of urethral connective tissue might be altered by vaginal trauma. In order to test these hypotheses, we designed the present study with the following aims: (1) examining LPP in C57BL/6 mice after VD and (2) identification of LOX expression and characterization of the metabolism of urethral connective tissue induced by vaginal trauma in a mouse model of SUI using immunofluorescence staining and Western blot analysis. We expect this information to offer clues to the pathogenesis of SUI and to open additional avenues for novel research and potential therapies.
Further, this mouse model is anticipated to be useful for further mechanistic investigations and targeted therapeutic interventions by taking advantage of genetic manipulation.
Materials and methods
Experimental animals and design
Eighteen virgin female C57BL/6 strain mice, aged approximately 6–8 weeks, were randomized into three groups as follows: two groups undergoing VD for 1 h with 3 mm and 8 mm dilators (compatible with the diameter of mouse newborn head) each, and a non-instrumented control group. Two days after VD, the mice from these three groups underwent suprapubic bladder tubing (SPT) placement. Four days after VD, LPP was assessed in these mice under urethane (1 g/kg, i.p.) anesthesia. The non- instrumented control group did not undergo VD but did undergo SPT placement and LPP measurement. After measuring LPP, the animals were sacrificed, and the urethras were removed for histological examination, immunofluorescence staining and Western blot analysis. All experimental protocols were approved by the Institutional
Animal Care and Use Committee of China Medical University.
Vaginal distension
Mice in the 8 mm and 3 mm VD groups were anesthetized with 1.5% isoflurane.
To avoid rupturing the vagina, vaginal accommodation of Hegar’s dilators was achieved by sequentially inserting and removing different increasing sizes of Hegar’s dilators that were lubricated with Surgilube (Fougera, Melville, NY). Finally, for the 8 mm VD group, an 8 mm dilator was lubricated and inserted into the vagina [20]. After 1 h, the 8-mm dilator was removed, and the animal was allowed to awaken from the
anesthesia spontaneously. Mice in the 3 mm VD group were anesthetized and only were subjected to 3 mm dilator for 1 h [20]. The non-instrumented control group did
not undergo vaginal dilation.
Suprapubic tube implantation
The surgical procedure was carried out under 1.5% isoflurane anesthesia according to the methods described by Lin et al.[20] or Cannon and Damaser [21].
Two days after VD, an SPT (PE-10 tubing, Clay Adams, Parsippany, NJ) was implanted in the bladder. Key points of the operation were as follows: (1) a midline longitudinal abdominal incision was made, 0.5 cm above the urethral meatus; (2) a small incision was made in the bladder wall, and PE-10 tubing with a flared tip was implanted in the bladder dome; and (3) a purse-string suture with 8-0 silk was tightened around the catheter, which was tunneled subcutaneously to the neck, where
it exited the skin.
Leak point pressure measurement
Two days after implanting the bladder catheter (4 days after VD), the LPP was assessed in these mice under urethane anesthesia. The bladder catheter was connected to both a syringe pump and a pressure transducer. Pressure and force transducer signals were amplified and digitized for computer data collection at 10 samples per second (PowerLabs, AD Instruments, Bella Vista, Australia). The mice were placed supine at the level of zero pressure while bladders were filled with room temperature
saline at 1 mL/h through the bladder catheter. If a mouse voided, the bladder was emptied manually using Crede’s maneuver. The average bladder capacity of each mouse was determined after 3–5 voiding cycles. Subsequently, the LPP was measured in the following manner [20,21]. When half-bladder capacity was reached, gentle pressure with one finger was applied to the mouse’s abdomen. Pressure was gently increased until urine leaked, at which time the externally applied pressure was quickly removed. The peak bladder pressure was taken as the LPP. At least three LPPs were
obtained for each animal, and the mean LPP was calculated.
