行政院國家科學委員會專題研究計畫 期末報告
神經新生與受損海馬迴之功能恢復
計 畫 類 別 : 個別型 計 畫 編 號 : NSC 100-2314-B-004-001- 執 行 期 間 : 100 年 08 月 01 日至 101 年 10 月 31 日 執 行 單 位 : 國立政治大學神經科學研究所 計 畫 主 持 人 : 賴桂珍 計畫參與人員: 碩士班研究生-兼任助理人員:林曉涵 公 開 資 訊 : 本計畫涉及專利或其他智慧財產權,2 年後可公開查詢中 華 民 國 102 年 01 月 31 日
中 文 摘 要 : 海馬迴是腦中負責學習與記憶的重要部位, 也是許多神經疾 病的發病點,像是阿茲 海莫症,失憶症,中風,癲癇,長期壓力 等等。如果能找到方法來修復腦部的病變, 將可造福很多 人。在成人或成鼠的大腦裡有兩個地方可以持續的進行神經 細胞新生, 海馬迴的粒細胞是其中一個。之前我發展出一套 雞尾酒治療方式 (包含神經滋養分子, 神經生長分子, 以及 豐富有刺激的環境) 來促進神經細胞新生及生存,並恢復海馬 迴 功能(Lai et. al., 2013a, submitted)。延續先前研究 成果, 本研究主要要利用我之前建立的模範系統來研究是否 神經細胞新生在恢復腦部功能扮演一個非常重要的角色。這 個研究主要包括下列四部份: (1)同時作雞尾酒治療並抑制神 經細胞新生,觀察在 沒有神經細胞新生的狀況下,是否雞尾酒 治療仍能恢復海馬迴功能,若不能恢復海馬 迴功能,表示在這 種治療下,神經細胞新生是必須的;若仍能恢復海馬迴功能, 表示 可能透過促進既有神經的重新連結來達到治療效果, (2)觀察並分析新神經細胞的分佈 狀況及形態(長度,分枝及 突觸)(3)在行為學習記憶測試完畢,短時間內利用分析具突 觸活化專一性之 immediate early gene 的表現來了解是否 新神經細胞參與了行為學習記憶測試,也就是說新神經細胞是 否已融入神經網路中。 移除腎上腺可以很專一的造成海馬迴粒細胞死亡而不影響腦 部任何其他區域, 我以此作為研究系統來進行神經細胞新生 及恢復腦部功能的研究, 並借此更加了解神經細胞新生與恢 復腦部功能的機制。 中文關鍵詞: 成鼠神經新生
英 文 摘 要 : The hippocampus is a brain region central to learning and memory and is a key target of many neurological diseases that have dramatic cognitive consequences, including Alzheimer's and other forms of dementia, stroke, epilepsy, and chronic stress. Discovering methods that reverse damage would dramatically improve health for many people. Hippocampal granule cells are one of the two cell pools that produce new neurons continuously in adult mammalian brains. Previously, I used a simple mammalian model system for ablation and regeneration of specific neurons (granule cells) for studying the ability of
regenerated neuron to restore cognitive and
al. 2013a, b. submitted). In this proposal, I take one step further to study if neurogenesis plays a critic role in the function recovery of damaged hippocampus.
Corticosterone, an adrenal stress hormone, is essential for the survival of granule cells. Bilateral removal of adrenal glands(ADX) leads to extensive granule cell death and causes memory deficits. This surgery is used to specifically eliminate granule cells in the hippocampus to study the regeneration of granule cells and restoration of functional brain circuitry. Many substances are known to accelerate neurogenesis, but there are few data regarding the restoration of functional brain network after increased neurogenesis. A cocktail treatment that I developed promoted neurogenesis and functional recovery of damaged hippocampus (Lai et. al. 2013a, submitted). The same model will be used in this proposal to address question further.
To determine whether neurogenesis is essential for the cocktail treatment to be effective, neurogenesis blocking strategies were used to decide if the
deficit re-emerged when neurogenesis is blocked. The morphology of the new granule cells were analyzed in detail after treatment, including length of neurites, density of mature spine, and post synaptic density. It is important for new granule cells to be
integrated to the circuitry to be functional.
Immediate early gene expression analysis is performed shortly after the behavior test to understand if the new granule cells are integrated in the network. To be specific, a synaptic-activation activated
immediate early gene expression were used as a marker.
