R e s e a R c h a R t i c l e
Podophyllin, but not the constituents quercetin
or kaempferol, induced genotoxicity
in vitro and
in vivo through ROS production
Mei Chih Lin
1,2, Hui Wen Cheng
1, Yi Chen Tsai
1, Po Lin Liao
3, Jaw Jou Kang
3, and Yu Wen Cheng
11College of Pharmacy, Taipei Medical University, Taipei, Taiwan
2Bureau of Food and Drug Analysis, Department of Health, Executive Yuan, Taipei, Taiwan 3Institute of Toxicology, College of Medicine, National Taiwan University, Taipei, Taiwan
Address for Correspondence: Yu-Wen Cheng, 110, No. 250, Wu-Hsing Street, Taipei Medical University, Taipei, Taiwan; Fax: +886 2 27374622; E-mail: ywcheng@tmu.edu.tw. Equal contribution to correspondence: Jaw-Jou Kang, 100, No.1, Jen-Ai Road, Section one, National Taiwan University, Taipei, Taiwan; Fax: 886 2 23410217; E-mail: jjkang@.edu.tw
(Received 11 June 2008; accepted 26 August 2008)
Introduction
Herbal medicines are gradually becoming accepted by people as legitimate pharmaceuticals and are generally thought of as dietary supplements in most parts of the world. Many people believe that these so-called herbal medicines are natural and harmless. These misconceptions have encouraged people to use herbal medicines without precaution. Chinese herbal nephropathy (CHN) was first reported in 1993 as patients ingested Chinese herbal medicines to lose weight (Vanherweghem et al., 1993). Upon closer examination, the true cause of the CHN was the misuse of Stephaniae Radix in place of Aristolochiae species. Aristolochiae species contain aristolochic acid (AA), was shown to be a strong rodent carcinogen (Arlt et al., 2002), and was listed as a known carcinogen by the International Agency for Research on Cancer
(IARC, 2002). Because of the serious toxicity induced by AA, the plants of the Aristolochiae species were withdrawn from the market in some countries. Due to the above incidences, the use of herbal medicine for the public health needs to be evaluated, especially as increasing amounts of herbal product are distributed through the world’s markets.
Podophyllin (PD) is an alcoholic plant extract obtained from the dried rhizomes and roots of the common plants of Podophyllum emodi (Indian
Podophyllum) and Podophyllum peltatum (Mayapple or Mandrake). The plants of Podophyllum were
used as traditional medicines for the treatment of cathartics and skin disorders, and also as antihelmintics and antifungals (Rivera and Tyring, 2004). Those are usually combined with henbane or belladonna to prevent side-effects. It is often used as pills or tinctures. The dried ripened fructus of
ISSN 0148-0545 print/ISSN 1525-6014 online © 2009 Informa UK Ltd DOI: 10.1080/01480540802433757
abstract
The genotoxic potential of podophyllin (PD) was investigated in this study. PD increased bacterial revertants and abnormal chromosomal structures in a concentration-dependent manner, both with and without metabolic activating enzymes, and increased the incidence of micronuclei in imprinted control region mouse reticulocytes. Results from three studied constituents of PD, such as podophyllotoxin, kamp-ferol, and quercetin, suggested that the mutagenic effect of PD was not due to the presence of podophyl-lotoxin, kampferol, and quercetin and might be related to other components and the formation of reactive oxygen species. The detailed mutagenic mechanisms need further investigation, and the medicinal use of PD needs to be cautioned against.
Keywords: Podophyllin; Genotoxicity; Ames test; Chromosome aberration; Micronucleus; Reactive oxygen
species
http://www.informapharmascience.com/dct
Drug and Chemical Toxicology Downloaded from informahealthcare.com by Taipei Medical University on 08/08/11
Podophyllum were formulated as pills to cure stasis,
dysmenorrhea, dystocia, and stillbirth in the Chinese Pharmacopoeia (2005). In addition to the aforemen-tioned Podophyllum species, Podophyllum
pleian-thum, a folk medicine distributed in Taiwan, was
traditionally used to treat snake bites. Because of their diverse biological actions, their widespread use without caution has resulted in severe poisoning or fatal cases (West et al., 1982). The common reaction was neuropathy, which lasted for several years. PD is an irritant to the eyes and mucus membranes. It can result in severe systematic toxicity after ingestion or topical application, which is usually reversible but has been fatal (Reynold and Prasad, 1996). Symptoms of toxicity include nausea, vomiting, abdominal pain, and diarrhea. Though it has a number of adverse effects, it is still included in pharmacopeias (e.g., the 2006 United States Pharmacopoeia; USP), but its clinical use is limited to applying on warts.
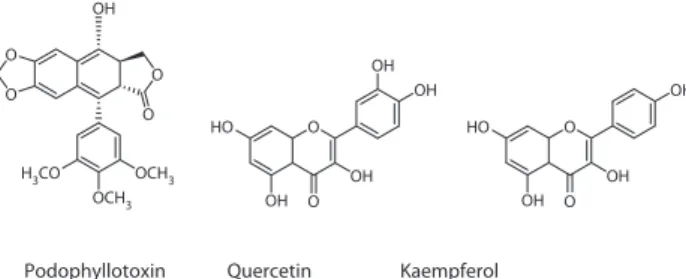
Podophyllotoxin (Figure 1), the major constituent of PD, was separated and identified in 1880. Subsequently, a series of aryltetralin-type ligands were isolated, which included -peltatine, −peltatine, podophylltoxone, and 6-methoxypodophyllotoxin, etc. (Xiao et al., 2002). In addition to these ligands, flavonoids such as querce-tin and kampferol (Figure 1) also exist in PD.
