Characteristics and Levels of Sophistication: An Analysis
of Chemistry Students
’ Ability to Think with Mental
Models
Chia-Yu Wang&Lloyd H. Barrow
Published online: 31 July 2010
# Springer Science+Business Media B.V. 2010
Abstract This study employed a case-study approach to reveal how an ability to think with mental models contributes to differences in students’ understanding of molecular geometry and polarity. We were interested in characterizing features and levels of sophistication regarding first-year university chemistry learners’ mental modeling behaviors while the learners were solving problems associated with spatial information. To serve this purpose, we conducted case studies on nine students who were sampled from high-scoring, moderate-scoring, and low-scoring students. Our findings point to five characteristics of mental modeling ability that distinguish students in the high-, moderate-, and low-ability groups from one another. Although the levels of mental modeling abilities have been described in categories (high, moderate, and low), they can be thought of as a continuum with the low-ability group reflecting students who have very limited ability to generate and use mental models whereas students in the high-ability group not only construct and use mental models as a thinking tool, but also analyze the problems to be solved, evaluate their mental models, and oversee entire mental modeling processes. Cross-case comparisons for students with different levels of mental modeling ability indicate that experiences of generating and manipulating a mental model based on imposed propositions are crucial for a learner’s efforts to incorporate content knowledge with visual-spatial thinking skills. This paper summarizes potential factors that undermine learners’ comprehension of molecular geometry and polarity and that influence mastery of this mental modeling ability.
Keywords Chemistry . Molecular geometry . Molecular polarity . Mental models . Mental modeling ability
DOI 10.1007/s11165-010-9180-7
C.-Y. Wang (*)
Institute of Education, National Chiao Tung University, 1001 Ta-Hsueh Road, Hsinchu 30010, Taiwan e-mail: cwg25@mail.nctu.edu.tw
L. H. Barrow
Introduction
Understanding conventional representations, visualizing a spatial structure of a molecule from a symbolic or a two-dimensional (2D) representation, and relating properties of a matter with its molecular structure require learners to be able to transform freely among macroscopic, submicroscopic, and symbolic representations (Dori and Barak 2001; Johnstone 1991; Treagust et al. 2003). These are essential skills that chemistry learners are expected to develop in chemistry education. However, chemistry concepts are often taught with symbolic representations and with emphases mostly on propositions and principles (Yang et al. 2003). Learners may not have enough opportunities to work with physical or virtual models and to practice using them as a thinking tool.
Findings of previous studies have shown that chemistry learners may encounter difficulties in interpreting symbolic representations and transforming them into three-dimensional (3D) structures (Bodner and Domin2000; Furió and Calatayud1996; Furió et al.2000; Tuckey et al. 1991). They also experience difficulties in connecting properties of a molecule with its formula and geometric structure (Marais and Jordaan2000; Treagust et al.2003).
Briggs and Bodner (2005) argued that visualization and construction of mental models help supply students with a tool for reasoning. These researchers found that second-year organic chemistry students employed a set of visualization operations to make sense of an input from their eyes and to manipulate a constructed mental model for solving a problem. It was reported that some students were able to perform visualization operations such as rotating, relating, zooming in and out, and manipulating a mental model by applying rules or syntax, as well as performing inspection on or reading results of an inference from a mental model (Briggs2004; Reiner and Gilbert2000).
Instructional research has helped learners to form mental models of molecules in 3D or to visualize chemical processes at the submicroscopic level using concrete models (Dori and Barak2001) and computer animation (Rundgren and Tibell2009; Urhahne et al.2009; Yang et al. 2003). Findings from Dori and Barak (2001) indicate that by acquiring more opportunities to work with physical models and virtual animation, general chemistry students were more capable of undertaking transformations among chemistry formulas, 2D representations, and 3D representations than were the students’ counterparts who received traditional instruction. Other evidence shows that learning with 3D computer animation might merely benefit students who have not yet formed or have difficulties in constructing mental models with suitable spatial features. When learners have possessed or are able to construct appropriate mental models, learning with 3D simulations or with 2D static illustrations yielded no difference in learning outcomes (Urhahne et al.2009). Instruction that concerned the formation of mental models also aided students’ reading-comprehension performance (Leutner et al.2009). Findings of the aforementioned research suggest that opportunities to form a mental model and to use it as a tool of thinking facilitate comprehension and problem-solving relative to chemistry concepts involving spatial information. Nevertheless, attempts are needed to delineate attributes of this mental modeling ability and to investigate how lacking this ability may influence learning in chemistry.
Theoretical Framework
Mental models are internal representations that an individual constructs to understand or to give a rational explanation for an experienced phenomenon (Greca and Moreira 2002). Although theorists of mental models have not formulated a universally accepted definition
of what constitutes a mental model, it is generally agreed that a mental model encompasses visual-pictorial and propositional components (Greca and Moreira 2002; Mayer 2001; Schnotz and Bannert2003).
When learning a new concept that incorporates both texts and pictures, an individual selects relevant information from both texts and visual components. The selections of relevant text-based and visual information are text-based on the individual’s existing knowledge and experiences (Mayer2001; Schnotz and Bannert2003; Schnotz and Küerschner2008). The individual then processes text-based information to generate propositional representations and to form a system of semantic relations (Schnotz and Bannert 2003; Schnotz and Küerschner2008). Meanwhile, visual information is perceived and processed through a separated channel (Mayer2001). The individual forms a visual-based mental model by identifying components and structural organizations of the perceived graphic entities (Schnotz and Bannert 2003). Comprehension is considered a process of structural mapping between the system of semantic relations and the visual-based mental model (Schnotz and Bannert 2003; Schnotz and Küerschner2008).
In general, reasoning with mental models requires integration of propositional representations and a visual-based mental model in working memory (Mayer2001; Ramadas2009; Schnotz and Bannert 2003; Schnotz and Küerschner 2008; Seel et al. 2008). In a problem-solving situation, a learner constructs a mental model on the basis of his or her knowledge schema for a specific goal. The learner can then explore and inspect the mental model to describe and explain properties of a system. He or she is also able to stimulate actions in imagination (e.g., perform a thought experiment) or test a hypothesis on a mental model for inference and prediction by adding additional propositional representations to the mental model (Greca and Moreira2002; Ramadas2009; Seel et al.2008).
At the same string of thought, if chemistry learners are to develop understanding of a chemistry concept or to solve a problem in a new situation, instruction has not only to assist development of appropriate propositional representations and formation of an adequate mental model, but also to facilitate integration between the two (Ramadas2009). Students’ comprehension or problem-solving ability may be hindered if the following three impediments are not addressed:
(1) Students possess inaccurate or fragmented conceptual knowledge (Nicoll2003; Peterson and Treagust 1989; Peterson et al. 1989); therefore, they may use inadequate propositional representations or intuition for reasoning (Furió et al.2000; Ramadas2009). (2) Students may construct a mental model with inadequate components or faulty structure that would lead to an improper result of reasoning (Bodner and Domin
2000). Sometimes, students may either not understand conventions of chemical representations (Justi et al. 2009) or experience difficulties in visualizing a 3D geometrical structure of a molecule from a formula or a 2D representation (Ferk et al.