1
行政院國家科學委員會補助專題研究
計畫
□期中進度報告
n
期末報告
神經新生與受損海馬迴之功能恢復
計畫類別:n個別型計畫 □整合型計畫
計畫編號:NSC 100-‐2314-‐B-‐004-‐001-‐
執行期間:100 年 8 月 1 日至 101 年 10 月 31 日
執行機構及系所:國立政治大學神經科學研究所
計畫主持人:賴桂珍
共同主持人:
計畫參與人員:林曉涵
本計畫除繳交成果報告外,另含下列出國報告,共 _0__ 份:
□移地研究心得報告
□出席國際學術會議心得報告
□國際合作研究計畫國外研究報告
處理方式:除列管計畫及下列情形者外,得立即公開查詢
n涉及專利或其他智慧財產權,□一年n二年後可公開查詢
中 華 民 國 102 年 1 月 25 日
目錄
中文摘要--- ii
英文摘要--- iii
前言--- 2
研究目的--- 2
文獻探討--- 2
研究方法--- 5
結果與討論--- 7
參考文獻--- 9
摘要 海馬迴是腦中負責學習與記憶的重要部位, 也是許多神經疾病的發病點,像是阿茲 海莫症,失憶症,中風,癲癇,長期壓力等等。如果能找到方法來修復腦部的病變, 將可 造福很多人。在成人或成鼠的大腦裡有兩個地方可以持續的進行神經細胞新生, 海 馬迴的粒細胞是其中一個。之前我發展出一套雞尾酒治療方式 (包含神經滋養分子, 神經生長分子, 以及豐富有刺激的環境) 來促進神經細胞新生及生存,並恢復海馬迴 功能(Lai et. al., 2013a, submitted)。延續先前研究成果, 本研究主要要利用我之前建 立的模範系統來研究是否神經細胞新生在恢復腦部功能扮演一個非常重要的角色。 這個研究主要包括下列四部份: (1)同時作雞尾酒治療並抑制神經細胞新生,觀察在 沒有神經細胞新生的狀況下,是否雞尾酒治療仍能恢復海馬迴功能,若不能恢復海馬 迴功能,表示在這種治療下,神經細胞新生是必須的;若仍能恢復海馬迴功能,表示 可 能透過促進既有神經的重新連結來達到治療效果,(2)觀察並分析新神經細胞的分佈 狀況及形態(長度,分枝及突觸)(3)在行為學習記憶測試完畢,短時間內利用分析具突 觸活化專一性之 immediate early gene 的表現來了解是否新神經細胞參與了行為學 習記憶測試,也就是說新神經細胞是否已融入神經網路中。 移除腎上腺可以很專一的造成海馬迴粒細胞死亡而不影響腦部任何其他區域, 我以 此作為研究系統來進行神經細胞新生及恢復腦部功能的研究, 並借此更加了解神經 細胞新生與恢復腦部功能的機制。 ii
Abstract
The hippocampus is a brain region central to learning and memory and is a key target of many neurological diseases that have dramatic cognitive consequences, including Alzheimer’s and other forms of dementia, stroke, epilepsy, and chronic stress.
Discovering methods that reverse damage would dramatically improve health for many people. Hippocampal granule cells are one of the two cell pools that produce new
neurons continuously in adult mammalian brains. Previously, I used a simple mammalian model system for ablation and regeneration of specific neurons (granule cells) for
studying the ability of regenerated neuron to restore cognitive and physiological
functions of the hippocampus (Lai et. al. 2013a, b. submitted). In this proposal, I take one step further to study if neurogenesis plays a critic role in the function recovery of
damaged hippocampus.
Corticosterone, an adrenal stress hormone, is essential for the survival of granule cells. Bilateral removal of adrenal glands(ADX) leads to extensive granule cell death and causes memory deficits. This surgery is used to specifically eliminate granule cells in the hippocampus to study the regeneration of granule cells and restoration of functional brain circuitry. Many substances are known to accelerate neurogenesis, but there are few data regarding the restoration of functional brain network after increased neurogenesis. A cocktail treatment that I developed promoted neurogenesis and functional recovery of damaged hippocampus (Lai et. al. 2013a, submitted). The same model will be used in this proposal to address question further.
To determine whether neurogenesis is essential for the cocktail treatment to be effective, neurogenesis blocking strategies were used to decide if the deficit re-emerged when neurogenesis is blocked. The morphology of the new granule cells were analyzed in detail after treatment, including length of neurites, density of mature spine, and post synaptic density. It is important for new granule cells to be integrated to the circuitry to be functional. Immediate early gene expression analysis is performed shortly after the behavior test to understand if the new granule cells are integrated in the network. To be specific, a synaptic-activation activated immediate early gene expression were used as a marker.
This model system provides the opportunity for regeneration of selectively and gradually lost neurons, regrowth of synaptic connectivity, and recovery of cognitive function. This work offers the promise to repair brain damage through neural circuit regeneration.
2 一前言
Objectives:
The hippocampus (HPC) is a key target of many neurological diseases, such as Alzheimer’s and other form of dementia, stroke, epilepsy, chronic stress which have dramatic cognitive consequences. The dentate gyrus(DG) subgranular zone of the hippocampus is the source of new born granule cells and is one of the 2 well known regions that continuously generate new neurons in adult mammalian brains. Previously, I used a simple mammalian model system for ablation and regeneration of specific neurons (granule cells) for studying the ability of regenerated neuron to restore cognitive and physiological functions of the hippocampus (Lai et. al. 2013a, b. submitted). The cocktail treatment used in the study is a combination of neurotrophic factors, growth factors, and environmental enrichment. This is a model system for studying the regeneration of selectively and gradually lost neurons, regrowth of synaptic connectivity, and recovery of cognitive function. This work offers the promise to repair brain damage through neural circuit regeneration. I would like to take one step further to expand this research for understanding the required conditions for replacing neural circuitry in the brain. 二研究目的
Overall Hypothesis: There are 3 possible explanations for the functional recovery of the
hippocampus after the cocktail treatment. (1) It promotes neurogenesis. Thus, new granule cells replace the function of lost neurons. (2) It increases the survival of granule cells (3) It improves the networking of existing neurons. Even though the treatment did increase the number of granule cells, one can’t rule out the possibility of improved networking.