Quercetin, a well-known flavonoid and minor constituent of PD, is found in most herbs, vegeta-bles, fruits, and in beverages such as teas and wines. Studies have shown that flavonoids have multiple biological activities, such as acting as an antioxidant (Rice-Evans, 2001; Fujisawa and Kadoma, 2006), anti-inflammatory, antiviral, platelet-aggregation inhibi-tor (Landolfi, et al., 1984; Hertog and Hollman, 1996), antiaging agent (Rice-Evans, 2001), and for preventing cardiovascular disease (Cao et al., 1998; Rice-Evans, 2001; Carollo et al., 2007). However, in the 1970s, quercetin was reported as mutagenic in the Ames test (Bjeldanes and Chang, 1977; Nagao et al., 1981), chromosome aberration test (Silva et al., 1996, 1997), and micronucleus test (Sahu et al., 1981; Caria et al., 1995). Kampferol also was shown to have mutagenic activity in the Ames and chromosome aberration tests (Nagao et al., 1981; Silva et al., 1996, 1997).
The aim of this study was to clarify PD’s genotoxic potential and investigate the genotoxic mechanisms ascribed to the constituents, podophyllotoxin, kampferol, and quercetin. Two in vitro short-term mutagenicity bioassays, the Ames Salmonella assay and the chromosome aberration test with mammalian cells, were conducted. In addition, a micronucleus test was used to evaluate genotoxicity in vivo. Our results showed that PD was strongly mutagenic in
vitro and in vivo, and the mutagenic effect was not
from quercetin and kampferol.
Materials and methods
Materials
PD, podophyllotoxin, quercetin, kampferol, Aroclor 1254, D(+)-glucose, sodium chloride, acridine orange, glucose-6-phosphate (G6P), -nicotinamide adenine dinucleotide phosphate (−DP), colcemid, hydro-gen peroxide (H2O2), dimethyl sulfoxide (DMSO), 2’, 7’-dichlorofluorescin diacetate (DCFH-DA),
4-nitro-O-phenylenediamine (4-NOP), 2-aminoanthracene
(2-AA), benzo[a]pyrene (BaP), mitomycin C (MMC), and sodium azide were all obtained from Sigma Chemical Co. (St. Louis, Missouri, USA). Giemsa stain solution was purchased from Mute Chemical Co., Ltd. (Tokyo, Japan). Fetal calf serum, penicillin/strepto-mycin/glutamine, F-12K medium, and trypsin were obtained from Gibco BRL (Grand Island, New York, USA). PD constituent analysis was kindly provided by Dr. Cheng Hui-Wen, Ph. D. Results from the liquid chromatography/mass spectrometry/mass spectrom-etry (LC/MS/MS) examined the constituents of PD and showed that the relative amounts of the constituents were 31.9, 3.2, and 1.8% of podophyllotoxin, kamp-ferol, and quercetin, respectively (Lin et al., 2007). Experimental animals
For the Ames test, Wistar rats (200 g) were used for the preparation of the liver microsomal (S9) fraction. For the micronucleus test, male imprinting control region (ICR) mice, aged 8–9 weeks and weighing 30–40 g, were employed. All animals were purchased from the Animal Center of the College of Medicine, National Taiwan University. The animals were allowed at least a 1-week acclimation period in their animal hous-ing at 25°C and a 12-h light-dark cycle. All animal treatments were approved by the Institution Animal Care and Use Committee (IACUC) of the College of Medicine, National Taiwan University, which follows the Animal Welfare Protection Act of the Department of Agriculture, Executive Yuan (Taipei, Taiwan).
Figure 1. Structures of podophyllotoxin, quercetin and kampferol.
O O O O OCH3 OCH3 H3CO OH O HO OH OH OH OH O O HO OH OH OH O
Podophyllotoxin Quercetin Kaempferol
Drug and Chemical Toxicology Downloaded from informahealthcare.com by Taipei Medical University on 08/08/11
Rat-liver microsome (S9) preparation
Rat-liver microsomes (S9) used for metabolic activation were prepared, as described previously (Matsuoka et al., 1979; Maron and Ames (1983). To obtain the liver microsomal fraction, each of the Wistar rats was injected intraperitoneally (i.p.) with Aroclor 1254 (30 mg/kg body weight), and 4 days later, the rats were sacrificed by cervical dislocation. The livers were homogenated, diluted 1:4 with 0.15 M of KCl, and centrifuged for 10 min at 900 × g. The super-natant was pooled and diluted (to a protein concen-tration of 30 mg/mL), frozen in small aliquots, and stored at –70 to –80°C until use. The final preparation of the S9 mixture was made in accordance with the protocol of Ames et al. (1975). The composition and final concentrations of the S9 reaction mix used for the CHO-K1 cell chromosome aberration test were as follows: G6P, 4.4 mM; −NADP, 0.84 mM; KCl, 30 mM; NaHCO3, 0.032%; and S9 fraction, 10%.
Ames salmonella/microsome test
The method we used followed that of Maron and Ames (1983) and Organization for Economic Cooperation and Development guidelines (OECD, 1997). The
Salmonella typhimurium bacteria and histidine
auxotrophic strains, TA98, TA100, and TA102, were obtained from MOLTOX (Molecular Toxicology, Annapolis, Maryland, USA) and grown for 14 h at 35 ± 2°C with continuous shaking. Bacteria were grown to a density of 1–2 × 109 cells/mL with a OD600
absorb-ance of 0.2–0.3. Top agar, containing 2 mL of heated agar, 0.1 mL of test chemical, 0.1 mL of bacteria, and 0.5 mL of S9 solution, were mixed and added to three different minimal glucose agar plates. All plates were incubated at 37°C for 48 h, and the number of bacteria colonies was determined. The entire experiment was replicated again on a different day, with a total of six plates for each concentration of PD or test chemicals with and without S9. Each tester strain was routinely checked to confirm its features for optimal response to known mutagenic chemicals with the following agents: 4-NOP (0.5 g/plate), MMC (0.5 g/plate), and 2-AA (5 g/plate). A test compound was judged to be mutagenic in the plate test if it produced, in at least one concentration and one strain, a response equal to three times (or more) of the control incidence with a positive dose-response relationship (de Serres and Shelby, 1979; Suter et al., 2002). The only exception was strain TA102, which has a relatively high spon-taneous revertant number, whereby an increase by a factor of 1.5 above the control level was taken as an indication of a mutagenic effect.
