C38
Journal of Organometalk Chemirtry. 36X (19X9) C38-C40 Elsevier Sequoia S.A., Lausanne - Printed in The Netherlands JOM 9927PC
Preliminary communication
Structure of
(meso-cis-2,lO-diphenyl-6-aza-k2,lO-diphospha-
k
2P-bicyclo[9.4.0]pentadeca-ll(
1) ,12,14-triene) -
(bicyclo[ 2.2. llheptadiene) rhodium( I) hexafluorophosphate
Shiuh-Tzung Liu *, Ming-Chu Cheng and Shie-Ming Peng *
Department of Chemistry. National Taiwan University Taipei. Taiwan 10764 (R.O.C.) (Received November 28th, 1988: in revised form March 14th, 1989)
Abstract
The structure of the title compound has been determined. All three donor atoms in the macrocycle are coordinated to the metal center to form a novel penta-coordi- nated rhodium(I) species. The N-Rh distance is 2.317(4) A, slightly longer than normal ones.
Introduction
Recently, due to the research interest in the field of asymmetric catalytic hydrogenation [l], the structural analyses of rhodium(I) complexes have received considerable attention [1,2,3]. Whereas many tetra-coordinated rhodium(I) com- plexes are known, few penta-coordinated species are reported [4]. Here, we would like to present the structure of an unusual square-pyramidal rhodium(I) compound. Results and discussion
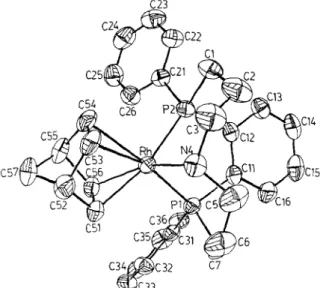
A solution of (NBD),RhPF, (NBD = norbornadiene) in dichloromethane was added under nitrogen to a solution of the macrocyclic ligand [5] in dichloromethane. Crystals of the yellow complex were grown from the above solution with addition of a small portion of ethanol. The ORTEP plot of the title complex is shown in Fig. 1 *. Selected bond distances and angles are listed in Table 1. As shown in Fig. I the rhodium center is surrounded in a plane by two phosphorus atoms and two double bonds (assuming that each double bond occupies a single coordination site), since the nitrogen atom is apical with respect to the plane, the rhodium metal is in square-pyramidal geometry. The rhodium-phosphorus distances (2.233(Z) and
* Crystal data: C,,H,,F,NP,Rh CH,CI,, M = 816.4, monoclinic, P2,/c, a 12.878(2), b 14.874(g). ( 17.725(2) A, p 97.02(l)“, V 3369.71 A3, Z= 4, 0, 1.61 Mg m-3, D, 1.60 Mg m--j, p 0.70 mm -I, h(Mo-K,) 0.7107 A. F(OOO) = 1516. T 300 K, R = 0.048 for 4748 observed reflections.
c39 C23
Fig. 1. ORTEP Plot of the cation of the rhodium(I) complex.
2.296(2) A), as well as the P2-Rh-Pl angle (85.39(5) o ) are similar to those reported in [(PPFA)Rh(NBD)]+ (PPFA = cu-(2-diphenylphosphinoferrocenyl)ethyldimethyla- mine) (2.28(l) A) and [(S, S-chiraphos)Rh(COD)]+ (S, S-chiraphos = (2S,3S)-2,3- bis(diphenylphosphino)butane; COD = 1,Scyclooctadiene) (2.275(l) and 2.266(l) A, 83.82(6) “) [2a,3].