I propose that the functional recovery of the hippocampus after the granule cell
degeneration is due to the regeneration of new granule cells from the endogenous pools of neurogenic stem/progenitor cells in the hippocampus and form appropriate connections in such a way as to restore normal function. The purpose of this research is to provide evidences to support the proposed hypothesis.
三 文獻探討
Background and Significance: The capacity of the brain to exhibit plasticity when cells
are lost due to injury or disease has been a core area for many decades in neuroscience research. Despite an impressive list of plastic processes, the progress on restoring neural circuits is strikingly limited. This reflects the extraordinary difficulty of the problem rather than shortcomings of the investigators. Nearly all of the work has involved one of two strategies: 1) acting on transmitter, modulator, or neurotrophin receptors, or 2) transplanting embryonic tissue or grafts derived from stem cells from a donor. The former strategy likely facilitates compensation in spared circuitry and there is no evidence for replacement of lost cells. The latter approach has been unimpressive in several ways: lack of long-term survival of grafted neurons,
lack of evidence that grafted cells function as neurons, lack of evidence for integration of transplants into normal pre- and post-synaptic information processing positions in
networks, and lack of availability of embryonic tissue (Lindvall & Hagell, 2002). Each of these is an important limitation. Even in instances of excellent graft survival with
multiple transplant locations, there are clear persisting functional deficits (Helene et al., 2003; Shetty and Turner, 1996; Turner and Shetty, 2003).
Recently a new set of opportunities has opened up based upon the surprising discovery that in the adult brain there are two pools of cells that continuously generate new cells, including neurons( Aimone et. al., 2010). One of these pools of neurogenic stem cells is centred on the subventicular zone of the lateral ventricular wall and the other, the focus of this proposal, is located in the dentate subgranular zone of the hippocampus(HPC) . Previously, I successfully developed a rat model which can: (1) selectively eliminate the dentate gyrus granule cells; (2) induce behavioral deficits associated with granule cell death (Lai et. al., 2013b, submitted); (3) promote generation of new neurons functional recovery by treating the animals with selected substances and/or environmental
stimulation (Fig. 1, Lai et. al., 2013a, submitted).
Selective elimination of hippocampal granule cells
Moderate level of the corticosterone (CORT), secreted by adrenal gland, is essential for hippocampal granule cell survival. Removal of adrenal glands
(adrenalectomy, ADX) can lead to specific granule cell death in the hippocampus within a few weeks without significant effects to other regions of the brain (Sloviter et al., 1989, 1993a, 1993b; Sousa et al., 1997). Thus, we will use this model to selectively remove granule cells. After ADX, corticosterone levels, weight gain, and salt intake, which are all affected by ADX, will be assessed in each ADX-rat to confirm that the adrenal glands were successfully removed. The lack of stimulation of the high-affinity glucocorticoid receptors leads to apoptotic cell death, only in granule cells. Thus, unlike with some other lesion methods there is a procedure available to evaluate side effects unrelated to granule cell death. Given the differences between abrupt vs. prolonged lesion processes, another attractive feature of ADX-induced granule cell death as a model, involves the relatively gradual loss of neurons, a feature more in line with neural degenerative diseases.
Deficits after hippocampal granule cell loss
Certain learning and memory processes are known to depend upon HPC circuit activity in rodents. Examples of tasks that show a large deficit after HPC damage in rats include the hidden platform version of the Morris water task (Morris et al 1982), Pavlovian
conditioned fear of context (Kim and Fanselow, 1992; Phillips and LeDoux, 1994; Sutherland and McDonald, 1990), and delayed recognition memory tasks involving visual or odor cues (Dudchenko et al. 2000; Prusky et al., 2004a). Even within these tasks it is possible to arrange variations in procedure that make the task more or less sensitive to HPC damage. Another issue concerns whether selective loss of granule cells produces behavioral deficits similar to those after complete (or nonselective) HPC damage.
Reliable deficits after lesions largely confined to the DG have been reported in the Morris water task, radial arm maze, Hebb-Williams maze, spatial pattern separation contextual fear conditioning, general activity levels, and exploration (Czurko et al., 1997; Gilbert et al., 2001; Jeltsch et al., 2001; Lee & Kesner 2004a & b 1983; Tandon et al., 1991; Xavier et al., 1999 ). Both home-based open field and object-context recognition tests showed there are robust significant differences between ADX and control animals (Lai et. al. 2013b, submitted). Although not all of these same behaviors have been measured in rats with loss of granule cells due to ADX. Sloviter and colleagues showed that residual CORT level after ADX predicts both extent of granule cell loss and the magnitude of impairment in spatial learning. Incomplete removal of adrenal tissue is almost certainly
4
the cause of spared granule cells. (McCormick, et al., 1997; Sloviter et al., 1989, 1993a, 1993b). The biggest effect of ADX appears on the first three trials of Morris water task training, a time when rats are displaying a variety of prepotent escape strategies and very little real “place” learning. some of the behavioural changes after ADX should be
reversible by
replacement CORT and some should not: the former reflecting deficits due to lack of glucocorticoid receptor stimulation and the latter reflecting the degeneration of granule cells and loss of information processes through dentate to HPC subfield CA3. The electrophysiological changes in DG occur with the degeneration of granule cells is another interesting issue which will not be studied in this proposal.