Chromosomal aberration test
The test was conducted by using the method of Cheng et al. (2004, 2005) and Matsuoka et al. (1979), with some modification. Chinese hamster ovary epithelial cells (CHO-K1, ATCC: CCL-61) were plated into 6-cm dishes at 5 x 105 cells/plate; after overnight
incuba-tion, cells were treated with DMSO (solvent), MMC (1 g/mL), and various concentrations of test chemi-cals for 3 h with or without S9, followed by 0.1 g/mL colcemid for 3 h. After trypsinization, cells were treated with hypotonic solution (0.03 M of potassium chlo-ride and 0.01 of M sodium citrate) at 37°C for 10 min, fixed in Carnoy’s solution (methanol:acetic acid, 3:1), and spread on glass slides by the air-drying method. Specimens were stained with a 3% Giemsa solution in 0.07-M phosphate buffer (pH 6.8) for 30 min. For determination of both of the chromosome aberrations, 100 metaphases per experimental group were scored. Structural chromosome aberrations observed in each experimental group were classified into seven types as follows: type gap (G), chromosome-type break (B), chromosome-chromosome-type ring (R), chromo-some-type dicentric (D), chromatid-type gap (g), chromatid-type break (b), and chromatid-type exchange (e).
Micronucleus assay
The micronucleus assay from peripheral blood cells was performed, as described (Cheng et al., 2004; Hayashi et al., 1990). Male ICR mice, weighing 30–40 g, were employed and divided into five groups. The PD suspension was prepared in distilled water and admin-istered by gavage, using a stomach tube. MMC (1 mg/ kg) was administrated by i.p. injection to serve as a pos-itive control. Then, 24, 48, and 72 h after the treatment, 3–4 L of peripheral blood was collected from the tail vein and transferred onto a slide prestained with acridine orange (1 mg/mL) and then covered with a coverglass. The number of micronucleated cells were counted in 1,000 reticulocytes per animal. Slides were analyzed by using a Zeiss Axiophot 2 fluorescent microscope (Carl Zeiss, Inc., Thornwood, NY, U.S.A.) with a combination of a blue excitation (e.g., 488 nm) and a yellow-to-orange barrier filter (e.g. 515 nm long pass), with an X100 objective lens.
Analysis of ROS production by flow cytometry Intracellular reactive oxygen species (ROS) generation was measured by a flow cytometer with an oxidation-sensitive DCFH-DA fluoroprobe (Rothe and Valet,
Drug and Chemical Toxicology Downloaded from informahealthcare.com by Taipei Medical University on 08/08/11
1990). CHO-K1 cells, (2 x 106 /mL) were treated with
various concentrations of PD (25, 50, and 100 g/ mL) for 2 h, then trypsinized and stained with 20 g/ mL of DCFH-DA for 30 min at 37°C in the dark. Cells were then collected after phosphate-buffered saline (PBS) washing for fluorescence measurements. The level of intracellular ROS was determined with a FACS CaliburTM flow cytometer (Becton Dickinson,
San Jose, California, USA) at an excitation wavelength of 488 nm and an emission wavelength of 525 nm. For each treatment, 104 cells were counted and the
experiment was performed in triplicate. Statistical analysis
All experiments were performed at least in triplicate, and data are presented as the mean ± standard error of the mean. Statistical analysis, including the Student’s
t-test, were performed, using SigmaPlot (Systat
Software Inc., Point Richmond, California, USA), and p < 0.05 was considered as significantly different.
Results
PD-induced bacterial revertants in ames test
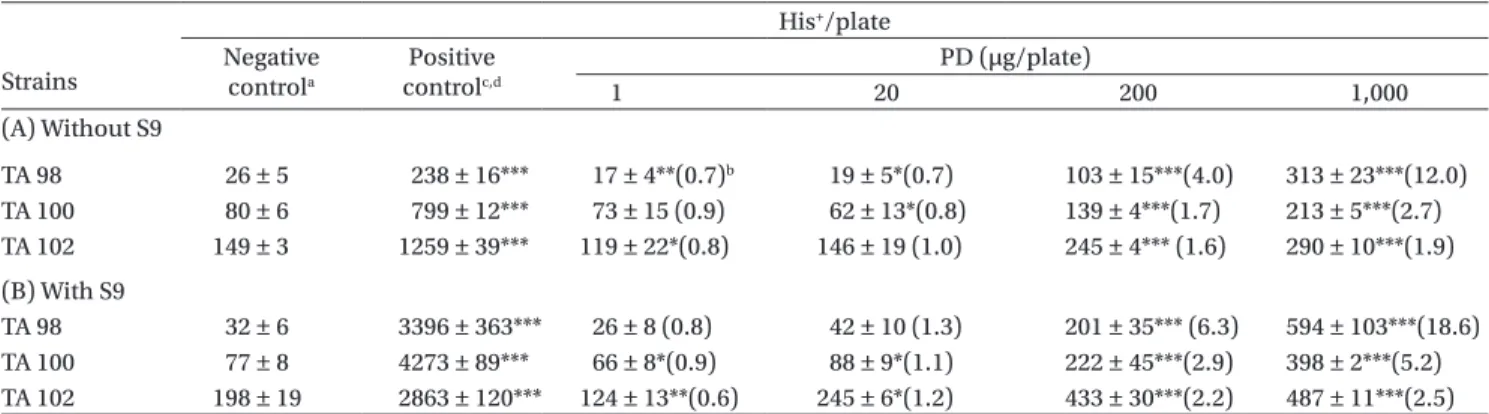
The data for the PD-induced histidine revertants in the three strains of Salmonella typhimurium were listed in Table 1. Our data showed that PD increased colony formation in strains TA98, TA100, and TA102 in a concentration-dependent manner (Table 1A). The increase at a PD concentration of 1,000 g/plate in TA98, TA100, and TA102 was 12.0 2.7 and 1.9-fold, respectively. In the presence of S9, the increases were
significantly enhanced, reaching 18.6-, 5.2-, and 2.5-fold, respectively (Table 1B). The induction of the TA98 strain to greater than three times relative to con-trol values either with or without S9 was regarded as a positive mutagenic effect (FDA, 2004).