Histological examination and immunofluorescence staining
The mice in all three groups were sacrificed immediately after completing the measurements of LPP, and the urethras were harvested. The middle one-third of the urethras was histologically examined along with immunofluorescence staining examination. Half of each urethral tissue sample was fixed in 10% buffered formalin (pH 7.4) and used for Masson Trichrome staining. Cross-sections of the specimens were examined using light microscopy and photographed. The mean thickness of the four regions of the striated muscle near the two diagonal lines in the transverse sections of the mid-urethra [20] was evaluated in detail in each mouse using Image- Pro Plus 5.1 image analysis software (Silver Spring, MD).
The other half of each tissue sample was embedded in Tissue-Tek O.C.T.
Compound (Torrance, CA) and frozen in liquid nitrogen, and cryostat sections of 8 μm were cut and used for immunofluorescence staining of collagen, elastin and LOX.
For immunofluorescence staining, the sections were permeabilized with 0.05% Triton X-100 for 5 min and blocked with 5% normal horse serum in PBS for 1 h at room temperature. The sections were then incubated with the primary antibody derived from rabbit (anti-collagen antibody, 1:4000 dilution, Abcam, Cambridge, UK; anti- elastin antibody, 1:250 dilution, Abcam, Cambridge, UK; anti-LOX antibody, 1:150 dilution, Abcam, Cambridge, UK) for 1 h at room temperature. After being washed with PBS three times, the sections were incubated with fluorescein isothiocyanate conjugated secondary antibody derived from goat (1:300, Zymed, CA) for 1 h at room temperature and viewed under fluorescence microscopy. The images were then
analyzed with Image-Pro Plus 5.1 image analysis software.
Western blot analysis
Urethral tissue samples were prepared by homogenization of cells in a lysis buffer containing 1% IGEPAL CA-630, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, aprotinin (10 μg/mL), leupeptin (10 μg/mL), and phosphate-buffered saline (PBS). Cell lysates containing 100 μg of protein were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to a polyvinylidene fluoride membrane (Millipore Corp, Bedford, MA, USA). The
membrane was stained with Ponceau S to verify the integrity of the transferred proteins and to monitor the unbiased transfer of all protein samples. Detection of collagen, elastin, LOX and β-actin on the membranes were performed with an electrochemiluminescence kit (Amersham Life Sciences Inc, Arlington Heights, IL, USA) with the use of the antibody derived from rabbit (anti-collagen antibody, 1:500 dilution, Abcam, Cambridge, UK; anti-elastin antibody, 1:2000 dilution, Abcam, Cambridge, UK; anti-LOX antibody, 1:200 dilution, Abcam, Cambridge, UK; anti-β- actin antibody, 1:2000 dilution, Abcam, Cambridge, UK). The intensity of each band
was quantified using a densitometer (Molecular Dynamics, Sunnyvale, CA).
Statistical analysis
The analysis and experiments were performed by different authors, who were blinded to the sample status. The data are presented as mean ± standard deviation for each group. Statistical differences among groups were determined by one-way analysis of variance (ANOVA) followed by Fisher’s LSD as a post-hoc test. All statistical tests were two-sided. A P-value less than 0.05 was considered statistically significant. All calculations were performed using the Statistical Package for Social
Sciences (SPSS for Windows, release 8.0, SPSS Inc, Chicago, IL, USA).
Results
LPP values on the fourth day after VD were significantly decreased in the 3 mm and 8 mm VD groups (21.3 ± 4.2 and 8.8 ± 1.9 cmH2O, respectively) as compared to
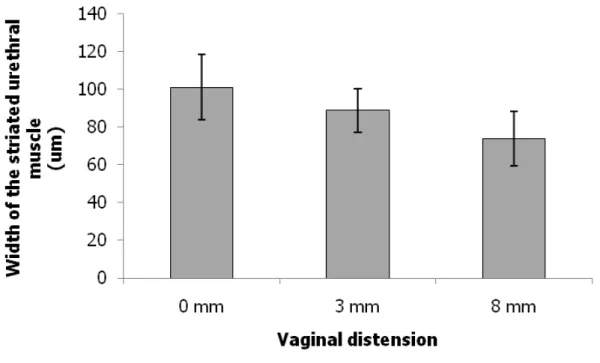
that in the non-instrumented control group (48.5 ± 12.8 cmH2O) (Fig. 1A). Although the mean thickness of the four regions of the striated muscle near the two diagonal lines in the transverse sections of the mid-urethra showed a trend toward a decrease with an increase in VD size, no significant difference was observed between the
groups (Fig. 1B).