Hippocampal Neurogenesis
There are at least two brain regions in which neurogenesis in adult animals has been demonstrated, a forebrain subventricular zone (SVZ) and the HPC subgranular zone (SGZ) (Alvarez-Buylla and Garcia-Verdugo, 2002; Alvarez-Buylla and Lim, 2004; Erickson et al., 1998). It is the latter that is directly relevant to the aims of this proposal, although the majority of work so far on adult neurogenesis has been focused on the SVZ cells. It has been suggested that the SGZ cells be referred to a multipotent progenitors, or progenitors. There are several progenitor cell stages. 1) Type –1 cells have a radial-glia-like morphology and express the astrocytic antigen glia fibrillary acidic protein (GFAP). Type-1 cells can also give rise to GFAP positive S-100ß positive astrocytes. The
daughters of Type-1 cells either express S-100ß or neuron-specific doublecortin (DCX, expressed in immature neurons), but not both. 2) Type-2 cells give the appearance of migrating along the SGZ (Kempermann, et al., 2004). They are GFAP negative. 3) Type-3 cells represent a further maturation developing a much more rounded nucleus and cell body. They are still proliferative and express DCX. Type-3 cells after further
development become immature granule cells that are postmitotic. Mature granule cells are present 2-3 weeks after becoming postmitotic. They stop expressing DCX and calretinin, instead they express calbindin, and the postmitotic neuronal marker NeuN. The number of new cells that survive to maturity is a small fraction of the total that were born. The majority of expansion of this population occurs due to proliferation of cells after Type-1. (Brandt, et al., 2003). It is this huge surplus of proliferating cells neuronal lineage precursors that offers the opportunity to restore lost granule cells in the present proposal.
Regulation of the rate of neurogenesis is controlled by a variety of behaviorally derived factors and endogenous regulators. Wheel-running increases the rate of proliferation of Type-2 cells, but not Type-3 cells (Kempermann, et al., 2004a; Kronenberg, et al., 2003). Environmental enrichment can dramatically increase the number of new neurons in DG, but it may not increase proliferation of any of the cell types. environmental enrichment may primarily increase the proportion of neuronal precursors that are selected to survive to granule cell maturity (Kronenberg, et al., 2003).
A variety of neurotransmitters, hormones, modulators, and neurotrophins affect adult HPC neurogenesis. antagonists at the NMDA receptor increase proliferation (Seri and Alvarez-Bullya, 2002). Not all agents that affect proliferation have a similar effect on long-term survival of new cells. For example, estrogen, which decreases extracellular glutamate concentrations in DG via increase NMDA receptor number, increases
proliferation but does not have a reliable effect on long-term survival (Seri and Alvarez-Bullya, 2002). Certain serotonergic agonists increase proliferation and long-term survival of new granule cells. (Banasr, et al., 2004; Djavadian, 2004; Malberg et al., 2000). The basis for the stimulation of proliferation and survival in the dentate is unclear. CORT levels also determine proliferation rate. Elevating circulating CORT level decreases both proliferation and long-term survival; ADX increases proliferation (Cameron and Gould, 1994, 1996). Several neurotrophins and related factors have a potent effect in accelerating proliferation. These include epidermal growth factor (EGF), brain-derived neurotrophic factor (BDNF), insulin-like growth factor (IGF-2), fibroblast growth factor (FGF-2 or bFGF), vascular endothelial growth factor (VEGF), erythropoietin (EPO), and sonic hedgehog (shh) (Alvarez-Buylla and Lim, 2004). It should be noted that my previous work on using combinations of proliferation/neuronal survival factors with environmental enrichment stimuli in vivo with HPC neurogenesis to promote functional recovery after granule cell death (Lai et.al, 2013a submitted) is one of the pioneer works if not the first in the field have been done.
Are new granule cells functional? It is clear that adult neurogenesis produces cells that are in the correct locations, express granule cell specific antigens, normal dendritic arbour, and extend mossy fiber-type axons into CA3, all characteristics of mature granule cells (Kempermann, et al., 2004b; Stanfield and Trice, 1988). It was demonstrated in HPC slices that weeks after birth, new granule cells acquired the electrophysiological properties of normal older granule cells (DG. Van Praag et al. 2002). Taking advantage of the activity-dependent expression of c-fos, it was shown that over 80% of new granule cells show c-fos activation by Morris water task training (Jessberger, et al., 2003).
However, c-fos is an activity dependent immediate early gene in general, it is not synaptic activation dependent. Arc is a plasticity-related gene whose induction occurs soon after synaptic activation (Kawashima et. al., 2009, Yilmaz-Rastoder et. al., 2010). The transcripts of Arc (Activity-regulated cytoskeleton-associated protein) are
transported to the dendrites and protein synthesis occurs there.
In summary, there are several practical routes to enhance proliferation and long-term survival of new neurons in the HPC DG.
Significance and Implications.
The goal of the proposed project is to systematically evaluate whether the cocktail treatment repair damaged brain circuitry by promoting neurogenesis. If this is the case, then the results of the proposed project will be pivotal for research aimed at reversing the effects of neurological degenerative diseases and brain injury. Also, this project, by evaluating the effects of damage restricted to the DG on memory, will contribute to a greater understanding of general hippocampal function.
四研究方法
Methodology: Male Long Evan Rats, 3 months of age, will be used for surgery. Animals
are housed in pairs before surgery. They are under normal 12 hour light cycle with food and water ad libitum. All animal procedures will be performed according to protocols approved by appropriate Animal Care and Use Committees.