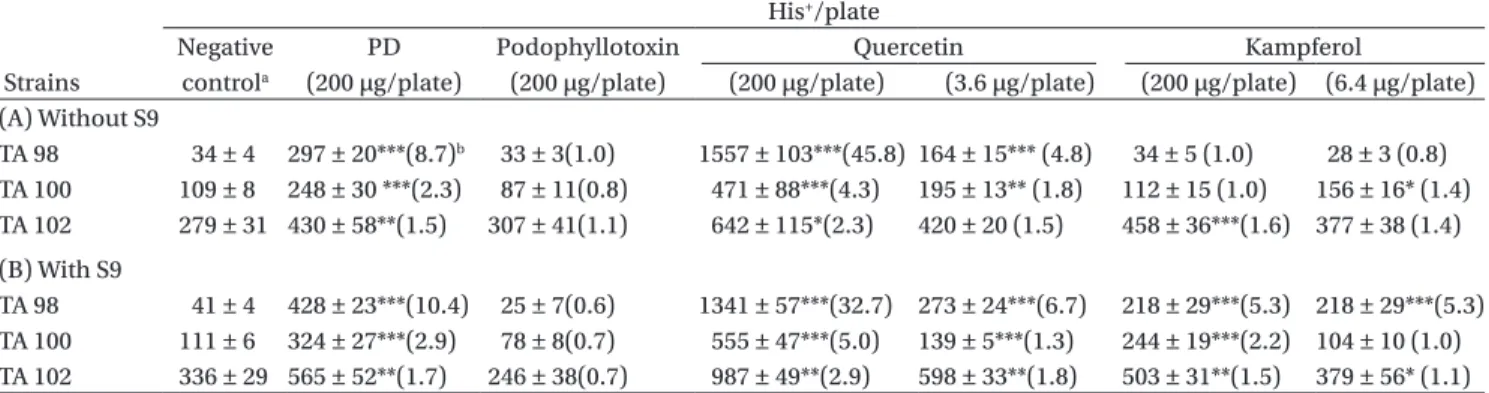
Three major constitutive compounds of PD were simultaneously examined in the Ames Salmonella/ microsome test in order to clarify the mutagenic effects. Table 2 showed the number of revertants induced by dif-ferent compounds at the same concentration. Quercetin significantly increased colony formation in strains TA98, TA100, and TA102 with or without S9. The increases in-duced by quercetin (200 g/plate) ranged from 32.7- to 45.8-fold over the negative control in the TA98 strain (either with or without S9), and the mutagenicity was at-tenuated in the presence of S9. Kampferol increased col-ony formation in all strains with S9, and TA102 without S9. Podophyllotoxin did not show a mutagenic effect in the Ames test. Based on the chemical analysis (Lin et al., 2007), the percent composition of quercetin and kamp-ferol in PD were 1.8 and 3.2%, respectively. The amount of quercetin and kampferol in 200 g of PD were 3.6 and 6.4 g. This amount also induced revertants in the TA98 strain both with or without S9 (Table 2).
Induction of chromosome aberration by PD in CHO-K1 cells
PD-induced abnormal chromosome structures were examined with CHO-K1 cells. In Table 3, MMC (1 g/ mL) and BaP (5 g/mL), were used as the positive con-trols, and they significantly induced abnormal chromo-some structures in the absence (Table 3A) or presence (Table 3B) of S9. PD (25, 50, and 100 g/mL) increased the abnormal structure of chromosomes at 3 h, 24 h
Table 1. Induction of His+ revertants in three strains of Salmonella typhimurium by podophyllin (PD) with and without metabolic
activation (by S9). Strains His+/plate Negative controla Positive controlc,d PD (µg/plate) 1 20 200 1,000 (A) Without S9 TA 98 26 ± 5 238 ± 16*** 17 ± 4**(0.7)b 19 ± 5*(0.7) 103 ± 15***(4.0) 313 ± 23***(12.0) TA 100 80 ± 6 799 ± 12*** 73 ± 15 (0.9) 62 ± 13*(0.8) 139 ± 4***(1.7) 213 ± 5***(2.7) TA 102 149 ± 3 1259 ± 39*** 119 ± 22*(0.8) 146 ± 19 (1.0) 245 ± 4*** (1.6) 290 ± 10***(1.9) (B) With S9 TA 98 32 ± 6 3396 ± 363*** 26 ± 8 (0.8) 42 ± 10 (1.3) 201 ± 35*** (6.3) 594 ± 103***(18.6) TA 100 77 ± 8 4273 ± 89*** 66 ± 8*(0.9) 88 ± 9*(1.1) 222 ± 45***(2.9) 398 ± 2***(5.2) TA 102 198 ± 19 2863 ± 120*** 124 ± 13**(0.6) 245 ± 6*(1.2) 433 ± 30***(2.2) 487 ± 11***(2.5)
Values are presented as the mean ± standard error (N ≥ 6). *p < 0 .05, **p < 0.01, ***p < 0.001 vs. the negative control.
aThe 2-L dimethyl sulfoxide plate was used as the negative control. bMultiples of increase relative to the negative control.
cPositive control in the –S9 plate: TA 98, 4-NOP 2 g/plate; TA 100, sodium azide 5 g/plate; TA102, MMC 0.5 g /plate. dPositive control in the +S9 plate: TA 98, TA 100, and TA102, 2-AA: 5 g/plate.