2003). These students may perform algorithms or work with formulas and definitions lacking reference to mental models (Greca and Moreira2002; Stieff et al.2005). (3) Students may be unable to integrate propositional representations into a mental model
(Ramadas2009; Reiner and Gilbert2000). Findings from a study by Rundgren and Tibell (2009) indicate that even students who had appropriate background knowledge might not understand mechanisms or dynamic processes that an animation conveyed because these students could not associate their knowledge with depictive information in the animation. Gilbert (2005,2008) identified a metacognitive aspect describing this sophistication as it inheres in an ability to think with mental models. Gilbert (2005) stated that a fluent performance in visualization (in his term, metavisualization or metavisual capability) involves
the capability to acquire, retain, retrieve, and amend a stored visual representation for a specific purpose, as well as the ability to reflectively monitor the corresponding process and outcomes while learning from and with the representation. He further divided this metavisual capability into two levels: at the lower level, a learner is capable of reflecting on a task or a problem to be addressed and the solution that follows. At the upper level, a learner is capable of reflecting on his or her own reasoning process as feedback for self-improved learning and metavisual capability. The major difference between the two levels lies in the metacognitive skillfulness that an individual performs. Still, empirical evidence is needed for delineating this metavisual capability and supporting the acquisition of it.
Development and mastery of the ability to construct and to reason with mental models often requires a lot of practice and mental effort (Seel et al.2008). We feel that there is a need to portray and to characterize this ability and, in addition, to explore difficulties that learners encounter while using mental models as a thinking tool. In this study, we investigated general chemistry college students’ thinking processes while the students were solving molecular geometry and polarity problems. Concepts of molecular geometry and polarity are usually introduced in the middle of a sequence of general chemistry courses. Learning these two concepts requires an understanding of prerequisite concepts. Also, it requires a learner to visualize a spatial structure of a molecule and to reason with the structure in 3D. Thus, it is a difficult concept for students to understand; yet research on this topic is limited to identifying chemistry students’ common misconceptions about molecular polarity and its prerequisite concepts (Jang2003; Nicoll2003; Peterson and Treagust1989; Peterson et al.1989). Only Furió et al. (1996,2000) attributed one learning impediment to students’ common-sense reasoning.
By investigating learners’ thinking relative to molecular geometry and polarity problems, we hope to delineate characteristics and levels of sophistication about general chemistry students’ ability to construct and use mental models as a tool for thinking. Therefore, this study was designed to explore the following research question: how does an individual’s ability to think with mental models influence his or her thinking and understanding relative to molecular geometry and polarity? Specifically, we describe the features and levels of sophistication characterizing the mental modeling ability possessed by high-scoring, moderate-scoring, and low-scoring students. By carefully comparing the mental modeling processes of general chemistry students across high-, moderate-, and low-scoring groups, we hope to reveal learning impediments of the moderate- and low-low-scoring students and, in turn, to provide insights with which chemistry instructors can effectively teach this abstract concept.
Methods
Subjects and Data Collection
The design and the implementation of the current study rest largely on a theoretical framework of personal constructivism and a case-study methodology. The study took place in the second course of a three-course general chemistry sequence at a research-intensive institute in the American Midwest during the fall 2006 semester. We adopted three diagnostic instruments on electronegativity (Taber2002), chemical bonding (combining selected items from Jang2003; Peterson et al.1989), and molecular geometry and polarity (Furió et al.2000; Peterson et al.
1989) to diagnose students’ understanding of these prerequisite concepts. To understand how
understanding of molecular geometry and polarity, we conducted case studies on nine students who were sampled from high-scoring students (JS, RE, and CR), moderate-scoring students (SD, KU, and TA), and low-scoring students (AM, KA, and JT) on the basis of the percentage of their correctly answered questions regarding diagnostic instruments. High-scoring students were individuals whose percentages of questions answered correctly were≥70% for two out of three diagnostic instruments. Low-scoring students were individuals whose percentages of questions answered correctly were ≤50% for two out of three diagnostic instruments. All interviews took place after the participants’ completion of lessons on molecular geometry, polarity, and relevant concepts.
We used a combination of a think-aloud protocol and an interview-about-events (White and Gunstone 1992) to collect data on the nine students’ explanations and their
constructions of artifacts (including drawings and model constructions). Students were interviewed by the first author, who also designed the interview. Interviews were conducted on a one-on-one basis, and each interview was approximately 50 minutes in length. The interview participation was voluntary. Participants gave permission for video-taping and were assured that this study was independent of their classroom assessment and that their responses would not be identified to the instructor.
The interviewer started by asking participants to draw or to build a model of H2S, SCl2,
and BF3 molecules using play-dough and straws to represent the mental model in their
mind. Each participant was asked to describe features of his or her mental model including shape of lone pair electrons, bond polarity, and attraction or repulsion among bonding and lone pair electrons. Also, the individual determined the geometrical structure, bond angles, and polarity of the molecule. Meanwhile, the interviewer asked the participants constantly about whether or not they were using a mental image while thinking through the tasks, and probed for details about features of the mental model and actions of applying these models. The nine student interviews were video-taped for data analysis.
Data Analysis
In order to determine features of mental modeling ability possessed by high-, moderate-, and low-scoring students, we randomly sampled one student from each scoring group to begin our analysis. We carefully reviewed the three interview videos to examine and annotate participants’ verbal and nonverbal moves that, during problem-solving, were associated with construction and use of mental models. We specifically focused on common features of mental actions (visualizations, metacognitive skills, etc.), excluding factors associated with content knowledge, that performed across the three scoring groups but illustrated differences of quality among the three groups. Using a grounded theory (Strauss and Corbin 1998), we identified a coding scheme consisting of five characteristics and identified exemplary excerpts from each high-, moderate-, and low-scoring student that clearly illustrated these five characteristics for high and low levels of quality.
At the second step of the analysis, we used this scheme to examine the remaining six interviews, moving from videos of the high-scoring group to those of the moderate- and low-scoring groups. As we analyzed each video, we compared it to the exemplary excerpts and reflected on the meaning of each characteristic. We continued to move back and forth between our data (for example, a video of a high-scoring student) and the coding scheme to examine whether or not observed mental moves in the video aligned with exemplary excerpts of high-scoring examples but departed from excerpts of moderate- and low-high-scoring examples. We continued this constant-comparative process (Strauss and Corbin1998) for within- and cross-case analyses, while we modified the meaning of each characteristic to satisfaction.
These characteristics were assembled to form a protocol that depicts the degree of sophistication for an individual’s mental modeling ability. We revisited each video and scored each characteristic with a 2, 1, or 0 point based on the quality of each characteristic revealed during the participant’s thinking processes. To determine the consistency of our analysis, we had an independent coder simultaneously code all nine videos. The independent coder was not involved in this project, and was blind to both the identity and the score of the participants. This analysis indicated 93% agreement in the coding of the types of characteristics and 87% agreement in scores on quality of the characteristic. The divergent results of coding between the researchers and the independent coder were resolved by discussion.
On the basis of their total scores, the nine participants were assigned to high mental modeling ability (HMMA), moderate mental modeling ability (MMMA), and low mental modeling ability (LMMA) groups. This re-categorization enabled us to conduct comparisons across high-, moderate-, and low-ability groups regarding how the five characteristics surfaced while the participants thought with mental models. We then discussed broader ideas that encompassed the five characteristics depicting students’ mental modeling behaviors within and across the three ability levels. It is from these broader ideas that our assertions emerged. We returned to the data with these assertions to find specific examples that illustrate how possessing a different level of mental modeling ability influences an individual’s understanding of molecular geometry and polarity.