Surgery: bilateral adrenal removal (ADX) will be performed at age of 3 month. Sham
6
removed. At all times during all experiments rats will be housed in pairs or in condoes (6 animals, enriched environment) with continual access to water and 0.9% saline. All rats will be weighed each week. At the end of the experiment and before initiating CORT replacement, a blood sample will be taken and plasma CORT will be measured using a radioimmunoassay procedure (Maclennan et al., 2003). Because of the length of these experiments and that ectopic adrenal tissue or incomplete ADX have been shown in the literature, any rat who does not have a body weight that is 1 SD or more below the mean of the sham controls at 8 wk post-ADX, and preference for drinking saline over water, and CORT level <1µg/dl plasma will be considered to be incomplete ADX and will not participate in further experimentation (McCormick et al., 1997; Sousa et al., 1997).
Promote Neurogenesis and survival: Factors that can promote neurogenesis will be
carried in an osmotic pump which is surgically set up under the skin and the treatments are infused into ventricle. Assorted environmental enrichment will be used to increase the rate of new neuron survival. The treatments will combine with CORT replacement. It is necessary to give CORT in the ADX rats for the survival of the new neurons. CORT replacement alone is not expected to have an impact on recovery of circuitry or memory (Lai et. al., 2013a).
Measuring Neurogenesis: BrdU is packed in the osmotic pump to be infused into the
brain with the cocktail treatment together (Zhao and Lang, 2009). Animals will be perfused with 4% paraformaldehyde after done with behavior tests. Section of the hippocampus will be stained with neuron maker (NeuN) to determine the total number of granule cells. Antigen-positive cells will be counted in Z-sectioned 40 µ sections acquired using a Zeiss microscope, only in the granular layers and adjacent subgranular zones. Proliferation after ADX, ADX-CORT replacement, and all of our treatments will be quantified immunohistochemically in two ways: with BrdU and with Ki67. Ki67 is an antigen expressed by cycling cells for approximately 12-24 hr. Numbers of new neural precursors will be measured by labeling the DCX antigen and the number of these that are actively cycling will be assessed using DCX + Ki67 double-labeling. the number of new mature neurons will be assessed in separate groups of rats who receive BrdU injections. BrdU+NeuN and BrdU+S-100ß double-labeling will be applied to quantify new mature neurons and glia. The morphology analysis will be quantified by
fluorescence microscope with Stereology and Neurolucida software from MBF (Micro Bright Field Inc.).
Behavioral Tests: (1) Morris water task: rats receive 8 trials per day with a hidden
platform. Rats are released from the 4 cardinal compass points twice each day in a pseudorandom sequence. On odd-numbered days the platform is repositioned in a new hidden location, remaining there for all trials of that day, and on even-numbered days the platform remains in the same location as the previous day. On every trial we
automatically record time to find the platform, swim path length, heading error,
proportion of path in each quadrant and annulus, and swim speed. (2) Object recognition test: Two different shape of boxes will be used. Each one contains two identical objects inside. Animals will spend some time to get familiar with the box and associate the box with certain object. Then one of the object will be replaced with a different type of object. A normal animal will spend more time on the new object. The time animals spend on two different objects will be compared between treated animals and control animals.
Immediate early gene expression:
Many immediate early genes are translated in the soma. However, the transcripts of activity-regulated cytoskeleton associated protein(Arc), are transported to the dendrites and protein synthesis there. Arc is a plasticity-related gene whose induction occurs soon after synaptic activation (Kawashima et. al, 2009). The animals are euthanized 30minutes after the behavior test. The animals are perfused as described in”Measuring
Neurogenesis” section. The brain sections are immunostained with Arc antibody and secondary fluorescence antibody.
五結果與討論
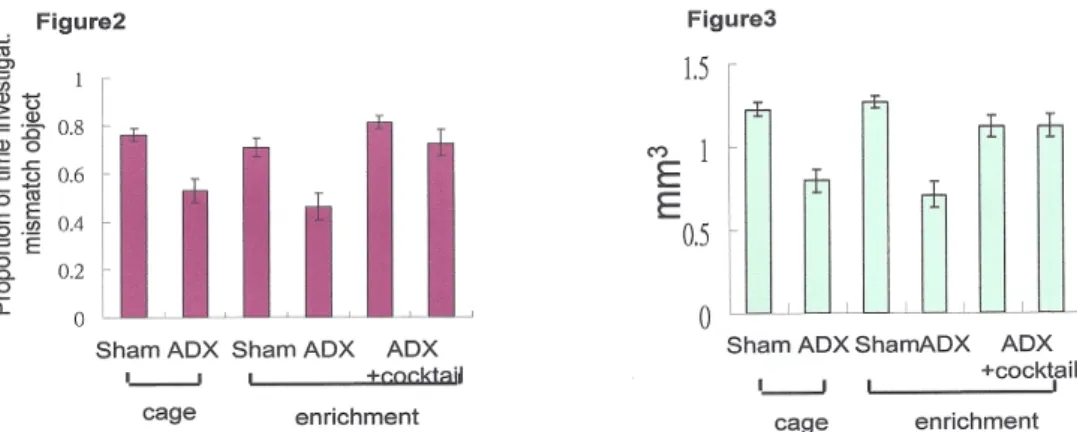
From my previous studies (figure1, 2 ), I have shown that ADX caused granule cell death in the hippocampus and lead to behavior deficit. The behavior deficit was reversed after re-‐population of the granule cells by the cocktail treatment (Lai et al 2013a, submitted). The volumes of dentate gyrus in the ADX animals were
repopulated as shown in figure 3.