Drug and Chemical Toxicology Downloaded from informahealthcare.com by Taipei Medical University on 08/08/11
without S9, and at 3 h with S9 in a dependent manner (Table 3A and 3B). Gap and break defects were the main abnormal chromosome structures in PD-induced aberrations. Quercetin and kampferol, in concentrations found in 100 g/mL of PD (1.8 and 3.2 g/mL, respectively), did not induce abnormal structures in chromosomes in CHO-K1 cells. In vivo induction of micronuclei by PD
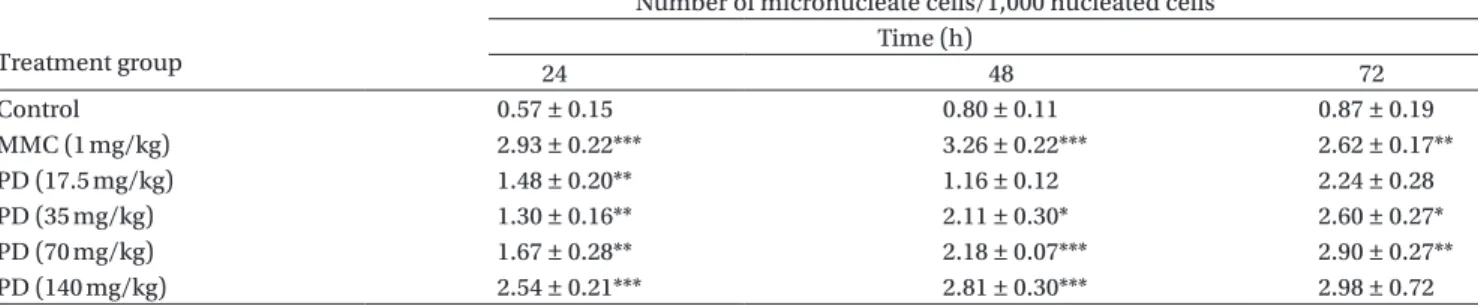
The in vivo micronucleus induction in mice by PD is shown in Table 4. The positive control MMC sig-nificantly induced micronucleus formation. PD demonstrated a concentration dependent (from 17.5
to 140 mg/kg) increase in micronucleus formation at 24, 48, and 72 h. The highest induction was 2.81 ± 0.30 of micronucleated reticulocytes at 48 h, which was a 3.51-fold increase relative to the control value of 0.80 ± 0.11. Quercetin (2.52 mg/mL) and kampferol (4.48 mg/mL) were added according to their corre-sponding concentrations in PD (140 mg/mL) and had no effect on micronucleus formation up to 72 h. ROS induced by PD in CHO cells
CHO-K1 cells loaded with the fluorescence probe, DCFH-DA, were used to measure the generation of ROS with flow cytometry. PD increased the DCF
Table 2. Induction of His+ revertants in three strains of Salmonella typhimurium by podophyllin (PD) and PD components with and
without metabolic activation (by S9).
His+/plate
Negative PD Podophyllotoxin Quercetin Kampferol
Strains controla (200 µg/plate) (200 µg/plate) (200 µg/plate) (3.6 µg/plate) (200 µg/plate) (6.4 µg/plate)
(A) Without S9 TA 98 34 ± 4 297 ± 20***(8.7)b 33 ± 3(1.0) 1557 ± 103***(45.8) 164 ± 15*** (4.8) 34 ± 5 (1.0) 28 ± 3 (0.8) TA 100 109 ± 8 248 ± 30 ***(2.3) 87 ± 11(0.8) 471 ± 88***(4.3) 195 ± 13** (1.8) 112 ± 15 (1.0) 156 ± 16* (1.4) TA 102 279 ± 31 430 ± 58**(1.5) 307 ± 41(1.1) 642 ± 115*(2.3) 420 ± 20 (1.5) 458 ± 36***(1.6) 377 ± 38 (1.4) (B) With S9 TA 98 41 ± 4 428 ± 23***(10.4) 25 ± 7(0.6) 1341 ± 57***(32.7) 273 ± 24***(6.7) 218 ± 29***(5.3) 218 ± 29***(5.3) TA 100 111 ± 6 324 ± 27***(2.9) 78 ± 8(0.7) 555 ± 47***(5.0) 139 ± 5***(1.3) 244 ± 19***(2.2) 104 ± 10 (1.0) TA 102 336 ± 29 565 ± 52**(1.7) 246 ± 38(0.7) 987 ± 49**(2.9) 598 ± 33**(1.8) 503 ± 31**(1.5) 379 ± 56* (1.1)
Values are presented as the mean ± standard error (N ≥ 6). *p < 0.05; **p< 0.01;***p < 0.001 vs. the negative control.
aThe 2-L dimethyl sulfoxide plate was used as the negative control. b Multiples of increase relative to the negative control.
Table 3A. Chromosome aberrations of CHO-K1 cells treated with podophyllin (PD) and PD components with and without metabolic activation (by S9).