Although both double bonds are in truns position to phosphorus atoms, their distances to metal center appear to be unequal. The Rh-C dittances are 2.125(5) and 2.103(5) A for one double bond and 2.275(5) and 2.282(5) A for the other (i.e.,
Table 1
Selected bond lengths (A) and bond angles (O ) Rh-Pl 2.2326(U) Rh-N4 2.317(4) Rh-c53 2.275(5) R&C56 2.103(5) Pl-c7 1.822(6) Pl-c31 1.822(5) PI-Cl1 1X28(5) Cl-C2 1.525(9) C3-N4 1.486(8) CS-C6 1.462(10) Pl-Rh-P2 85.39(5) P2-Rh-N4 90.51(12) Rh-PI-C7 114.00(19) Rh-P2-Cl2 108.62(17) Rh-N4-C5 119.6(3) N4-C5-C6 114.1(5) Pl-C7-C6 114.7(4) P2-Cl-C2 112.5(4) RI-P2 Rh-c54 Rh-c51 P2-Cl P2-c21 P2-Cl2 C2-C3 N4-C5 C6-C7 PI-Rh-N4 N4-Rh-C56 Rh-P2-Cl Rh-N4-C3 C3-N4-C5 C5-C6-C7 C2-C3-N4 Cl-C2-C3 2.2959(U) 2.282(5) 2.125(5) 1.820(6) 1.832(5) 1.839(5) 1.465(10) 1.458(S) 1.539(9) 90.18(12) 148.15(19) 114.64(20) 114.9(3) 111.9(5) 117.46) 117.8(5) 117.9(6)
c40
Table 2
Selected torsional angles ( o )
P2-Rh-N4-C3 39.5 PI-RhpN4-CS -12.5 Rh-N4-C3-C2 - 64.0 RI-N4-C5-C6 - 41.9 N4-C3-C2-Cl 78.0 N4-CS--C6-C7 84.3 C3-C2-Cl-P2 - 64.5 CS-C6-C7-PI -~ 46.9 C2-Cl-P22Rh 46.7 C6-C7-Pl--Rh -- IX.3 Cl-P2-Rh-N4 - 32.0 C7-Pl-RI-N4 37.8
the distances of Rh to double bonds are 1.992 and 2.176 A, respectively). Such unequal metal-olefin distances have been reported in many systems, such as ([n5-(t-Bu)2P(C,H,)]2Fe)Rh(NBD) cation [2b], but the difference of 0.18 A in our complex is somewhat larger than that previously reported (see below).
Another unique feature of this structure is that the distance of the nitrogen atom to metal is 2.317(4) A, indicating the bonding between these two atoms. Although the distance is slightly longer than the reported value 2.26(3) A in [(PPFA)Rh(NBD)]+ species, this longer distance is probably due to the influence of its position as an axial group.
Focusing on the 6-membered ring, it is noteworthy that the torsional angles around the Rh, P2, Cl, C2, C3, N4 atoms have the typical +R, -g alternation, characteristic for a chair conformation (Table 2). The other ring with four negative and two positive torsional angles is attributed to a twist-boat conformation. The conformations of the two six-membered chelate rings are similar to those in the NiCl, analog [5], due to the “flagpole” interactions between H2B and H6B. Additionally, such an arrangement can also explain the unequal distances between 0 rhodium and carbons in the crystal structure. The H3B-H53 distance is 2.26 A for this particular conformational arrangement. Inspection of the Dreiding model of this complex with equal Rl-C distances indicates that the H3B-H53 distance becomes relatively close, thus the increase in this distance relieves the interaction. However, this unequal behavior in crystal structure is not observed in CDCl, solution by NMR spectroscopy. The “P NMR spectrum only shows a doublet at 56.0 ppm with J(Rl-P) 121 Hz.
Acknowledgment. This work was supported by the National Science Council Grants NSC77-0208-M002-14 (SMP) and NSC77-0208-M002-49 (STL).
References
1 (a) J. Halpem Pure Appl. Chem., 55 (1983) 99; (b) W.S. Knowles Act. Chem. Res., 16 (1983) 106. 2 (a) W.R. Cullen. F.W.B. Einstein, C.-H. Huang, AC. Willis, E.-S. Yeh J. Am. Chem. Sot., 102 (1980)
988; (b) W.R. Cullen, T.-J. Kim, F.W.B. Einstein, T. Jones Organometallics, 2 (1983) 714. (c) W.R. Cullen, T.-J. Kim, F.W.B. Einstein, T. Jones, ibid., 4 (1985) 346. and ref. therein.
3 R.G. Ball, N.C. Payne Inorg. Chem., 16 (1977) 1187.
4 F.A. Cotton, G. Wilkinson Advanced inorganic Chemistry, 4th edit., 1980. .John-Wiley & Son, New York.