Results
Five Characteristics of Mental Modeling Ability
Five characteristics of mental modeling ability emerged from the interview analyses, and these characteristics distinguish students from one another according to the high, moderate, and low mental modeling ability groups. The first characteristic describes whether a student could generate a 3D mental model of a given chemical formula without having to draw a Lewis structure. Depending on their familiarity with a given molecule, sometimes students were able to determine the Lewis structure of the molecule mentally while generating a 3D mental model; yet other times, they relied on creating a 2D Lewis dot structure on paper to provide cues for visualizing a 3D mental model. This characteristic is divided into two levels (characteristic 1a and characteristic 1b) depending on whether construction of a 2D representation on paper was needed for mental model construction.
The second characteristic describes degrees of a student’s abilities to reconstruct, manipulate, or adjust a generated mental model according to corresponding propositions or when a new condition was added to the model. Individuals with a higher level of this characteristic (characteristic 2a) not only knew how the conditions and propositions were associated with the generated mental model but also could impose propositions or conditions while adjusting the mental model. Thus, a mental model for these individuals was functional and could serve as a thinking tool. Students with a lower level of this characteristic (characteristic 2b) possessed a rigid mental model perhaps because they were unaware of connections between the mental model and corresponding propositions. Thus, these students concluded that the shape or structure of the mental model would not change when a new proposition was added to the model. When they had a concrete model in front of them and were prompted to reason with the model, some students could derive a correct conclusion by adjusting the concrete model on the basis of the conditions in the problem.
The third characteristic describes the presence and the degree of task analysis that a learner undertakes while approaching a task. Individuals with a higher level of this characteristic (characteristic 3a) analyze a problem to be solved and recognize conditions and propositions that are critical for solving the problem successfully. Actions of task analysis were also observed in students with a lower level of this characteristic (characteristic 3b); however, these students overlooked one or more of the conditions or propositions during task analysis or while thinking with mental models.
The fourth characteristic describes a learner’s possible constant monitoring of his or her own reasoning processes while constructing, manipulating, or adjusting a mental model. Approaches such as self-dialogue while solving a problem and evaluating the context and necessary information for problem-solving were observed in students in the HMMA group. The fifth characteristic describes actions of an individual’s self-checking using an alternative approach, such as drawing a Lewis structure on paper to verify his or her answers or performing inspections to identify errors from his or her mental model. Observed behaviors, like task analysis, monitoring, and self-checking (characteristics 3, 4, and 5, respectively), are discussed as manifestations of metacognitive skills in science education literature.
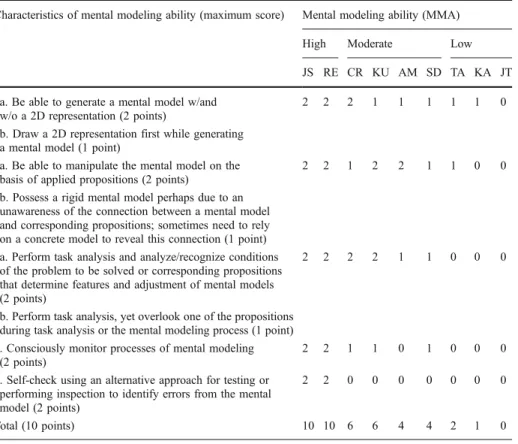
These five characteristics were assembled into a protocol of mental modeling ability, and scores of each participant on the five characteristics are shown in Table 1. Scores were assigned to the five characteristics for each participant according to the highest level and the most frequent level observed in a participant’s responses. Based on the sum of scores for the five characteristics, participants received 7~10, 4~6, and 0~3 points were assigned into
Table 1 Characteristics of mental modeling ability and scores of the mental modeling ability for nine participants Characteristics of mental modeling ability (maximum score) Mental modeling ability (MMA)
High Moderate Low
JS RE CR KU AM SD TA KA JT 1a. Be able to generate a mental model w/and
w/o a 2D representation (2 points)
2 2 2 1 1 1 1 1 0
1b. Draw a 2D representation first while generating a mental model (1 point)
2a. Be able to manipulate the mental model on the basis of applied propositions (2 points)
2 2 1 2 2 1 1 0 0
2b. Possess a rigid mental model perhaps due to an unawareness of the connection between a mental model and corresponding propositions; sometimes need to rely on a concrete model to reveal this connection (1 point) 3a. Perform task analysis and analyze/recognize conditions
of the problem to be solved or corresponding propositions that determine features and adjustment of mental models (2 points)
2 2 2 2 1 1 0 0 0
3b. Perform task analysis, yet overlook one of the propositions during task analysis or the mental modeling process (1 point) 4. Consciously monitor processes of mental modeling
(2 points)
2 2 1 1 0 1 0 0 0
5. Self-check using an alternative approach for testing or performing inspection to identify errors from the mental model (2 points)
2 2 0 0 0 0 0 0 0
high mental modeling ability (HMMA), moderate mental modeling ability (MMMA), and low mental modeling ability (LMMA) groups, respectively. Among the nine interview participants, CR (originally belonging to the high-scoring group), AM (originally belonging to the low-scoring group), and TA (originally belonging to the moderate-scoring group) were relocated from their original category according to their scores on the three diagnostic instruments. We attempted to explain the shifts of CR, AM, and TA from their original groups according to scores of the three diagnostic instruments; however, evidence to support the explanations was outside the scope of this study (Wang2007).
In the following subsections, we portray high, moderate, and low levels of sophistication in participants’ ability to think with mental models referring to the five characteristics in Table 1. We also provide excerpts from the interviews to demonstrate how possessing a different level of mental modeling ability influences a participant’s thinking processes while he or she is solving problems related to molecular geometry and polarity. Although the levels of mental modeling abilities are described as categories (high, moderate, and low), they can be thought of as a continuum with the LMMA group reflecting students who have no or very limited ability to generate and use mental models while the HMMA students reflect the most sophisticated ability (observed among the interview participants). HMMA students not only construct and use mental models, but also evaluate their mental models and monitor the mental modeling processes. Comparisons across HMMA, MMMA, and LMMA groups were organized into four assertions in the discussion section.