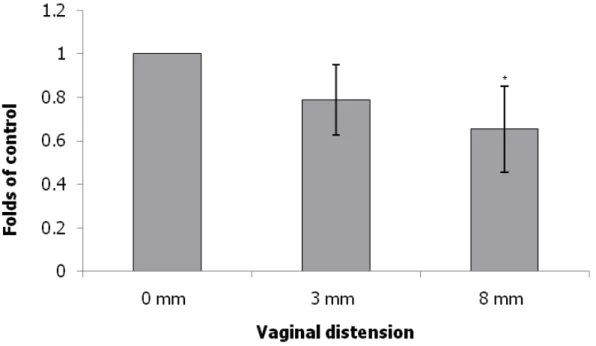
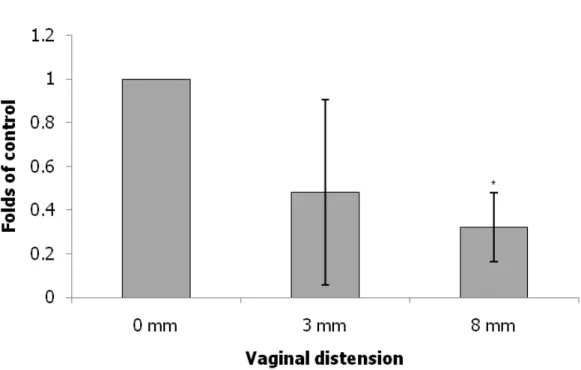
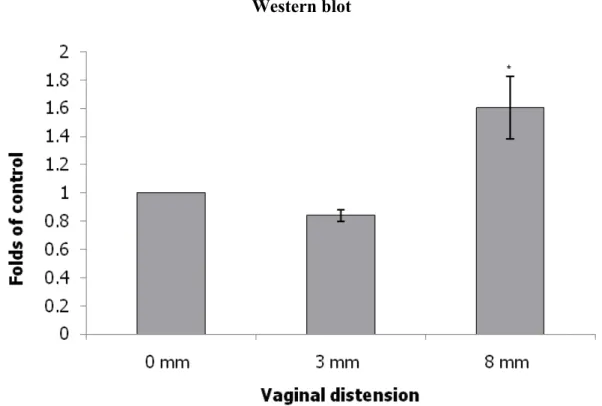
The expression levels of collagen, elastin and LOX were normalized to those of β-actin. Collagen expression in the urethra as indicated by Western blot and immunofluorescence staining examinations was significantly decreased in the 8 mm VD group and decreased in the 3 mm VD group as compared with that in the non- instrumented control group (Fig. 2). Elastin expression in the urethra as indicated by Western blot and immunofluorescence staining was significantly decreased in the 8 mm VD group and decreased in the 3 mm VD group as compared with that in the non-instrumented control group (Fig. 3). Lastly, LOX expression in the urethra was also significantly increased in the 8 mm VD group (as indicated by Western blot and immunofluorescence staining) and increased in the 3 mm VD group (as indicated by immunofluorescence staining) as compared with that in the non-instrumented control group (Fig. 4).
Comments
Birth trauma occurs as a direct result of a higher ratio of the baby’s head to the birth canal in humans. If active labour is maintained for more than 30 min, pressure on the vaginal sidewall can reach 240 cm H2O during the peak of contractions, which is sufficient for causing microcirculatory ischemia and overstretching of the muscles of the pelvic floor, the pubo-urethral ligaments and nerve tissue; these combined
events lead to SUI [22,23]. In light of the paucity of human urethral tissue available for analysis, our animal model of VD-induced SUI represents a reasonable proxy for
the study of the urethral effects of labour.