8
Figure 2. ADX animals have behavior deficit in compare to sham animals no matter
if they were kept in a cage or in the enriched environment. After the cocktail treatment in conjunction with environmental enrichment, the performance of ADX animals in the behavior test similar to sham.
Figure 3. The volumes of dentate gyrus were reduced in ADX animals due to neuron
death. The lost granule cells were repopulated after the cocktail treatment. The volumes of dentate gyrus were similar to sham animals after the cocktail treatment.
New granule cells integration in the hippocampus
After regeneration of granule cells, immediate early gene (Arc, a marker for synaptic activation) expression was used as a marker (Kawashima et. al. 2009) to evaluate the involvement of new neurons in the network of the circuitry. Cocktail treated animals were subjected to behavior test, which is sensitive to granule cell degeneration. Shortly after the behavior test, animals were sacrificed and prepared for immediate early gene expression analysis to determine if new granule cells are involved in this behavior test. The immunohistochemistry results showed that Arc was expressed in both new and existing granule cells (figure 4). However, the number of new neurons in the granule layer express Arc is higher in treated ADX animals than non- treated.
Figure 4
(a) (b)
In figure 4(a), the section were immunostained with BrdU (new born cells, blue), immediate early gene (Arc, red), and NeuN (for mature neurons, green). The arrows point at the mature new born neurons and they are recruited in the neuron activity. In figure 4(b), the section were immunostained with BrdU (new born cells, green), doublecortin (immature neuron, red), and DAPI (for nucleus, blue). The arrows point at the immmature new born neurons at age of 1-‐2 weeks.
V. Referances
Alvarez-Buylla A. and Garcia-Verdugo JM. (2002)Neurogenesis in adult subventricular zone. J Neurosci. 22(3):629-34
Alvarez-Buylla, A and Daniel A. Lim (2004). For the Long Run: Maintaining Germinal Niches in the Adult Brain. Neuron, 41, 683–686.
Banasr M, Hery M, Printemps R, Daszuta A (2004). Serotonin- induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone.
Neuropsychopharmacol 29, 450-460.
Brandt MD, Jessberger S, Steiner B, Kronenberg G, Reuter K, Bick-Sander A, Von der Behrens W, Kempermann G. (2003). Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol Cell Neurosci 24, no.3, 603-613.
Aimone JB, Deng W, Gage FH. (2010) Adult neurogenesis: integrating theories and separating functions. Trends Cogn Sci.;14(7):325-37.
Cameron, HA; Gould, E (1994). Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neurosci 61, 203-209.
Cameron, H; Gould, E (1996). Distinct populations of cells in the adult dentate gyrus undergo mitosis or apoptosis in response to adrenalectomy. J Comp Neurol 369, 56-63 Czurko, A; Czeh, B; Seress, L; Nadel, L; Bures, J (1997). Severe spatial navigation deficit in the Morris water maze after single high dose of neonatal x-ray irradiation in the rat. Proc Nat Acad Sci USA 94, 2766-2771.
Djavadian, RL (2004). Serotonin and neurogenesis in the hippocampal dentate gyrus of adult mammals. Acta Neurobiol Exp 2004, 64: 189-200.
Dudchenko, PA; Wood, ER; Eichenbaum, H (2000). Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory but produce significant impairments on spatial span, recognition, and alternation. J Neurosci 20, 2964-2977.
Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn A-M, Nordborg C, Peterson DA, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med 4: 1313-1317. Gilbert, PE; Kesner, RP; Lee, I (2001). Dissociating hippocampal subregions: A double dissociation between dentate gyrus and CA1. Hippocampus, 11, 626-636.
Helene, J; Jason, Y; Elisabeth, A; Patricia, MP; Sarah, S; Luc, G; Sophie, C; Eliane, M; Jean-Christophe, C (2003). Transplantation of neurospheres after granule cell lesions in rats: cognitive improvements despite no long-term immunodetection of grafted cells. Behav Brain Res 143, 177-191.
Jeltsch, H; Bertrand, F; Lazarus, C; Cassel, JC (2001). Cognitive performances and locomotor activity following dentate granule cell damage in rats: Role of lesion extent
10
and type of memory tested. Neurobiol Learn Mem 76, 81-105.
Jessberger S, Kempermann G. (2003). Adult-born neurons mature into activity-dependent responsiveness. Eur J Neurosci 18:2707-2712.
Kempermann, G Jessberger, S Steiner, B Kronenberg, G (2004a). Milestones of neuronal development in the adult hippocampus. Trends in Neurosci 27, 447-452.
Kempermann, G Wiskott, L Gage, FH (2004b) Functional significance of adult neurogenesis. Curr Opin Neurobiol 14, 186-191.
Kim JJ, Fanselow M. (1992). Modality-specific retrograde amnesia of fear. Science 256:675-676.