Levels of Sophistication about Mental Modeling Ability
High Level of Sophistication about Mental Modeling Ability (HMMA Group)
Students in the HMMA Group Could Generate a Mental Model of a Given Molecule with or without a 2D Representation (Characteristic 1a) HMMA students were able to generate a 3D mental model of a given chemical formula (e.g., BF3) without drawing a 2D Lewis dot
structure on paper. While the students talked-aloud during problem-solving processes, they gestured with their hands in the air to label each imaginary atom and correctly indicated relative positions of the atoms spatially. Once the imaginary molecule was constructed, the students were able to rotate the model to examine the spatial structure of the molecule from different angles if necessary. RE’s descriptions of visualizing a BF3molecule demonstrates
this characteristic:
What I did was I made a Lewis structure in my head. Like you have a boron and three fluorines. So the boron is the one that is going to be in the middle. Then I had three fluorines and I pictured, if I had three more atoms, what kind of shape is that going to make. And I pictured four dots with one in the middle making a shape, and I said, okay, that is going to put one here, one here, and one here. So I am going to have a triangular shape. (RE, interview)
RE explained how she visualized a 3D mental model from a 2D Lewis structure of a carbon tetrachloride (CCl4) molecule and examined its spatial structure from different angles to
understand its molecular polarity:
For a while, I pictured it in my head like the Lewis structure, like this (Fig.1). And I thought, well that must be a polar molecule because these three are all pulling down, and this one is pulling up, so that’s only canceling out this one [the pull pointing downward]. But then, when I learned that all the angles are 120°, I had to picture it in
my head and flip it upside down and turn it around, and see that it was all even on all the sides and the pull is going in all these opposite directions. (RE, interview)
Students in the HMMA Group Performed Task Analyses to Recognize Conditions of the Problem to be Solved or Corresponding Propositions that Determine Features and Adjustment of Mental Models (Characteristic 3a). By Doing so, they were Able to Reconstruct, Manipulate, or Adjust Parts of a Mental Model Accordingly by Imposing Related Propositions or Conditions of the Problem on the Model (Characteristic 2a) By performing task analysis, the HMMA students recognized and applied appropriate chemistry principles to constructing a mental model of a molecule. They also identified and imposed critical conditions to manipulate or adjust the model for inference. Students may have performed task analysis consciously or subconsciously while constructing or adjusting their mental model. Either way, they knew what chemical principles are appropriate to apply and how the principles are connected to the mental model. We use RE’s case as another example to illustrate this characteristic of mental modeling ability. While determining whether BF3is a polar or non-polar molecule, RE stated the following:
If I just think about it off the top of my head, I can picture the boron is going to be in the middle. There are three fluorides. And they are going to make a triangular shape. They are all going to be coming out from the bottom. No, it is going to be triangular planar, and it’s going to be in one plane most likely because if there’s no lone pairs, it’s all going to be in the same plane. And then I said, okay, if I had these three [bonded fluorine atoms] and they are going to be evenly spaced apart…you know, the angles are all going to be the same, then the pulls [dipole moments] are going to be towards the fluorines. And it’s going to pull, like one is pulling this way, one is pulling this way, one is pulling this way. And they are all going to cancel out. (RE, interview)
RE started off visualizing the BF3molecule with three B-F bonds coming from the bottom
of the boron atom. She soon realized that there were no lone pairs or bonds on top of the boron atom. Therefore, RE adjusted her mental model from a trigonal pyramidal shape to a trigonal planar shape by imposing the VSEPR principles on her model, thinking the three B-F bonds would repel each other and stay as far apart from each other as possible. In the next step, RE applied the concept of bond dipole, resulting from the electronegativity difference between boron and fluorine atoms, to each B-F bond with notions of the direction and magnitude of the bond polarity. Applying appropriate propositions to a functional mental model, RE was able to correctly infer molecular polarity by summing the overall dipole moments.
HMMA students’ mental models were functional. These students incorporated both conditions of a problem and the propositional statements they considered appropriate into the Fig. 1 RE’s drawing of a CCl4
mental model construction. Therefore, the mental model served to illustrate considerations that the individual imposed on the mental model. Once constructed, the mental model was flexible, which allowed students to apply an additional condition or proposition to the model and to adjust it accordingly. A functional mental model increases the chances for a student to derive a model that represents relatively accurate spatial information, if the propositions that the student possesses are accurate in the first place. With the functional mental model, both of the students in the HMMA group were able to use a model that contained accurate spatial information as a basis for an inference. In the following conversations, JS’s case provides strong evidence of how he applied appropriate propositions to a generated mental model at two different times and derived an answer that was beyond his existing knowledge:
I: What would be the bond angle [of H2S]?
JS: The bond angle? Between the two sulfurs it would be less than 109.5. Just a little less than 109.5.
I: Why is it less?
JS: Because if all of these were equally apart, then it would be 109.5. But we know that these electron pairs [lone pairs] push out more, so they squeeze these [H-S bonds] a little bit closer so it is a little bit less than 109.5.
I: If we replace the two hydrogen atoms of H2S with two chlorines, would the bond
angle change?
JS: I don’t think the bonding would—it does make sense that it would change. I don’t think I have ever been taught that it would, but it makes sense that it would because there would be more electrons on a chlorine atom than there are on a hydrogen. And you’d kind of think that those electrons would want to repel each other. (JS, interview)
An HMMA student, like RE or JS, would analyze and recognize key conditions and propositions during the problem-solving. They would then apply the identified propositions to adjusting the generated mental model accordingly and would draw inferences based on the mental model in the new condition. Performing appropriate task analysis may facilitate generation of a mental model with appropriate static and spatial features. It also leads to an identification of key conditions and propositions applicable to the following inference. By knowing what propositions to apply to a mental model with correct features, the HMMA student possesses a functional model that is available for reasoning and testing.
HMMA Students were Able to Recognize their Approaches to the Problem and Constantly Monitor their Processes for the Construction of and Reasoning in Mental Models (Characteristic 4) Throughout all the processes of mental model construction and problem-solving, these HMMA students consciously and constantly monitored their processes of reasoning. The two students in this group each employed different approaches. For example, when determining the polarity of BF3, RE had a series of self-dialogues, posing questions to
herself and then answering them during each step of the problem-solving process. Also, when RE and JS were asked to build a model of 1, 2-dichloroethane (C2H4Cl2), a molecule with
novel geometry to the students, they could recognize and describe their strategy of visualizing the 3D molecular geometry. For instance, RE stated,
I just say, okay, you know this has got four, it’s going to be a tetrahedral. What I did was I divided it [the C2H4Cl2molecule] in half. I said, okay, if I just look at this half
and then [I ask], how is that shape going to be? Instead of trying to picture the whole thing at once, I broke it up so that I could see this is how this side is going to be, and this side is just pretty much the same thing attached to it. (RE, interview)
A molecule composed of two central atoms, like C2H4Cl2, is considered novel for
mental model construction in the mind of a general chemistry student. However, RE managed her mental model construction consciously, starting by reorganizing a symmetrical feature of the molecule. On the basis of this condition, she then simplified the molecular structure by dividing it in half and visualized a tetrahedral shape attached to the identical one. We use JS’s descriptions about his mental model of BF3as another example. During
the mental modeling processes, JS examined the problem: to determine whether BF3is a
polar or nonpolar molecule. He then constructed and used a mental model that contained only essential features in order to solve the problem.
JS: This is what I picture. I picture a boron right here and then fluorine, fluorine, fluorine, and that is about it. Of course, I know that they are bonded, but that’s all I really need to know: that it is a trigonal planar. Because in my picture, if I were drawing electron pairs, I mean, if there were some, I would add that in my picture. But it’s not in my picture because I know that the unshared electron pairs don’t exist. So that is all I need to know: that they are a trigonal planar, that there are three forces, they are equal forces, so they equally repel.
I: When this image emerged in your mind, could you do something with it? Did you do something with it to help you think about the geometry of BF3?
JS: In my mind, as far as the geometry, it was pretty simple, just what I went through before. I knew that there were only three things outside and they were all the same, so I knew they were trigonal planar. And I didn’t even really…in my mind I didn’t care whether or not they were moving. It didn’t really make any difference to me.
In JS’s illustration of his mental model, he represented the shape of BF3with four dots
and consciously eliminated features that were irrelevant to the problem, such as chemical bonding or symbols of the boron and fluorine atoms. He could also articulate propositions associated with the BF3 molecule and, for determining the geometry, distinguish those
essential conditions from irrelevant information.
Also, these HMMA Students Self-Checked and Verified their Mental Models and Answers Using an Alternative Approach if the Given Molecule was Relatively Novel to them (Characteristic 5) By undertaking the conscious monitoring described as the previous characteristic, HMMA students generally affirmed the correctness of their mental model and the derived answers at the end of the mental modeling processes. When the students encountered a molecule that was relatively novel to them, after they derived a conclusion using their mental model, they often used an alternative approach: for example, drawing out a Lewis dot structure of the molecule step-by-step and counting numbers of lone pairs and bonding pairs to verify the answer they had derived earlier in their mind. Also, they used numbers of lone pairs and bonds presented in the 2D Lewis dot structure to inspect and verify their mental model. It was not clear, however, whether or not these students mentally applied principles of VSEPR models to the 2D Lewis dot structure to infer its geometry before using the 2D Lewis structure to verify their mental model. The following excerpts described RE’s self-checking processes after she had used her mental model to determine that BF3is a nonpolar
molecule, and she said,
Now, I could be wrong just thinking that off the top of my head because the things like that, that is the kind of thing that you may end up drawing. Let’s see, boron’s got three…plus 21. [She adds up the overall number of valence electrons.] You may end up drawing it and find that there is a lone pair and that is going to change it a little bit.