In the present study, the LPP was significantly decreased in the 3 mm and 8 mm VD groups as compared with that in the non-instrumented control group, indicating vaginal trauma injury to the urethra. This finding is compatible with that reported by Lin et al. [19]. VD (simulated birth trauma), which induces ischemia and stretch injury, may cause alterations in the composition and function of the lower urinary
tract (LUT) tissues [24].
Vaginal delivery can injure the nerve, muscle and connective tissues responsible for maintaining continence [2,3]. In the present study, collagen and elastin expression in the urethra was significantly decreased in the 8 mm VD group and decreased in the 3 mm VD group as compared with that in the non-instrumented control group, indicating decreased syntheses of the ECM components or increased proteolysis in the urethra. Our data suggest that decreased syntheses of the ECM components or increased proteolysis can be activated by vaginal trauma. There is evidence that connective tissue injury plays a role in the development of SUI. This study model characterizes an acute phase of post-trauma, representing lower urinary dysfunction immediately post-partum. Certain alterations in connective tissue metabolism are
known to be activated by vaginal trauma [25].
LOX expression is known to be regulated by hypoxia-inducible factors [26]. The present study shows that LOX expression in the urethra was significantly enhanced in the 8 mm VD group and increased in the 3 mm VD group as compared with that in the non-instrumented control group. Our data suggest that the LOX can be activated by vaginal trauma, which induces ischemia. LOX has been reported to be involved in the cross-linking of collagen and elastin, which is essential for the stabilization of collagen fibrils and for the integrity and elasticity of mature elastin [27]. We propose that the increase in LOX is a compensatory mechanism, which promotes the assembly and enhances the maturity of elastin/collagen fibers [15-19]. The assembly and cross- linking of elastic/collagen fibers is crucial for the recovery of urethral support after vaginal trauma. The majority of such alterations induced by a similar protocol of vaginal distension have been demonstrated to recover after 4-6 weeks following the
trauma [28].
Our study has certain limitations. First, the pelvic floor structure of the mouse, which is a quadruped and has a lax abdominal wall, is different from that of a human female; therefore, the results of this study need to be carefully applied to human subjects. Second, this study includes a relatively small sample size. Despite the small sample size, the LPP values were statistically significantly decreased in the 3 mm and
8 mm VD groups (21.3 ± 4.2 and 8.8 ± 1.9 cmH2O, respectively) as compared with that in the non-instrumented control group (48.5 ± 12.8 cmH2O). Further, urodynamic studies were conducted under anesthesia; fortunately, none of our subjects manifested any evidence of bladder instability, implying that detrusor overactivity was not present, and giving credence to our interpretation of fluid expulsion in the absence of increased bladder pressure as evidence of SUI. Third, this study only observed changes in LOX expression and in the metabolism of urethral connective tissue induced by vaginal trauma. The use of gene chip analysis and proteomic analysis would permit the exhaustive assessment of genome expression in the given tissues, possibly pointing to another mechanism of VD-induced incontinence; these methods should be used in future studies. Fourth, most of studies concerning the effects of birth trauma on the animal pelvic floor have used pregnant rats [5,29]. Pregnancy confers a different hormonal status, with other accompanying changes, as compared to non-gravid rats. As the pelvic structures affected by pregnancy hormones may respond differently to the trauma, this study reflects vaginal trauma and not birth trauma. Fifth, the histological assessments focused on striated muscle, but urethral smooth muscle is also affected by vaginal distension. In Fig. 3, the Western blot results after 3 mm distension show tremendous variability (high standard deviation). A further study evaluating smooth muscle outcomes and
functional genomics, which include proteomics and gene knockdown assays, with a larger sample of C57BL/6 mice after VD or post-partum is warranted for further
clarity.
In conclusion, our results suggest that VD can induce SUI in female mice. SUI following vaginal trauma involves over-expression of LOX and decreased syntheses of the extracellular matrix components or increased proteolysis in the urethra.
