PRENATAL DIAGNOSIS
Prenat Diagn 2007; 27: 431–434.
Published online 13 February 2007 in Wiley InterScience (www.interscience.wiley.com) DOI: 10.1002/pd.1702
Midtrimester maternal serum inhibin A levels after
multifetal pregnancy reduction
Heng-Ju Chen1,2, Lee-Wen Huang1,2, Yu-Hung Lin1,2, Kok-Min Seow1, Bih-Chwen Hsieh1,2, Jiann-Loung Hwang1,2,3* and Chii-Ruey Tzeng3
1Department of Obstetrics and Gynecology, Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan 2College of Medicine, Fu Jen Catholic University, Sinchuang City, Taipei, Taiwan
3Department of Obstetrics and Gynecology, Taipei Medical University, Taipei, Taiwan
Objective To investigate the relationship between the maternal serum inhibin A concentrations and the number of fetuses. Further, the maternal serum inhibin A levels for twin pregnancies and multiple pregnancies reduced to twins in the second trimester were compared.
Methods Three groups of women with pregnancies following in vitro fertilization and embryo transfer were recruited for this study. Groups 1, 2 and 3 included 20 singleton pregnancies, 37 twin pregnancies, and 35 multifetal pregnancies, respectively. In group 3, multifetal reduction was performed during 10 – 12 weeks of gestation. Blood samples were obtained longitudinally at 10th, 12th, 15th and 18th week of gestation. Results There was a significant association between the number of fetuses and maternal plasma inhibin A prior to multifetal reduction. The inhibin A levels were not significantly different between twin and multifetal reduced twin pregnancies at 15th and 18th weeks of gestation.
Conclusion In multifetal reduction to twin pregnancies, the maternal serum levels of inhibin A decrease to the level of twin pregnancies during the second trimester. Therefore, inhibin A may be effectively used as a marker for Down syndrome screening in cases of twin pregnancy following multifetal reduction. Copyright 2007 John Wiley & Sons, Ltd.
KEY WORDS: multifetal reduction; inhibin A; second-trimester pregnancy; Down syndrome screening
INTRODUCTION
Maternal serum inhibin A level is raised in Down syn-drome pregnancies as compared to normal pregnan-cies (Aitken et al., 1996; Wallace et al., 1996; Wen-strom et al., 1997) and has been shown to be a useful marker in prenatal screening for Down syndrome dur-ing the second trimester of pregnancy (Spencer et al., 1996). Traditionally, triple-test (alpha-fetoprotein (AFP), human chorionic gonadotrophin (HCG), and unconju-gated estriol (uE3)) has been used for Down syndrome
screening (Chard and Macintosh, 1995). This test can incorporate inhibin A as a fourth marker for Down syn-drome, such that the detection rate for Down syndrome can be improved from 59 to 70%. (Wald et al., 1996).
Data about the combined screening efficacy of AFP, HCG, and uE3 in twin pregnancies are less robust
than in singletons (Barnabei et al., 1995). Down syn-drome screening in twin pregnancies can be estimated based on mathematical modeling (Neveux et al., 1996). Inhibin A levels are twice as high in twin pregnancies compared with singleton pregnancies so that its values can be adjusted for Down syndrome screening in twin pregnancies (Watt et al., 1996).
*Correspondence to: Jiann-Loung Hwang, Department of Obstet-rics and Gynecology, Shin-Kong Wu Ho-Su Memorial Hospital, No. 95 Wen Chang Road, Shih Lin District, Taipei, Taiwan. E-mail: m004983@ms.skh.org.tw
The incidence of multiple pregnancies has increased since the development of assisted reproductive technolo-gies (Schenker et al., 1981). First-trimester multifetal pregnancy reduction from high-order gestations to twins has been often undertaken to decrease the perinatal mor-bidity and mortality (Evans et al., 1994). Several studies have found midtrimester maternal serum AFP levels to be remarkably elevated following multifetal reduction, suggesting that this analyte cannot be used for serum screening (Grau et al., 1990; Shulman et al., 1996). Goodwin et al. reported that a case following the loss of one fetus in a twin pregnancy had an extremely ele-vated second-trimester serum inhibin A level (Goodwin et al., 2000). Consequently, whether the serum inhibin A used as a marker for Down syndrome screening in twin pregnancy following multifetal reduction remained unknown.