(A) Without S9 Number of aberrant cells/100 cellsa
Treatment (µg/mL) Aberrant cells (%) G B D R g b e
3 h Negativea 1.0 ± 1.0 0 ± 0 0 ± 0 1.0 ± 1.0 0 ± 0 0 ± 0 0 ± 0 0 ± 0 control MMCb 5.7 ± 0.9*** 2.0 ± 0.6 0 ± 0 0.3 ± 0.3 0 ± 0 0 ± 0 3.0 ± 0.6 0.3 ± 0.3 PD (g/ml)b 25 7.3 ± 0.3** 0.7 ± 0.3 1.0 ± 0.6 1.0 ± 0.0 0.7 ± 0.7 2.3 ± 0.9 1.7 ± 0.9 0 ± 0 50 8.0 ± 3.6 0.7 ± 0.7 0.3 ± 0.3 1.0 ± 0.0 0.3 ± 0.3 3.7 ± 2.2 2.0 ± 1.0 0 ± 0 100 12.0 ± 1.0** 1.3 ± 1.3 0 ± 0 1.3 ± 0.3 0 ± 0 4.7 ± 1.5 4.0 ± 0.6 0.7 ± 0.7 Qc 1.8 1.3 ± 0.3 0 ± 0 0 ± 0 0 ± 0 0 ± 0 0.7 ± 0.3 0.7 ± 0.3 0 ± 0 K 3.2 1.7 ± 1.0 0 ± 0 0 ± 0 0 ± 0 0 ± 0 0.7 ± 0.3 1.0 ± 1.0 0 ± 0 24 h Negativea 1.0 ± 0.5 0 ± 0 0 ± 0 0 ± 0 0.7 ± 0.3 0.3 ± 0.3 0 ± 0 0 ± 0 control MMCb 11.7 ± 1.2*** 0.3 ± 0.3 0.3 ± 0.3 0.3 ± 0.3 0.7 ± 0.3 6.0 ± 0.6 2.0 ± 0.6 2.0 ± 0.6 PD (g/ml) 25 7.7 ± 0.3*** 0.3 ± 0.3 0 ± 0 2.3 ± 1.3 0 ± 0 1.0 ± 0.6 3.0 ± 1.2 1.0 ± 0.6 50 9.0 ± 2.9* 0.3 ± 0.3 0 ± 0 2.0 ± 0.0 0 ± 0 1.0 ± 0.6 2.7 ± 1.2 3.0 ± 1.7 100 14.0 ± 4.6* 2.3 ± 2.3 0.7 ± 0.3 2.7 ± 0.9 0 ± 0 2.3 ± 0.9 4.3 ± 0.9 1.7 ± 0.9 Q 1.8 2.7 ± 0.9 0 ± 0 0 ± 0 0.3 ± 0.3 0.3 ± 0.3 0.7 ± 0.3 1.3 ± 0.3 0 ± 0 K 3.2 2.7 ± 0.3 0 ± 0 0.3 ± 0.3 0.7 ± 0.3 0 ± 0 0.3 ± 0.3 1.0 ± 0.6 0.3 ± 0.3
Drug and Chemical Toxicology Downloaded from informahealthcare.com by Taipei Medical University on 08/08/11
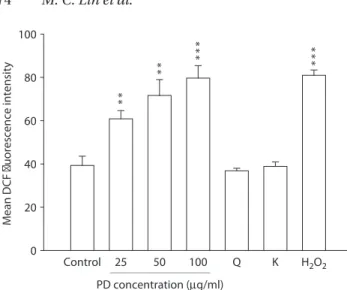
fluorescence intensity in a concentration-dependent manner (Figure 2), but quercetin and kampferol did not. The increased fluorescence intensity induced by PD (100 g/mL) was 79.7 ± 5.7% relative to the con-trol value of 39.3 ± 4.3%. H2O2 was used as the positive control.
Discussion
In this study, PD acted as a direct mutagen in bacteria and caused chromosome aberrations in CHO-K1 cells. PD increased the number of revertants in Salmonella
strains TA 98, TA100, and TA102 with or without S9. In the presence of S9, the mutagenic effect of PD was sig-nificantly enhanced, especially in the TA98 strain. Part of the PD-induced mutagenicity might be through metabolic activation. PD also increased the number of abnormally structured chromosomes both with and without S9. Although many reports examine the tox-icity of PD, comprehensive reports on the mutagenic effects of PD are few (Ferguson and Pearson, 1992).
Petersen and Weisman (1995) estimated the amounts of quercetin and kampferol in 20% PD were about 10% and recommended using pure podophyl-lotoxin or a lower percentage PD formulation to
Table 3B. Chromosome aberrations of CHO-K1 cells treated with podophyllin (PD) and PD components with and without metabolic activation (by S9).
(B) With S9 Number of aberrant cells/100 cells
Treatment Aberrant cells (%) G B D R g b e
3 h Negative 0.3 ± 0.3 0.3 ± 0.3 0 ± 0 0 ± 0 0 ± 0 0 ± 0 0 ± 0 0 ± 0 controla BaPb 15.5 ± 1.2*** 0.7 ± 0.3 0.3 ± 0.3 0.3 ± 0.3 5.3 ± 0.9 2.3 ± 0.3 2.3 ± 0.3 4.3 ± 0.9 PD (g/ml)b 25 9.0 ± 1.9* 1.5 ± 0.8 0 ± 0 3.2 ± 0.8 0.5 ± 0.2 2.2 ± 0.9 1.7 ± 0.6 0 ± 0 50 9.7 ± 1.7** 0.2 ± 0.2 0.3 ± 0.2 0.8 ± 0.3 0.2 ± 0.2 2.7 ± 0.9 5.2 ± 1.3 0.3 ± 0.3 100 14.0 ± 2.7** 1.0 ± 1.0 0 ± 0 2.3 ± 0.3 0.3 ± 0.3 5.3 ± 2.3 4.7 ± 0.9 0.3 ±0.3 Q 1.8 1.0 ± 0.0 0 ± 0 0.3 ± 0.3 0.3 ± 0.3 0 ± 0 0 ± 0 0.3 ± 0.3 0 ± 0 K 3.2 2.3 ± 0.3 0 ± 0 0 ± 0 0.3 ± 0.3 0.3 ± 0.3 0 ± 0 1.7 ±0.7 0 ± 0
aDimethyl sulfoxide (DMSO) was taken as the negative control.
bPD was dissolved in DMSO, and the solvent control (DMSO) did not exceed 0.1%. MMC at 1 g/mL and benzo(a)pyrene (BaP) at 5 g/
mL were used as the positive control without and with S9 medium. Quercetin at 1.8 g/mL and kampferol at 3.2 g/mL were added according to their contents existing in 100 g/mL PD.
cQ, quercetin; K, kampferol.
dValues are presented as the mean ± standard error (n = 3); *p < 0.05; **p < 0.01; ***p < 0.001 vs. the negative control. G, chromosome gap;
B, chromosome break; D, dicentric; R, ring; g, chromatid gap; b, chromatid break; e, exchange.