Kronenberg, G. et al. (2003) Subpopulations of proliferating cells of the adult
hippocampus respond differently to physiologic neurogenic stimuli. J. Comp. Neurol. 467, 455–463
Lai, G.J., S.H. Lin, S. Spanswick, R. Sutherland, Behavior deficit, neurogenesis, and functional recovery after granule cell death in the hippocampus. 2013a, submitted
Lai, G.J., S.H. Lin, R. Sutherland, Behavioral deficits induced by granule cell death in the hippocampus after adrenalectomy. 2013b, submitted
Lindvall O, Hagell P. (2002) Role of cell therapy in Parkinson disease. Neurosurg Focus. 13(5):e2
Lee, I Kesner, RP (2004a). Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear-conditioning. Hippocampus 14, 301-310.
Lee, I Kesner, RP (2004b). Encoding versus retrieval of spatial memory: Double dissociation between the dentate gyrus and the perforant path inputs into CA3 in the dorsal hippocampus. Hippocampus, 14, 66-76.
功能變數代碼變更
Kawashima T, Okuno H, Nonaka M, Adachi-Morishima A, Kyo N, Okamura M, Takemoto-Kimura S, Worley PF, Bito H. (2009). Synaptic activity-responsive element in the Arc/Arg3.1 promoter essential for synapse-to-nucleus signaling in activated neurons. Proc Natl Acad Sci U S A., 106(1):316-21.
Maclennan, KM; Zheng, YW; Sheard, PW; Williams, SM; Darlington, CL; Smith, PF (2003). Adrenalectomy-induced cell death in the dentate gyrus: Further characterisation using TUNEL and effects of the Ginkgo biloba extract, EGb 761, and ginkgolide B. Hippocampus, 13, 212-225.
Malberg JE, Eisch AJ, Nestler EJ, Duman RS (2000). Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20: 9104–9110.
McCormick, CM; McNamara, M; Mukhopadhyay, S; Kelsey, JE. (1997). Acute corticosterone replacement reinstates performance on spatial and nonspatial memory tasks 3 months after adrenalectomy despite degeneration in the dentate gyrus. Behav Neurosci 111, 518-531.
Morris, R.G.M., Garrud, P., Rawlins, J., O’Keefe, J. (1982) Place navigation is impaired in rats with hippocampal lesions. Nature 297:681-683.
Phillips RG, LeDoux JE. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn Mem 1994;1:34-45. Prusky, GT; Douglas, RM; Nelson, L; Shabanpoor, A; Sutherland, RJ (2004a). Visual memory task for rats reveals an essential role for hippocampus and perirhinal cortex. Proc Nat Acad Sci USA 101, 5064-5068.
Seri, B Alvarez-Buylla, A (2002). Neural stem cells and the regulation of neurogenesis in the adult hippocampus. Clin Neurosci Res 2, 11-16.
Shetty, AK; Turner, DA (1996). Development of fetal hippocampal grafts in intact and lesioned hippocampus Prog in Neurobiol. 50, 597-653.
Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic. (2010). Microglia shape adult hippocampal
neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. Oct 8;7(4):483-95.
Sloviter, RS Valiquette, G Abrams, GM Ronk, EC Sollas, AL Paul, LA Neubort, S (1989). Selective loss of hippocampal granule cells in the mature rat brain after adrenalectomy. Science, 243, 535-538.
Sloviter, RS Dean, E Neubort, S (1993a) Electron microscopic analysis of
adrenalectomy-induced hippocampal granule cell degeneration in the rat: Apoptosis in the adult central nervous system. J Comp Neurol 330, 337-351.
Sloviter, RS Sollas, AL Dean, E Neubort, S (1993b) Adrenalectomy-induced granule cell degeneration in the rat hippocampal dentate gyrus: Characterization of an in vivo model of controlled neuronal death. J Comp Neurol 330, 324-336.
Sousa, N Madeira, MD Paula-Barbosa, MM (1997). Structural alterations of the hippocampal formation of adrenalectomized rats: An unbiased stereological study. J Neurocytol 26, 423-438.
Stanfield BB, Trice JE (1988). Evidence that granule cells generated in the dentate gyrus of adult rats extend axonal projections. Exp Brain Res 72, 399-406.
Sutherland, R. J. (1985). The navigating hippocampus: an individual medley of movement, space, and memory. In G. Buzsáki & C. H. Vanderwolf (Eds.), Electrophysiology of the Archicortex. Budapest: Akadémiai Kiadó, pp. 255-279. Sutherland, R. J. & McDonald, R. J. (1990). Hippocampus, amygdala, and memory deficits in rats. Behavioural Brain Research, 37, 57-79.
Sutherland, R. J., Whishaw, I. Q., & Kolb, B. (1982). Spatial mapping: definitive
disruption by hippocampal and frontal cortex damage in the rat. Neuroscience Letters, 31, 271-276.
Sutherland, R. J., Whishaw, I. Q., & Kolb, B. (1983). A behavioural analysis of spatial localization following electrolytic, kainate-, or colchicine-induced damage to the hippocampal formation in the rat. Behavioural Brain Research, 7, 133-153. Tandon, P Barone, S Drust, EG Tilson, HA (1991). Long-term behavioral and
neurochemical effects of intradentate administration of colchicine in rats. Neurotoxicol 12, 67-78.
Turner, DA; Shetty, AK (2003). Clinical prospects for neural grafting therapy for hippocampal lesions and epilepsy. Neurosurgery 52, 3, 632-641.