The purposes of this study were to investigate the second-trimester inhibin A levels in twin pregnancies and multiple pregnancies reduced to twins; to examine whether the procedure of multifetal reduction has an effect on maternal serum inhibin A levels; and to evaluate the relationship between the maternal serum inhibin A levels and number of fetuses. To the best of our knowledge, this is the first study to examine the effect of multifetal reduction on maternal serum inhibin A levels in the second-trimester pregnancy.
Copyright 2007 John Wiley & Sons, Ltd. Received: 3 August 2006
Revised: 18 December 2006 Accepted: 20 December 2006 Published online: 13 February 2007
432 H.-J. CHEN ET AL.
MATERIALS AND METHODS
Subjects and samples
Three groups of Taiwanese women with pregnancies achieved following superovulation and in vitro fertil-ization and embryo transfer were recruited for studies. Body mass index was between 21 and 28 kg/m2. Groups 1 included 20 singleton pregnancies (age range 25–36, median 30) and blood samples were obtained at 10th week of gestation. Group 2 included 37 twin pregnan-cies (age range 27–39, median 32) and blood sample obtained longitudinally at 10th, 12th, 15th and 18th week of gestation. Group 3 comprised 35 women with multife-tal pregnancies (age range 24–39, median 31), undergo-ing fetal reduction to twin pregnancies (three fetuses, n= 24; four fetuses, n = 6, five fetuses, n = 5). In this group ultrasound-guided embryo reduction to twins was performed by transabdominal injection of potassium chloride into the fetal heart between 10 and 12 week of gestation. Maternal blood samples were obtained before embryo reduction at 10 weeks and at subsequent vis-its at 12, 15 and 18 weeks. Ovarian stimulation was performed in accordance to a gonadotrophin releas-ing hormone analogues (Supremon, Hoechst; Frankfart, Germany) desensitization protocol. All patients gave informed consent to participate in the study. The blood samples were stored at−40◦C until assay.
Assays
Dimeric inhibin A concentrations were measured using a commercial available kit which is a solid phase sandwich enzyme-linked immunosorbent assay (ELISA) (Serotec, Oxford Bio-Innovarion Ltd, UK). It used the monoclonal antibody specific for the βA subunit of inhibin. Before
the ELISA, samples and standards were mixed with 6% sodium dodecyl-sulphate and heated to 100◦C for 3 min, then cooled and incubated at the room temperature with 10% hydrogen peroxide for 30 min. These procedures can enhance the specificity and sensitivity of the ELISA, and even allow analysis of hemolysed samples. The blood samples were assayed in duplicate. The intra-plate and inter-plate coefficients of variation were <10% and sensitivity of the assay was <20 pg/mL.
Statistics
Inhibin A levels were expressed as median values (range). Nonparametric test with Kruskal-Wallis was used to determine whether the number of fetuses was significantly associated with inhibin A concentration. Mann–Whitney U test was performed to compare the difference of inhibin A level at 12th, 15th and 18th week of gestation between twin and multifetal reduced twin pregnancies. Statistical analyses were performed with the software SPSS Pack 10.0, differences were considered significant when P < 0.05.