[She is drawing a Lewis structure of BF3on the paper.] Okay, so it does not have any
lone pairs so…it’s going to be the same thing as what I said. (RE, interview) She further specified the situation when she would perform this self-checking: But if there was one that had lone pairs, I mean except for some of the ones that you see all the time like water, like that one [H2S], I know exactly what the Lewis
structure looks like in my head. But stuff that maybe you do not see everyday, I might do this in my head, but I would probably second guess myself. So I would have to sit down and draw it out just to make sure I was right and I didn’t leave something out and get the wrong answer. (RE, interview)
Moderate Level of Sophistication about Mental Modeling Ability (MMMA Group)
Students in the MMMA Group Constructed a 2D Lewis Structure Before Generating a Mental Model. When the Students were Familiar with the Geometry of a Given Molecule, they Could Visualize the Shape of a Molecule Without Seeing the 2D Representation (Characteristic 1b) Before generating a 3D mental model, most students in the MMMA group had to draw a 2D Lewis dot structure of a given chemical formula in order to count numbers of lone pairs and bonding pairs in the molecule. Information about numbers of lone pairs and bonding pairs was used to cue and visualize a corresponding geometrical structure from memory. Once the imaginary molecule was generated, these students could perform visualization operations similar to those of their peers in the HMMA group to rotate and to examine the imaginary molecule from different angles. This idea was interpreted from interview excerpts similar to the following excerpt, in which KU described his thinking process while determining the geometry of an H2S molecule:
I can picture what a tetrahedron looks like, so that is how I would base it… It’s kind of the pyramid at the bottom and this is sticking off the top (Fig.2), so I do picture the tetrahedron. When I first draw this [a Lewis dot structure, Fig.3], I just draw them out, and I don’t really know until I get the whole model drawn out of what the shape is going to be. Then after I determine that this would [be a] tetrahedral, then I can picture a tetrahedron and that would help me draw this diagram (Fig.2). But initially when I do the Lewis structure, I just draw it very two-dimensional. (KU, interview)
Evidence also shows that some students in this group experienced difficulties with perceiving the spatial structure of some molecular geometry in 3D. These students were able to visualize a 3D mental image of a molecule as if the molecule had a geometry that they saw frequently in the
Fig. 2 KU’s drawings of an H2S molecule
textbook or in class, for example, a bent molecule that had the same shape as a water molecule. Visualizing a 3D mental model became more difficult for these MMMA students if the molecule had a 3D geometry that was relatively novel to the individual. For instance, visualizing trigonal planar geometry was particularly difficult for SD. She said,
…they talk about this planar one all the time, which I don’t have a good concept of, the planar ones. Those are kind of hard because it almost seems like they are flat, but I’m not sure because we didn’t do a whole lot of examples where they are like that. (SD, interview) SD understood the VSEPR model and was able to apply it to some other geometric structures, but did not comprehend the shape of a trigonal planar. Therefore, SD had difficulty visualizing a 3D mental model by applying the VSEPR model to adjustments of her mental model and to inferences about a possible geometry. For SD, whether a specific molecular geometry (e.g., a trigonal planar) was available to recall and use in her long-term memory was critical for visualizing a 3D mental model.
Students in the MMMA group had some degree of ability to manipulate their mental model when compared to students in the HMMA group (Characteristic 2a). However, the MMMA students often held onto a rigid mental model rather than modify or adjust it on the basis of the problem’s new condition because the students were unaware of connections between the mental model and applied propositions (Characteristic 2b). Circumstances in which MMMA students neglected to perform task analysis or overlooked one condition of the problem during task analysis (Characteristic 3b) may also have contributed to these students’ possession of a rigid mental model. In either case, these students concluded that shape or structure of the mental model would not change when a new proposition would enter into the model. Some of the students who had a concrete model in front of them and were prompted to reason with the concrete model could adjust it on the basis of the problem’s conditions.
Mental models of the MMMA students had limited functions where the MMMA students could impose conditions of a problem and propositional statements they considered appropriate onto the mental model during the model construction. Once the mental model was constructed, students often concluded that the shape of the model would not change when an additional condition or proposition was added. For instance, in a previous interview question, AM had already constructed a mental model of H2S and stated that the bond angle of H2S would be
smaller than 109.5° because the lone pairs had greater repulsion than the H-S bonds. Thus, we assigned 2 points to AM concerning characteristic 2 (Table1) because she was able to infer the bond angle of H2S on the basis of the greater repulsion of lone pairs.
We then created a new condition—“If we replaced two hydrogen atoms [in H2S] with two
chlorine atoms, how would the bond angle change?”—to check whether or not AM would adjust her mental model by considering this new condition. AM started answering the question by drawing a Lewis structure for SCl2. Once she determined the number of lone
pairs and bonding pairs from the Lewis structure, she concluded that the geometry of SCl2
would be the same as that of H2S. She did not think that the bond angle would change
“because the sulfate is still tetrahedral, so the bond angle would be the same as with the hydrogens” (AM, interview). Instead of using the prior mental model of H2S as a template
Fig. 3 KU’s drawings of an H2S molecule
and modifying it for SCl2, AM started a new mental model construction. While constructing a
new mental model for SCl2, she neglected to consider the new condition of the problem, that
the S–Cl bonds would have greater repulsion between each other and would change the bond angle. Therefore, AM received 1 point on task analysis for characteristic 3.
The other MMMA student, SD, exhibited a similar cognitive path, saying that the bond angles for SCl2and for H2S would be the same since only changing the numbers of lone
pairs and bonds in a molecule could change the bond angle and geometry of the molecule. AM’s and SD’s cases revealed that they had followed a routine to approach a problem and had failed to understand how to apply chemical propositions to a 3D molecular structure and how to adjust its spatial geometry accordingly.
After being prompted by the interviewer, AM and SD reconsidered their answers regarding the size of chlorine atoms. They constructed a concrete model incorporating the new information about the size of chlorine atoms, and then adjusted the model to determine how the size of chlorine atoms influenced the bond angle. AM misinterpreted the context of the problem; thus, she considered the influence of the chlorine atom in terms of the atom’s electronegativity, rather than its size, which actually has a greater impact on bond angle. SD held onto her original mental model owing her partial understanding of the VSEPR model. AM’s and SD’s situations may imply that being able to generate a mental model and to perform operations of visualization is insufficient for the task at hand. To have a mental model that is functional for reasoning, an individual also needs to have a correct understanding of associated concepts, as well as have a correct understanding of how chemistry principles and propositions are connected to the mental model.
Limited or No Monitoring Relative to Their Mental Modeling Processes was Observed Among the MMMA Students (Characteristic 4). Also, Neither Self-Checking of Mental Models Nor Answers Based on an Alternative Approach were Observed in These Students (Characteristic 5) Students in the MMMA group often did not question the correctness of their mental model. The absence of monitoring during construction of mental models or during attempted applications of a mental model perhaps led to MMMA students’ possession of a rigid mental model rather than to the students’ use of the model as a thought-enhancing tool. Sometimes these students were uncertain about their answers that they had derived from their “mental model”-based inferences. These students suspected inaccuracies in their mental model, but undertook no further modification of a constructed model.