Therefore, the urethra actively undergoes remodeling in response to VD stimuli.
Conflits of interests
The authors do not have any conflicts of interest.
Acknowledgements
The authors would like to thank the National Science Council of the Republic of China and the China Medical University Hospital for financially supporting this research under Contract NSC 100-2314-B-039-007 and DMR-100-091.
References
1. MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery.
BJOG 2000;107:1460–70.
2. Thom DH, van den Eeden SK, Brown JS. Evaluation of parturition and other reproductive variables as risk factors for urinary incontinence in later life. Obstet Gynecol 1997;90:983–9.
3. Handa VL, Harris TA, Ostergard DR. Protecting the pelvic floor: obstetric management to prevent incontinence and pelvic organ prolapse. Obstet Gynecol 1996;88:470–8.
4. Feifer A, Corcos J. The use of synthetic sub-urethral slings in the treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 2007;18:1087–95.
5. Sievert KD, Emre Bakircioglu M, Tsai T, Dahms SE, Nunes L, Lue TF. The effect of simulated birth trauma and/or ovariectomy on rodent continence mechanism.
Part I: functional and structural change. J Urol 2001;166:311–7.
6. Hijaz A, Daneshgari F, Sievert KD, Damaser MS. Animal models of female stress urinary incontinence. J Urol 2008;179:2103–10.
7. Cannon TW, Wojcik EM, Ferguson CL, Saraga S, Thomas C, Damaser MS.
Effects of vaginal distension on urethral anatomy and function. BJU Int
2002;90:403–7.
8. Hijaz A, Daneshgari F, Huang X, Bena J, Liu G, Saffore L, et al. Role of sling integrity in the restoration of leak point pressure in the rat vaginal sling model. J Urol 2005;174:771–5.
9. Boyles SH, Li H, Mori T, Osterweil P, Guise JM. Effect of mode of delivery on the incidence of urinary incontinence in primiparous women. Obstet Gynecol 2009;113:134–41.
10. Conner E, Margulies R, Liu M, Smilen SW, Porges RF, Kwon C. Vaginal delivery and serum markers of ischemia/reperfusion injury. Int J Gynaecol Obstet.
2006;94:96-102.
11. Retzky SS, Rogers Jr RM. Urinary incontinence in women. Clin Symp 1995;47:2–
32.
12. Altman D, Ekstrom A, Forsgren C, Nordenstam J, Zetterstrom J. Symptoms of anal and urinary incontinence following cesarean section or spontaneous vaginal delivery. Am J Obstet Gynecol 2007;197:512–7.
13. Chen B, Wen Y, Zhang Z, Guo Y, Warrington JA, Polan ML. Microarray analysis of differentially expressed genes in vaginal tissues from women with stress urinary incontinence compared with asymptomatic women. Hum Reprod 2006;21:22–9.
14. Chen BH, Wen Y, Li H, Polan ML. Collagen metabolism and turnover in women with stress urinary incontinence and pelvic prolapse. Int Urogynecol J Pelvic Floor Dysfunct 2002;13:80–7.
15. Kerkhof MH, Hendriks L, Brölmann HA. Changes in connective tissue in patients with pelvic organ prolapse--a review of the current literature. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:461-74.
16. Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem 2003;88: 660–72.
17. Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, et al. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet 2004;36:178– 82.
18. Liu X, Zhao Y, Pawlyk B, Damaser M, Li T. Failure of elastic fiber homeostasis leads to pelvic floor disorders. Am J Pathol 2006;168:519-28.
19. Drewes PG, Yanagisawa H, Starcher B, Hornstra I, Csiszar K, Marinis SI, et al.
Pelvic organ prolapse in fibulin-5 knockout mice: pregnancy-induced changes in elastic fiber homeostasis in mouse vagina.Am J Pathol 2007;170:578-89.