RESULTS
Before multifetal reduction, there was a significant association between the number of fetuses and maternal plasma inhibin A (P < 0.001); the median (range) inhibin A concentration for fetus numbers are shown in Table 1. Data for inhibin A levels after fetal reduction based on the number of fetuses at conception were shown in Table 2. The circulating levels of inhibin A in women after multifetal pregnancies reduced to twins (Group 3) and nonreduced twin pregnancies (Group 2) pregnancies at 12th, 15th and 18th weeks of gestation were shown in Table 3. The concentrations of inhibin A in Group 2 and Group 3 were converted to multiple of median were presented in Table 4. The inhibin A concentrations were gradually decreased during this period. The inhibin A levels between twin and multifetal reduced twin pregnancies were significant different at only 12th weeks of gestation (P < 0.05). Subsequently, inhibin A levels were not significantly different between
Table 1 — Maternal serum concentrations of inhibin A (pg/mL) at 10 week of gestation by starting number of fetuses. Values are given as median with the range in parentheses
Number of fetuses N Inhibin A concentration 1 20 338.2 (102.8 – 881.8) 2 37 703.6 (158.2 – 1723.3) 3 24 1137.7 (551.3 – 2300.4) 4 6 1640.2 (1335.6 – 2498.4) 5 5 1801.1 (989.5 – 2095.3)
Table 2 — Maternal serum concentrations of inhibin A (pg/mL) after fetal reduction based on the number of fetuses at conception. Values are given as median with the range in parentheses
Number of fetuses Gestation (week) 3→ 2 (n = 24) 4→ 2 (n = 6) 5→ 2 (n = 5) P values 12 980.5 (461.2 – 2010.9) 1021.1 (588.2 –1981.4) 755.3 (392.4 – 1354.7) 0.387 15 467.7 (203.1 – 1258.6) 708.5 (412.5 – 939.5) 368.9 (236.3 – 580.9) 0.109 18 343.6 (208.5 – 762.4) 395.0 (321.5 –594.0) 307.9 (135.0 – 425.5) 0.130 Kruskal-Wallis test.
Copyright 2007 John Wiley & Sons, Ltd. Prenat Diagn 2007; 27: 431–434.
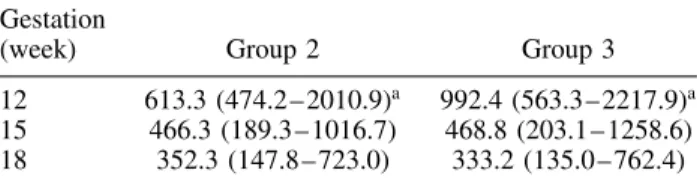
INHIBIN A IN FETAL REDUCTION 433 Table 3 — Maternal plasma levels of inhibin A (pg/mL) in
nonreduced twin pregnancies (Group 2, n= 37) and multifetal pregnancies reduced to twins (Group 3, n= 35). Values are given as median in parentheses. The range and number of cases are shown
Gestation
(week) Group 2 Group 3
12 613.3 (474.2 – 2010.9)a 992.4 (563.3 – 2217.9)a
15 466.3 (189.3 – 1016.7) 468.8 (203.1 – 1258.6) 18 352.3 (147.8 – 723.0) 333.2 (135.0 – 762.4)
aP < 0.05 (Mann–Whitney U test).
Table 4 — Maternal serum levels of inhibin A in nonfetal reduced twin pregnancies (Group 2, n= 37) and selective fetal reduced to twin pregnancies (Group 3, n= 35). The values are expressed as multiples of the median (range) for gestation of the singleton pregnancy following in vitro fertilization and embryo transfer
Gestation
(week) Group 2 Group 3
12 2.05 (1.60 – 6.78)a 3.35 (1.89 – 7.48)a
15 2.07 (0.84 – 4.51) 2.08 (0.90 – 5.59) 18 2.04 (2.87 – 4.25) 1.96 (0.79 – 4.78)
aP < 0.05 (Mann–Whitney U test).
Table 5 — Obstetric complications in nonfetal reduced twin pregnancies (Group 2, n= 37) and selective fetal reduced to twin pregnancies (Group 3, n= 35)
Group 2 (n)
Group 3 (n) Birth weight <10th percentile 8.1% (3) 11.4% (4) Preterm delivery (<37 weeks) 21.6% (8) 17.1% (6)
Preeclampsia 0 0
Gestational diabetes 2.7% (1) 0
twin and multifetal reduced twin pregnancies at 15th and 18th weeks of gestation.
Obstetric complications in Group 2 and Group 3 were shown in Table 5. There was no significant difference between the two groups in any of complications consid-ered.
DISCUSSION
The finding of this study indicated that in multifetal pregnancies circulating levels of inhibin A increase with number of fetuses, suggesting that feto-placenta unit is the source of inhibin A, confirming previous report (Lockwood et al., 1998). Subsequent to multifetal pregnancy reduction to twins, the maternal serum levels of inhibin A were significantly higher than those of twin pregnancies at 12 weeks of gestation and then fall to the level similar to those of nonreduced twin pregnancies at 15 and 18 weeks of gestation. These data suggest that fetal reduction causes fetoplacental death and therefore some decline in the production of inhibin A by the
surviving placental tissue is similar to the nonreduced twins.