Table 4A. Micronucleus formation in peripheral blood cells of mice treated with podophyllin (PD) and PD components in vivo. Treatment group
Number of micronucleate cells/1,000 nucleated cells Time (h) 24 48 72 Control 0.57 ± 0.15 0.80 ± 0.11 0.87 ± 0.19 MMC (1 mg/kg) 2.93 ± 0.22*** 3.26 ± 0.22*** 2.62 ± 0.17** PD (17.5 mg/kg) 1.48 ± 0.20** 1.16 ± 0.12 2.24 ± 0.28 PD (35 mg/kg) 1.30 ± 0.16** 2.11 ± 0.30* 2.60 ± 0.27* PD (70 mg/kg) 1.67 ± 0.28** 2.18 ± 0.07*** 2.90 ± 0.27** PD (140 mg/kg) 2.54 ± 0.21*** 2.81 ± 0.30*** 2.98 ± 0.72
Table 4B. Micronucleus formation in peripheral blood cells of mice treated with podophyllin (PD) and PD components in vivo. Treatment group
Number of micronucleated cells/1,000 nucleated cells Time (h) 24 48 72 Control 0.50 ± 0.09 0.53 ± 0.21 0.76 ± 0.09 MMC (5 mg/kg) 3.88 ± 0.48*** 5.44 ± 0.70*** 5.92 ± 0.63*** Quercetin (2.52 mg/kg) 0.43 ± 0.12 0.24 ± 0.02** 0.28 ± 0.06* Kampferol (4.48 mg/kg) 0.42 ± 0.12 0.58 ± 0.12 0.39 ± 0.09*
Data are expressed as the mean ± standard error from the six independent experiments. Quercetin at 2.52 mg/mL and kampferol at 4.48 mg/mL were added according to their contents existing in 140 mg/mL of PD. *p , 0.05; **p , 0.01; ***p , 0.001 vs. the control.
Drug and Chemical Toxicology Downloaded from informahealthcare.com by Taipei Medical University on 08/08/11
prevent toxicity. Although PD formulations may con-tain variable amounts of the active ligand podophyl-lotoxin, the dry weight of this constituent is almost never identified.
Due to the uncertainty of the exact constituents in PD, we characterized the constituents in PD based on their mutagenic effects. As a resin mixture, the per-centages of podophyllotoxin, quercetin, and kampfer-ol in PD were detected by LC/MS/MS as 31.9, 1.8, and 3.2%, respectively (Lin et al., 2007). Podophyllotoxin, the main component in PD, did not show a mutagen-ic effect in the Ames test. Podophyllotoxin is a white crystalline substance isolated from PD. It has several biological activities similar to PD, including an antivi-ral activity against type I herpes simplex and measles (Rivera and Tyring, 2004) and has shown antitumor activity (Canel, et al., 2000).
Quercetin is a widely distributed flavonoid, mainly found in vegetables (e.g., onion and broccoli), fruits (e.g., apples, grapes, cherries, and berries), tea, red wine, and herbs (e.g., Ginkgo and sophora). Our study showed that quercetin (1.8% in PD) is a potent muta-gen in the Ames test but not in the chromosome aber-ration test. Although the mutagenic effects of querce-tin have been reported in many cell types (Caria et al., 1995; Oliveira et al., 1997; van Duursen et al., 2004), the underlying mechanism of its action is still unclear.
Like quercetin, kampferol is a well-known anti-oxidant that prevents ROS-mediated apoptosis in
cerebellar granule cells of Wistar rats (Samhan-Arias et al., 2004). Kampferol (3.2% in PD) exhibited a mutagenic effect in the Ames test but not in the chromosome aberration test. From the results of our study, quercetin and kampferol both induced bacte-rial revertants with or without S9 but had virtually no effect in the chromosome aberration test. Direct ROS generation was measured by flow cytometry with DCF fluorescence and showed that PD, but not quercetin and kampferol, significantly induced ROS generation in CHO-K1 cells.
There are a number of contradicting results examining the role of quercetin in ROS generation. Quercetin induced ROS generation in WIL2-NS cells (Saito et al., 2004; Mertens-Talcott et al., 2005) and suppressed ROS generation in HL-60 and PC12 cells (Wang et al., 1999; Wang and Joseph, 1999). Most researchers agree that ROS play a key role in human cancer development (Monari et al., 2006; ten Kate et al., 2006, Knaapen et al., 2006; Jenkins et al., 2007). ROS include oxygen radicals (e.g., superoxide, hydroxy, and peroxyl and alkoxyl radicals) and cer-tain nonradicals that are either oxidizing agents and/ or are easily converted into radicals (e.g., hypochlo-rous acid, ozone, and peroxynitrite), singlet oxygen, and hydrogen peroxide. Several in vitro studies have reported the characteristic DNA damage caused by ROS. Quercetin can produce H2O2 in the medium, and chromosome damage induced by H2O2 has been demonstrated in WIL2-NS cells (Saito et al., 2004). Further, quercetin and kampferol also increase 8- oxodG formation (Yamashita, et al., 1999). Previous studies have shown that ROS might be involved in the mutagenic mechanism induced by quercetin and kampferol. In our study, ROS were not involved in quercetin- and kampferol-induced mutagenesis either in the Ames test or in abnormal chromosomal structure assays, since no ROS were detected by DCFH-DA fluorescence probe.
The in vivo micronucleus test (Table 4) showed that PD induced micronucleus formation. Surprisingly, quercetin exerted a genotoxic effect in the Ames test but did not show a clastogenic effect in the in
vivo micronucleus test. These data suggest a
mini-mal role of quercetin in PD-induced mutagenesis. A safety review of quercetin for clinical applications was reported in 2005 (Okamoto, 2005). The International Agency for Research on Cancer (IARC) concluded in 1999 that quercetin could not be classified as carci-nogenic to humans. Although quercetin is genotoxic to Salmonella, its safety in certain human applica-tions has been approved. Although one report has indicated that quercetin and kampferol could induce micronuclei in vivo (Sahu et al., 1981), this effect did not occur in our study.