20. Lin YH, Liu G, Daneshgari F. A mouse model of simulated birth trauma induced stress urinary incontinence. Neurourol Urodyn 2008;27:353–8.
21.Cannon TW, Damaser MS. Effects of anesthesia on cystometry and leak point pressure of the female rat. Life Sci 2001;69:1193–202.
22.Rempen A, Kraus M. Measurement of head compression during labor:
preliminary results. J Perinat Med 1991;19:115–20.
23.Sultan AH, Monga AK, Stanton SL. The pelvic floor sequelae of childbirth. Br J Hosp Med 1996;55:575–9.
24. Capolicchio G, Aitken KJ, Gu JX, Reddy P, Bägli DJ. Extracellular matrix gene responses in a novel ex vivo model of bladder stretch injury. J Urol 2001;165:
2235–40.
25. Chen B, Yeh J. Alterations in connective tissue metabolism in stress incontinence and prolapse. J Urol 2011;186:1768-72.
26. Erler JT, Bennewith KL, Nicolau M, Dornhöfer N, Kong C, Le QT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 2006;440:1222-6.
27. Klutke J, Stanczyk FZ, Ji Q, Campeau JD, Klutke CG. Suppression of lysyl oxidase gene expression by methylation in pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct 2010;21:869-72.
28. Rahn DD, Acevedo JF, Word RA. Effect of vaginal distention on elastic fiber synthesis and matrix degradation in the vaginal wall: potential role in the pathogenesis of pelvic organ prolapse. Am J Physiol Regul Integr Comp Physiol 2008;295:R1351-58.
29. Lin G, Shindel AW, Banie L, Deng D, Wang G, Hayashi N, et al. Molecular mechanisms related to parturition-induced stress urinary incontinence. Eur Urol 2009;55:1213-23.
B
0 mm 3 mm
8 mm
Fig. 1 (A) LPP values on the fourth day after VD in the different groups. Each bar represents the mean ± standard deviation of six individual mice. # Significantly different from the value in the non-instrumented control group (P < 0.05). * Significantly different from the values in the non-instrumented control and 3-mm VD groups (P < 0.05). (B) Alterations in width of the striated urethral muscle in transverse sections of the mid-urethra as indicated by Masson Trichrome staining after VD in the different groups. E, epithelium; M, muscularis. Each bar represents the mean ± standard deviation of six individual mice.
A B
0 mm 3 mm
C D
8 mm Western blot
Collagen β-actin
0 mm 3 mm 8 mm
Immunofluore
scence
staining
Western blot
Fig. 2 Alterations of collagen expression in transverse sections of the mid-urethra as indicated by immunofluorescence staining(collagen: green) (A: 0 mm; B: 3 mm C: 8 mm) and Western blot (D) after VD in the different groups. The values are calculated by fold of control and expressed as mean ± standard deviation of six individual mice.
* Significantly different from the value in the non-instrumented control group (P <
0.05).
A B
0 mm 3 mm
C D
8 mm Western blot
Elastin β-actin
0 mm 3 mm 8 mm
Immunofluorescence staining
Western blot
Fig. 3 Alterations of elastin expression in transverse sections of the mid-urethra as indicated by immunofluorescence staining(elastin: green) (A: 0 mm; B: 3 mm C: 8 mm) and Western blot (D) after VD in the different groups. The values are calculated by fold of control and expressed as mean ± standard deviation of six individual mice.
* Significantly different from the value in the non-instrumented control group (P <
0.05).
A
B
0 mm
3 mm
C D
8 mm Western blot
LOX
β-actin
0 mm 3 mm 8 mm
Immunofluorescence staining
Western blot
Fig. 4 Alterations of LOX expression in transverse sections of the mid-urethra as indicated by immunofluorescence staining (LOX: green) (A: 0 mm; B: 3 mm C: 8 mm) and Western blot (D) after VD in the different groups. The values are calculated by fold of control and expressed as mean ± standard deviation of six individual mice.
* Significantly different from the value in the non-instrumented control group (P <
0.05).