Maternal serum inhibin A levels during pregnancies following multifetal reduction differ from serum AFP levels. In multifetal pregnancies, AFP increase with the number of fetuses. However, following fetal reduction to twins, the AFP concentration remains increased for at least 8 weeks. The most likely explanation for this is that AFP, which is stored in fetal tissues, is released into the amniotic fluid during tissue break down, from where it is absorbed in the maternal circulation (Abbas et al., 1994). Although the levels of inhibin A and AFP are proportionate to the number of fetuses, the differences in maternal circulating levels of inhibin A and AFP following fetal reduction may reflect their main different origins, which are placenta and fetus respectively.
Triple-test screening (AFP, HCG, and unconjugated estriol (uE3)), performed during the second trimester,
has become a common clinical practice to identified pregnancies at risk for chromosomal anomalies (Cuckle et al., 1987). Multifetal reduction of high-order fetal numbers to twins results in elevated serum AFP lev-els at midtrimester maternal blood sampling, whereas hCG and uE3 levels remain essentially unaffected (Rot-mensch et al., 1999). Maternal serum inhibin A levels for Down syndrome pregnancies are reported to be sig-nificantly elevated during the second trimester of preg-nancy (Spencer et al., 1996). Further, serum inhibin A levels are around twice as great for twin pregnancies as is the case for singleton pregnancies, such that the value of inhibin A levels can be adjusted for Down syn-drome screening in twin pregnancies (Watt et al., 1996). In the present study, serum inhibin A levels did not appear to differ significantly between twin and multi-fetal reduced twin pregnancies at 15th and 18th weeks of gestation. Although one study has already reported that inhibin A concentrations are not different between reduced and nonreduced twins in the second trimester (Maymon et al., 2006), we provide the novel data con-cerning the levels of inhibin A at several time points following fetal reduction. This result is an important contribution toward our understanding of the degener-ation of inhibin A in human serum. Therefore, inhibin A may be substitute for AFP as a marker for Down syn-drome screening in twin pregnancy following multifetal reduction in the second trimester.
This study was restricted to in vitro fertilization preg-nancies and it is unclear whether maternal serum inhibin A levels may be influenced by method of conception. Wald et al. found no significant differences in second-trimester maternal serum levels of inhibin A between singleton IVF and spontaneous conceived pregnancies Wald et al., 1999. However, other studies showed that inhibin A levels are increased in IVF pregnancy versus spontaneous singleton pregnancies (Lambert-Messerlian et al., 2006; Maymon et al., 2006). In twins, the mater-nal inhibin A level in the second trimester was no difference between IVF and spontaneous pregnancies (Maymon et al., 2006). Therefore, it would appear that inhibin A could be effectively used as a serum maker for Down syndrome in IVF twin pregnancies during the second trimester.
Copyright 2007 John Wiley & Sons, Ltd. Prenat Diagn 2007; 27: 431–434.
434 H.-J. CHEN ET AL.
In conclusions, this study demonstrated that maternal serum inhibin A concentrations increase with number of fetuses. For cases of multifetal reduction to twin pregnancies, maternal serum levels of inhibin A fall to those of twin pregnancies in the second trimester. Unlike AFP, mutifetal reduction dose not cause elevation of inhibin A. Therefore, inhibin A may be effectively used as a marker for Down syndrome screening in twin pregnancies following multifetal reduction.
ACKNOWLEDGEMENT
This study was sponsored by grant SKH-TMU-92-18 from the Shin Kong Wu Ho-Su Memorial Hospital.
REFERENCES
Abbas A, Johnson M, Bersinger N, Nicolaides K. 1994. Maternal alpha-fetoprotein levels in multiple pregnancies. Br J Obstet Gynaecol 101: 156–158.
Aitken DA, Wallace EM, Crossley JA, et al. 1996. Dimeric inhibin a as a marker for Down’s syndrome in early pregnancy. N Engl J Med 334: 1231–1236.