CR, for instance, applied the VSEPR model to constructing a 3D mental model of BF3that
contained only partially correct spatial information. He did not realize a flaw in his mental model: that the shape of BF3was a Y-shape rather than the shape of evenly spaced bonds. CR
concluded that BF3was a polar molecule because in his mental model, the sum of two dipole
moments pulling upward was greater than the single dipole moment pulling downward. The interviewer then asked him to build a concrete model. Building a concrete model of BF3
provided CR an opportunity to go through his thinking processes again as an alternative to examining the mental model. On the basis of the VSEPR model, he built a concrete model with a trigonal planar shape and with all bond angles equal to 120°. The interviewer prompted him to reconsider the difference between spatial information presented by the concrete model and spatial information presented by his mental model. He said,
CR: I never thought of that before, because…I mean I never made a model before. But this way [upward] to this way [downward] is essentially equal, same as this way [left] to this way [right], so now I think about that, kind of just another thought in my head, it makes me wonder.
I: Now would you change your answer saying this is polar or nonpolar?
CR: I still think it is polar. Wait, wait, wait, now I think about that, no I don’t. I think it is nonpolar now. Because they are all pulling in opposite directions symmetrically and they would cancel out. So I kind of changed my mind at this moment.
I: As you change your answer, do you use the image in your mind, to help you think about it?
CR: Yes I do. Because...see, before I was kind of…I forgot that, when I drew it, I drew these ones [two bonds pointing upward] closer together and I didn’t draw it symmetrically, I kind of confused myself and tricked myself into thinking it was polar. But like when you actually do it equally like this and symmetrically have a model in my head, oh, they are all pulling the same way. I’m, like, wait a second, this [up and down] and that [left and right] is the same span, so...
Metacognitive actions, such as monitoring or self-checking, did not take place when CR initially constructed and drew the Y-shape BF3molecule. CR’s reasoning with a rigid, 2D
Lewis structure with inaccurate spatial information led to a faulty conclusion that BF3was a
polar molecule. When CR was asked to work through the problem using an alternative approach, this symmetrical concrete model became a useful tool to provide him an opportunity to reconsider the balance of the dipole moment and to verify his unsymmetrical mental model. Evidence of students’ thinking flaws due to a lack of conscious or subconscious monitoring or evaluation may be hard to disclose in the excerpts. Yet the absence of both self-monitored mental modeling processes and critical evaluation of a mental model or conclusions is evident in light of a comparison between the MMMA students and the HMMA students.
Low Level of Sophistication about Mental Modeling Ability (LMMA Group)
Students in the LMMA Group Constructed a Mental Model by Recalling the Geometry of a Given Molecule Algorithmically on the Basis of Cues Such as Numbers of Lone Pairs and Bonds in a 2D Lewis Structure. When Recalling the Geometry from Long-Term Memory, these Students Often Drew out the Molecular Structure on a Paper and Rarely Visualized it as a Mental Representation (Characteristic 1) Similar to the student SD in the MMMA group, some students in the LMMA group had difficulties visualizing a certain molecular geometry. For example, when KA was asked to build a model of BF3, he built a model with
three B-F bonds in a T-shape. He said the following:
KA: So they are all single bonds. So it is just the trigonal, right? Like in geometry, this arrangement? I don’t have it memorized that well anymore.
I: Why is it not like this [a right trigonal planar], but looks like a T?
KA: Umm, I think it’s bent. It’s probably just something I don’t really know. Like I know how it looks like…. Like if it was tetrahedral, I wouldn’t know how it looked like, and if it’s octahedral I wouldn’t be sure. But I just know that’s how a bent molecule looks like. Just like the plane.... After it’s been bent, it’s going to look like it would be even, like a square box or whatever. (KA, interview)
Even though KA could name several geometrical shapes, such as trignoal, tetrahedral, or octahedral shapes, he did not conceptualize and visualize these structures in 3D. Instead, he memorized the associations between names and the corresponding geometrical structures in the textbook. Thus, memory about these associations faded away quickly. Although KA inferred that there were three single bonds in BF3from its Lewis structure and matched this information
with a name of a geometrical structure starting with the word‘trigonal’, this geometrical name did not lead to a correct pick of a 3D structure with correct spatial information.
While the LMMA students attempted to solve problems about molecular shape and polarity, drawing a 2D Lewis dot structure of a given formula was an essential step prior to the students’ construction of a mental model. The 2D Lewis structure provided different cues for visualizing a mental model depending on whether a specific molecular geometry was available to the memory. When a given molecule had a geometry that the students saw frequently in the textbook or in class (for example, a bent molecule), they used cues (for example, numbers of bonding and lone pairs in the Lewis structure) to recall its molecular geometry algorithmically. For instance, after TA drew a 2D Lewis structure of H2S, he
determined that the molecule shape would be bent. He said,
…because the molecular geometry will show up and that is the rule that it follows…. [The rule] is that if there are four bonding sites on a central atom and there are two [bonding pairs] that are there and then there are two sections of one, what would be lone pairs? So and that rule, for the molecular geometry would mean that it would be a bent molecule. (TA, interview)
Apparently, TA followed a certain rule to determine the molecular shape of H2S by
counting its numbers of lone pairs and bonds. In the following conversation, KA’s algorithmic strategy for dealing with molecular geometry was even more explicit. When KA used play-dough to show the geometry of H2S, he said the following:
KA: I can’t remember. So it’s tetrahedral. Two and two, so that’s bent, right? It’s a bent molecule?
I: Yeah. Tell me what just went through your mind?
KA: Just a graph. I counted up the total number of lone pairs and bonds and I know four is tetra. So it’s going to be a tetrahedral molecule. I think I just memorize the geometry. I see two and two and I see bent. That’s just my picture in my head. Something like that.
I: Okay. Tell me more about the details of the pictures in your mind. KA: It’s like a chart I memorized.
I: You refer to this one here [in the textbook]? KA: Yeah, that one.
[He then used a trigonal bipyramidal molecule with five electron pairs as an example to explain his strategy.]
KA: I pretty much just look at this column [with the number of lone pairs], I think. I know I’m going to have five [electron pairs] anyway. This is the number [of lone pairs] that’s important because that’s going to tell me if it’s seesaw, like one point there; and then like T [shape], the two points [lone pairs] there; and then three [lone pairs] for the linear. (KA, interview)
Earlier, KA had indicated that he could not visualize a geometric shape, such as a tetrahedral, in 3D. So what KA described here was based on his algorithmically memorizing 2D representations and word associations for both identifying the number of electrons and naming the geometry, such as“four is tetra” (KA, interview).
Some LMMA students neither understood the underpinning concepts, such as the VSEPR model, nor the rules of thumb to determine molecular geometry. In this case, these students either generated a mental model that had the same shape as the drawing of the 2D Lewis structure or simply used the drawing of the 2D Lewis structure as a tool for thinking without visualizing a mental model. When a novel molecule was given, such as BF3, the
LMMA students drew a 2D Lewis structure or generated a concrete model that had inaccurate spatial information. For these LMMA students, concepts of 3D molecular geometry had only a weak connection or no connection with the VSEPR model in their conceptual framework. They were not aware of which proposition (e.g., the VSEPR model) they should apply, why they should apply it, and what it would do to the model. For example, JT mistakenly applied propositions of electronegativity difference and bond polarity to a 2D, linear Lewis structure on paper. He determined that the geometry of H2S is
linear because the bonds on each side canceled each other and the lone pairs on each side also canceled each other. Similarly, KA and TA referred to the geometry of BF3as having a
T-shape, although the two students were uncertain about their own answers. This insufficient understanding may have been due to a lack of multiple types of prerequisite knowledge, which will be discussed in later sections on cross-case comparisons.