Van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH (2002). Functional neurogenesis in the adult hippocampus. Nature 415:1030-1034.
Xavier, GF; Oliveira, FJB; Santos, AMG (1999). Dentate gyrus-selective colchicine lesion and disruption of performance in spatial tasks: Difficulties in "place strategy" because of a lack of flexibility in the use of environmental cues? Hippocampus 9, 668-681.
Yilmaz-Rastoder E, Miyamae T, Braun AE, Thiels E. (2010), LTP- and LTD-inducing stimulations cause opposite changes in arc/arg3.1 mRNA level in hippocampal area CA1
12 in vivo. Hippocampus. Sep 7. [Epub ahead of print] Zhao M, Janson Lang AM, J Neurosci Methods. (2009)
184(2):327-31.Bromodeoxyuridine infused into the cerebral ventricle of adult mice labels nigral neurons under physiological conditions--a method to detect newborn nerve cells in regions with a low rate of neurogenesis.
國科會補助計畫衍生研發成果推廣資料表
日期:2013/01/31國科會補助計畫
計畫名稱: 神經新生與受損海馬迴之功能恢復 計畫主持人: 賴桂珍 計畫編號: 100-2314-B-004-001- 學門領域: 幹細胞/再生生物醫學無研發成果推廣資料
100 年度專題研究計畫研究成果彙整表
計畫主持人:賴桂珍 計畫編號: 100-2314-B-004-001-計畫名稱:神經新生與受損海馬迴之功能恢復 量化 成果項目 實際已達成 數(被接受 或已發表) 預期總達成 數(含實際已 達成數) 本計畫實 際貢獻百 分比 單位 備 註 ( 質 化 說 明:如 數 個 計 畫 共 同 成 果、成 果 列 為 該 期 刊 之 封 面 故 事 ... 等) 期刊論文 0 0 0% 研究報告/技術報告 0 0 100% 研討會論文 0 1 100% 篇 論文著作 專書 0 0 100% 申請中件數 0 0 100% 專利 已獲得件數 0 0 100% 件 件數 0 0 100% 件 技術移轉 權利金 0 0 100% 千元 碩士生 1 0 70% 博士生 0 0 100% 博士後研究員 0 0 100% 國內 參與計畫人力 (本國籍) 專任助理 0 0 100% 人次 期刊論文 0 1 70% 研究報告/技術報告 0 0 100% 研討會論文 0 1 100% 篇 論文著作 專書 0 0 100% 章/本 申請中件數 0 0 100% 專利 已獲得件數 0 0 100% 件 件數 0 0 100% 件 技術移轉 權利金 0 0 100% 千元 碩士生 0 0 100% 博士生 0 0 100% 博士後研究員 0 0 100% 國外 參與計畫人力 (外國籍) 專任助理 0 0 100% 人次其他成果
(
無法以量化表達之成 果如辦理學術活動、獲 得獎項、重要國際合 作、研究成果國際影響 力及其他協助產業技 術發展之具體效益事 項等,請以文字敘述填 列。) none 成果項目 量化 名稱或內容性質簡述 測驗工具(含質性與量性) 0 課程/模組 0 電腦及網路系統或工具 0 教材 0 舉辦之活動/競賽 0 研討會/工作坊 0 電子報、網站 0 科 教 處 計 畫 加 填 項 目 計畫成果推廣之參與(閱聽)人數 0國科會補助專題研究計畫成果報告自評表
請就研究內容與原計畫相符程度、達成預期目標情況、研究成果之學術或應用價
值(簡要敘述成果所代表之意義、價值、影響或進一步發展之可能性)
、是否適
合在學術期刊發表或申請專利、主要發現或其他有關價值等,作一綜合評估。
1. 請就研究內容與原計畫相符程度、達成預期目標情況作一綜合評估
□達成目標
■未達成目標(請說明,以 100 字為限)
□實驗失敗
□因故實驗中斷
■其他原因
說明:
原申請三年經費,但實際拿到一年經費2. 研究成果在學術期刊發表或申請專利等情形:
論文:□已發表 □未發表之文稿 ■撰寫中 □無
專利:□已獲得 □申請中 ■無
技轉:□已技轉 □洽談中 ■無
其他:(以 100 字為限)
3. 請依學術成就、技術創新、社會影響等方面,評估研究成果之學術或應用價
值(簡要敘述成果所代表之意義、價值、影響或進一步發展之可能性)(以
500 字為限)
海馬迴是腦中負責學習與記憶的重要部位,也是許多神經疾病的發病點,像是阿茲海莫 症,失憶症,中風,癲癇,長期壓力等等。如果能找到方法來修復腦部的病變,將可造福 很多人。在成人或成鼠的大腦裡有兩個地方可以持續的進行神經細胞新生,海馬迴的粒細 胞是其中一個。之前我發展出一套雞尾酒治療方式(包含神經滋養分子,神經生長分子, 以 及豐富有刺激的環境)來促進神經細胞新生及生存,並恢復海馬迴功能(Lai et. al., 2013a, submitted)。延續先前研究成果,本研究主要要利用我之前建立的模範系統來研 究是否神經細胞新生在恢復腦部功能扮演一個非常重要的角色。就未來應用層面來看,利用個體本身內生性'幹細胞'來再生細胞將可免除手術不便,幹 細胞來源的問題,以及異體移植所產生的排斥。