Barnabei VM, Krantz DA, Macri JN, Larsen JW Jr. 1995. Enhanced twin pregnancy detection within an open neural tube defect and Down syndrome screening protocol using free-beta hCG and AFP. Prenat Diagn 15: 1131–1134.
Chard T, Macintosh MC. 1995. Screening for Down’s syndrome. J Perinat Med 23: 421–436.
Cuckle HS, Wald NJ, Thompson S. 1987. Estimating a woman’s risk for having a pregnancy associated with Down syndrome using her age and serum alpha-fetoprotein level. Br J Obstet Gynaecol 94: 387–402.
Evans MI, Dommergues M, Timor-Tritsch I, et al. 1994. Transab-dominal versus transcervical and transvaginal multifetal pregnancy reduction: international collaborative experience of more than one thousand cases. Am J Obstet Gynecol 170: 902–909.
Goodwin KM, Sweeney PJ, Lambert-Messerlian GM, Canick JA. 2000. High maternal serum inhibin a levels following the loss of one fetus in a twin pregnancy. Prenat Diagn 20: 1015–1017.
Grau P, Robinson L, Tabsh K, Crandall BF. 1990. Elevated maternal serum alpha-fetoprotein and amniotic fluid alpha-fetoprotein after multifetal pregnancy reduction. Obstet Gynecol 76: 1042–1045. Lambert-Messerlian G, Dugoff L, Vidaver J, et al. 2006. First- and
second-trimester Down syndrome screening markers in pregnancies achieved through assisted reproductive technologies (ART): a FASTER trial study. Prenat Diagn 26: 672–678.
Lockwood GM, Ledger WL, Barlow DH, Groome NP, Muttukr-ishna S. 1998. Identification of the source of inhibins at the time of conception provides a diagnostic role for them in very early pregnancy. Am J Reprod Immunol 40: 303–308.
Maymon R, Cuckle H, Herman A. 2006. Maternal serum inhibin levels in twin and singleton pregnancies conceived by assisted reproduction. Hum Reprod 21: 1305–1308.
Neveux LM, Palomaki GE, Knight GJ, Haddow JE. 1996. Multiple marker screening for Down syndrome in twin pregnancies. Prenat Diagn 16: 29–34.
Rotmensch S, Celentano C, Shalev J, et al. 1999. Midtrimester maternal serum screening after multifetal pregnancy reduction in pregnancies conceived by in vitro fertilization. J Assist Reprod Genet 16: 8–12.
Schenker JG, Yarkoni S, Granat M. 1981. Multiple pregnancies following induction of ovulation. Fertil Steril 35: 105–123. Shulman LP, Phillips OP, Cervetti TA. 1996. Maternal serum analyte
levels after first-trimester multifetal pregnancy reduction. Am J Obstet Gynecol 174: 1072–1074.
Spencer K, Wallace EM, Ritoe S. 1996. Second-trimester dimeric inhibin-A in Down syndrome screening. Prenat Diagn 16: 1101–1110.
Wald NJ, Densem JW, George L, Muttukrishna S, Knight PG. 1996. Prenatal screening for Down’s syndrome using inhibin-A as a serum marker. Prenat Diagn 16: 143–153.
Wald NJ, White N, Morris JK, Huttly WJ, Canick JA. 1999. Serum markers for Down’s syndrome in women who have had in vitro fertilisation: implications for antenatal screening. Br J Obstet Gynaecol 106: 1304–1306.
Wallace EM, Swanston IA, McNeilly AS, et al. 1996. Second trimester screening for Down’s syndrome using maternal serum dimeric inhibin A. Clin Endocrinol 44: 17–21.
Watt HC, Wald NJ, George L. 1996. Maternal serum inhibin-A levels in twin pregnancies: implications for screening for Down’s syndrome. Prenat Diagn 16: 927–929.
Wenstrom KD, Owen J, Chu DC, Boots L. 1997. Elevated second-trimester dimeric inhibin A levels identify Down syndrome pregnancies. Am J Obstet Gynecol 177: 992–996.
Copyright 2007 John Wiley & Sons, Ltd. Prenat Diagn 2007; 27: 431–434.