The LMMA students preferred to draw a 2D Lewis dot structure on paper as a thought-enhancing tool and then to apply propositions or conditions of a problem to the 2D Lewis structure for drawing an inference. Unless redrawn, the 2D Lewis structure on paper offered little opportunity for learners to put the molecular shape to a test against associated chemical principles (Characteristic 2b). Performing task analysis was not observed in LMMA students; they were satisfied with employing algorithmic strategies rather than with performing reasoning skills based on learned principles while thinking through molecular polarity problems (Characteristic 3). When they had a concrete model in front of them and were prompted to reason with the concrete model, only one student could adjust it on the basis of the conditions in the problem.
Rather than generate a mental model and use it as a tool for thinking, the LMMA students preferred to draw out a 2D Lewis dot structure and then apply propositions manually on paper. TA stated, “I usually, just drawing things out better, it helps me out more than putting it in my head and thinking” (TA, interview). And rather than think about how the difference of electronegativity between two atoms can influence the electron cloud distribution, the LMMA students drew arrows along the bonds on paper pointing from the less electronegative atom to the more electronegative one. We use KA’s case to demonstrate an LMMA student’s algorithmic inference while the students attempted to determine polarity of H2S on the basis of a 2D Lewis structure:
I: Would you say hydrogen sulfide is a polar or nonpolar molecule?
KA: It would be polar because of its geometry. I know that since sulfur is more electronegative, it would be…I know that there would be a dipole moment in this direction [on one S-H bond], and a dipole moment in this direction [on the other S-H bond].
I: Why did you draw in that direction?
KA: I really don’t know why. It’s what one of my tutors said to do. He said that since sulfur is more electronegative, you just draw the arrow in that direction and draw the tail. I: Pointing toward the one that is more electronegative? Okay.
KA: So it’s polar. That’s the net total. I think that’s the way it would pull.
An LMMA student like KA did not understand or was not aware of connections between chemistry principles and the model of a given molecule. Without reconciling connections between a molecular structure and associated chemical principles, the LMMA students would not recognize the important conditions and how they affect molecular polarity during task analysis. Reasoning on the basis of 2D Lewis structures, the LMMA students often derived wrong conclusions owing to incorrect spatial information attached to the 2D Lewis structure. After constructing a concrete model and using it as a thinking tool, an LMMA student was able to
infer a correct conclusion by adjusting the shape of the concrete molecule while applying a proposition to the model. For example, TA drew a Lewis structure of BF3in a T-shape on
paper. He applied a notion of electronegativity difference to each B-F bond on the 2D Lewis structure and concluded that BF3was a polar molecule because “these two would
cancel each other out, and then there is nothing up here for the fluorine to pull against, so it would pull the whole molecule downward” (TA, interview). When TA was asked to construct a concrete model, he first built the model in a trigonal pyramidal shape and then switched to a T-shape. When TA was asked about whether or not he still thought that BF3
was a polar molecule, he answered thus:
TA: I would still think so because regardless if it’s in a plane or if it’s a pyramid that these are still pulling, and it’s just leaving that [B-F bond] there. But because this is pulling it down, I think that would be its [shape]…[He spoke while pulling the B-F bond and adjusting the 3D molecule from a T-shape to a trigonal planar]…. Wait, then it would not be polar; it would be set up like that [a trigonal planar shape]. I: Why do you say that?
TA: Because now they [the three B-F bonds] all have equal distance around each other, and they would all be pulling their own way. This way [the T-shape], these two [B-F bonds] would just cancel each other out and they would be left with this one [bond]. But this way [the trigonal planar shape], all three are…I would probably say they would go like this [the trigonal planar shape], and it would be a non-polar. TA applied the notion of electronegativity difference to the concrete model and thought that one force would be left pulling downward after the other two forces would cancel each other. While he was pulling the bond on the concrete model, the shape of the model was changed from a T-shape to a right trigonal planar shape. The new shape prompted TA to reconsider the balance of forces among three B-F bonds and to change his answer about the polarity of BF3to“nonpolar.”
TA’s case implies that an LMMA student who reasons on the basis of a 2D Lewis structure is mentally disadvantaged by the rigid structure and inaccurate spatial information of the drawings. Unless it is redrawn, the 2D Lewis structure permits less flexibility and adjustability with which students can modify the model accordingly when an additional proposition or condition is imposed. This disadvantage may be diminished if a learner has a concrete, adjustable, physical model to use as a thinking tool. In our case, when TA constructed a concrete model to infer the shape and polarity of the molecule against the net dipole moment, he could adjust the model according to imposed propositions and derive a correct answer.
The LMMA Students did not Monitor Their Processes of Mental Modeling (Characteristic 4). At the End of the Inference or Reasoning, these Students did not Perform Self-Checking, Using an Alternative Approach, to Test or Inspect Mental Models or 2D Representations (Characteristics 5) While solving a problem, each LMMA student in this group made errors. TA, for instance, added the number of valence electrons incorrectly; thus, he considered H2S an
exception to the octet rule with only seven valence electrons (TA, interview). After drawing directions of bond polarity for H2S with the hydrogen atom toward the sulfur atom, KA
thoughtlessly applied the same directions to sulfur dichloride, thinking the direction of bond polarity was from the chlorine atom toward the sulfur atom. Without reviewing the condition of the problem carefully (e.g., the electronegativity difference between S-Cl and S-H bond), KA neglected that chlorine atoms had greater electronegativity and that the directions of bond polarity should be in the opposite direction. Both TA and KA did not monitor their thinking process nor evaluate answers at the end; therefore, they were not aware of mistakes they made
or conflicts in their explanations unless noted by the interviewer. Observations of these interviews indicate that neither self-checking to identify errors from the 2D representations nor monitoring of a given modeling process occurred.
Conclusions and Discussion
We conducted cross-case comparisons among HMMA, MMMA, and LMMA students to provide insights into learning impediments affecting problem-solving. The comparisons also helped reveal the role of mental modeling ability during problem-solving processes. We organized results of the comparisons into four assertions. Based on results of cross-case comparisons, the following sections discuss implications for teaching molecular geometry and polarity concepts.
Assertion 1 Both HMMA and MMMA students performed visualization skills to generate and operate a 3D mental model. These visualization skills were not observed in LMMA students. The LMMA students relied on drawings of 2D Lewis structures for inference.
Visualization skills are essential for students to comprehend a 3D structure and to understand concepts involving 3D spatial information such as molecular geometry and polarity. In this study, the observed visualization skills included visualizing a 3D object from its 2D representation (spatial visualization, Ferk et al.2003), imagining what a 2D representation would look like from a different angle in 3D (spatial orientation), and visualizing effects of operations such as rotation, or mentally manipulating the object (spatial relations).
Visualization skills were reported by HMMA and MMMA students, but not by LMMA students when thinking about molecular polarity. It is important to clarify that LMMA students in this study did possess some mental modeling ability. For example, JT visualized electrons moving around the nucleus of a sulfur atom in relation to a solar system. However, when solving problems about molecular geometry and polarity, JT relied on a 2D Lewis structure and did not apply his visualization skills to the molecular structure. LMMA students’ reliance on 2D Lewis structures for inference often resulted in incorrect answers. This could be because the 2D Lewis structure loses the depth of spatial information; therefore, the 2D Lewis structure does not facilitate inference and reasoning of a spatial nature (Wu and Shah 2004). For some students in the MMMA and LMMA groups (e.g., TA, while determining the polarity of BF3), the availability of a concrete, functional model
comprising accurate spatial information increased the students’ ability to infer a correct answer, but only if the propositions applied to the model were correct.
Assertion 2 2D Lewis structures provided two types of visual cues for a student to construct a mental model. A student’s level of mental modeling ability and his or her familiarity with the geometric structure of the given molecule determined the type of visual cues the individual used. Students in the HMMA group constructed a 3D mental model of a given molecule by forming and labeling imaginary atoms without drawing a 2D Lewis structure. Simultaneously, these students accounted for the numbers of bonds and lone pairs so they could then apply the VSEPR model to arrange the relative position of each atom and determine its 3D molecular geometry. For molecules not frequently seen in the textbook or lectures, the HMMA students drew a 2D Lewis dot structure either prior to the mental model construction or at the end, as a means of verifying their mental model. For most students in the MMMA and LMMA groups, drawing a 2D Lewis dot structure of a given molecule to verify total numbers of lone pairs and bonding pairs was a necessary step before the mental model could be generated.
For students in the HMMA and MMMA groups, a 2D Lewis structure provided cues about numbers of bonds and lone pairs in the molecule to ensure the constructed 3D mental model contained features sufficient for applying the VSEPR model and then for using it to determine the molecular geometry. Students in the LMMA group either used the cues for numbers of bonds and lone pairs in the Lewis structure to recall a stored mental image of 3D molecular geometry algorithmically, or directly used the 2D Lewis structure as a visual cue to generate a similar mental model. For a molecule the student had seen frequently and perhaps had memorized, an LMMA student could algorithmically match the numbers of lone pairs and bonding pairs with a corresponding 3D geometric structure. If the molecule was unfamiliar, however, MMMA and LMMA students would employ the 2D Lewis structure as a basis for inferring and reasoning.
Assertion 3 Three types of knowledge may influence students’ ability to manipulate mental models on the basis of an imposed proposition (characteristic 2). This characteristic is crucial for incorporating an individual’s content knowledge into his or her visual-spatial thinking ability.
Manipulating a mental model according to applied propositions is a vital characteristic for chemistry students. This characteristic enables a learner to comprehend how chemical principles are enacted and to determine the structure of or spatial information about a 3D object: for example, a learner could use the VSEPR model to predict the molecular structure or to determine the polarity of a molecule by summing up the net dipole moment in 3D. Thus, this characteristic is crucial in that it enables chemistry learners to incorporate learned knowledge into visual-spatial thinking ability.
In our cross-case comparisons, students in the HMMA group were able to manipulate their mental models as cognitive tools for drawing inferences and for justifying adjustments to the model on the basis of appropriate chemistry principles. Students who had a low score regarding the second characteristic of mental modeling ability generally concluded that the structure of their mental model would not change when they were asked to add an additional condition (e.g., replacing two hydrogen atoms in an H2S molecule with two
chlorine atoms) to the mental model. Wu and Shah (2004) described their finding that students with high spatial ability tended to perform better on chemistry tasks because they were able to mentally manipulate information in 3D or represent complex information visually. The comparison of students’ mental modeling ability provides direct evidence to support Wu’s and Shah’s conclusion.
Findings from our comparisons of HMMA, MMMA, and LMMA students performing characteristics 1 and 2 imply that three types of knowledge may have influenced a learner’s ability to switch from a 2D representation to a 3D mental image or to manipulate a mental model on the basis of imposed propositions. This knowledge is of three types:
1. comprehension of a molecule’s geometrical structure in 3D, so a learner can visualize its structure spatially,
2. understanding various chemistry principles (e.g., the principle of electrostatic force, the VSEPR model) that determine the spatial arrangement of electron pairs, and
3. ability to connect and apply chemistry principles to the spatial structure of the molecule. Learners’ unfamiliarity with some geometrical shapes (for instance, SD’s insufficient understanding relative to the spatial features of trigonal planar) may affect the learners’ efforts to learn about molecular shape and polarity. Other impediments include students’ insufficient understanding of how chemical principles could be applied to a molecular structure and why these propositions would influence its spatial formation in 3D. Our observations of the MMMA and LMMA students regarding characteristics 1 and 2 suggest
that a student’s lack of any of the three types of knowledge could hinder the student’s ability to solve a molecular polarity problem successfully.
Furió and colleagues (Furió and Calatayud1996; Furió et al.2000) attributed learners’ errors in thinking through a molecular polarity problem to functional reduction or functional fixedness. Our findings provide further information suggesting that MMMA or LMMA students’ thinking flaws may be ascribed to two reasons: one is a lack of one of the above three types of knowledge, and the other reason is learners’ lack of metacognitive skillfulness during mental modeling processes. We will discuss this metacognitive aspect in the next assertion.
Assertion 4 Metacognitive skills (characteristics 3, 4, and 5) are critical for successful mental model reasoning. HMMA students performed a series of metacognitive actions including task analysis, monitoring, and evaluation throughout the mental modeling process, but these actions were observed for only some MMMA students and for no LMMA students. The LMMA students were satisfied with employing algorithmic strategies rather than perform reasoning skills based on learned concepts or principles while thinking through molecular polarity problems.
HMMA students performed several manifestations of metacognitive actions while solving molecular polarity problems. These metacognitive actions included the following: 1. analyzing and recognizing conditions of a problem as well as relevant knowledge of
chemistry (characteristic 3);
2. overseeing processes of mental model construction to ensure that the constructed model contained accurate features and spatial information to solve the problem, recognizing employed problem-solving approaches, monitoring steps of mental modeling, and overseeing quality and accuracy of their knowledge to apply to a mental model (characteristic 4);
3. using an alternative approach, such as drawing out a Lewis structure on paper instead of keeping it in their mind, to verify a mental model and thereby to address any uncertain steps or answers (characteristic 5).
These characteristics of mental modeling ability reflect such manifestations of metacognitive skillfulness as task analysis, monitoring, and evaluation. These metacognitive actions, however, were not demonstrated by some MMMA students or any LMMA students. In our observations, these students often neglected to analyze conditions of a problem carefully or to reduce the complexity of the problem by considering, for example, only the molecular shape or electronegativity differences between bonded atoms when determining the polarity of a molecule. While solving a problem, these MMMA and LMMA students failed to recognize the mistakes in their mental model and/or in their steps associated with mental or concrete models. Sometimes these students suspected or recognized that their mental model or knowledge was incorrect, but took no action to address the issue.
Differences observed among HMMA, MMMA, and LMMA students in terms of metacognitive skillfulness provide direct evidence to support Gilbert’s (2005,2008) descriptions regarding metavisual capability and a continuum of this metavisualization development among chemistry learners. Monitoring a mental modeling process metacognitively would reduce the possibility of missing steps or neglected considerations of propositions during the problem-solving processes. Performing this action of monitoring would also help a learner to identify conditions of a problem so that the learner could determine whether or not his or her mental model contains features sufficient for the problem-solving task at hand. In addition, verifying a previously derived answer using an alternative approach provides learners an opportunity to identify and to correct errors at the end of the mental modeling process. HMMA students’ possessing of these three characteristics reduced to a minimum the number of errors in the