Figure 2. Effects of podophyllin (PD) on reactive oxygen species (ROS) generation in CHO-K1 cells. PD dose dependently induced DCF fluorescence intensity. Cells were incubated with vari-ous concentrations of PD (25, 50, and 100 g/mL) for 2 h, then trypsinized and stained with 2’,7’-dichloroflyorescin diacetate (DCFH-DA) at 37°C for 30 min. Dimethyl sulfoxide and H2O2 (200 M) were used as the negative and positive controls, re-spectively. Quercetin (Q 1.8 g/mL) and kampferol (K 3.2 g/ml) were used as the paralleled test. Data are expressed as the mean ± standard error from six independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001 vs. the negative control.
Mean DCF uorescence intensity
0 20 40 60 80 100 Control PD concentration (µg/ml) ** ** *** *** Q 25 50 100 K H2O2
Drug and Chemical Toxicology Downloaded from informahealthcare.com by Taipei Medical University on 08/08/11
Conclusions
We conclude that PD could induce genotoxicity
in vitro and in vivo, and this effect might not come
from quercetin or kampferol. However, there are other constituents that could have contributed to the PD-induced mutagenesis. The lack of information on the carcinogenesis, bioavailability, metabolism, and tissue accumulation of PD in the animal requires further study. Although the genotoxic effect might not equal the carcinogenic effect, we have drawn atten-tion to the mutagenic effects of PD and will conduct further investigations to identify the precise mutagen in PD. Although this medicinal plant derivative is easily obtained, these data suggest that the use of PD should be done with caution.
Acknowledgments
The authors would like to express our appreciation to all who helped us with this research. The authors thank Dr. Jaw-Jou Kang of the Institute of Toxicology, College of Medicine, National Taiwan University, and Dr. George Hsiao of the Department and Graduate Institute of Pharmacology, College of Medicine, Taipei Medical University, who kindly gave us com-prehensive suggestions on the experiments. The authors also thank the Bureau of Food and Drug Analysis, Department of Health, Executive Yuan (Taipei, Taiwan) for supporting this work.
References
Ames BN, McCann J, Yamasaki E (1975). Methods for detecting carcinogens and mutagens with the Salmonella/mammali-an-microsome mutagenicity test. Mutat. Res. 31, 347–364. Arlt VM, Stiborova M, Schmeiser HH (2002). Aristolochic acid
as a probable human cancer hazard in herbal remedies: a review. Mutagenesis 17, 265–277.
Bjeldanes LF, Chang GW (1977). Mutagenic activity of quercetin and related compounds. Science 197, 577–578.
Canel C, Moraes RM, Dayan FE, Ferreira D (2000). Podophyllotoxin. Phytochemistry 4, 115–120.
Cao G, Russell RM, Lischner N, Prior RL (1998). Serum antioxi-dant capacity is increased by consumption of strawberries, spinach, red wine, or vitamin C in elderly women. J. Nutr. 128, 2383–2390.
Caria H, Chaveca T, Laires A, Rueff J (1995). Genotoxicity of quercetin in the micronucleus assay in mouse bone mar-row erythrocytes, human lymphocytes, V79 cell line, and identification of kinetochore-containing (CREST stain-ing) micronuclei in human lymphocytes. Mutat. Res. 343, 85–94.
Carollo C, Presti RL, Caimi G (2007). Wine, diet, and arterial hypertension. Angiology 58, 92–96.
Cheng YW, Kang JJ, Shih YL, Lo YL, Wang CF (2005). Cholesterol-3-beta, 5-alpha, 6-beta-triol induced genotoxicity through
reactive oxygen species formation. Food Chem. Toxicol. 43, 617–622.
Cheng YW, Lee WW, Li CH, Lee CC, Kang JJ (2004). Genotoxicity of motorcycle exhaust particles in vivo and in vitro. Toxicol. Sci. 81, 103–111.
Chinese National Pharmacopoeia Committee. (2005). Xiaoyelian, Fructus Podophylli. In, Chinese Pharmacopoeia, Volume I (p. 31). Beijing, China, Chemical Industry Press.
de Serres FJ, Shelby MD (1979). The Salmonella mutagenicity assay: recommendations. Science 203, 563–565.
FDA. (2004). FDA guidelines: introduction to the template for in vitro bacterial reverse mutation (Ames) test. CFSAN/Office of Food Additive Safety March 2004.
Ferguson LR, Pearson A (1992). Chromosomal changes in Chinese hamster AA8 cells caused by podophyllin, a common treat-ment for genital warts. Mutat. Res. 266, 231–239.
Fujisawa S, Kadoma Y (2006). Anti- and pro-oxidant effects of oxidized quercetin, curcumin, or curcumin-related com-pounds with thiols or ascorbate as measured by the induc-tion period method. In Vivo 20, 39–44.
Hayashi M, Morita T, Kodama Y, Sofuni T, Ishidate M (1990). The micronucleus assay with mouse peripheral blood reticulo-cytes using acridine orange-coated slides. Mutat. Res. 245, 245–249.
Hertog MG, Hollman PC (1996). Potential health effects of the dietary flavonol quercetin. Eur. J. Clin. Nutr. 50, 63–71. IARC (International Agency for Research on Cancer). (2002).
Some traditional herbal medicines, some mycotoxins, naphthalene, and styrene. IARC monographs on the evalu-ation of carcinogenic risks to humans. No. 82. Lyon, France, IARC.
Jenkins GJ, D’Souza FR, Suzen HS, Eltahir ZS, James SA, Parry JM, Griffiths PA, Baxter JN (2007). Deoxycholic acid at neutral and acid pH, is genotoxic to oesophageal cells through the induction of ROS: the potential role of antioxidants in Barrett’s oesophagus. Carcinogenesis 28, 136–142.
Knaapen AM, Güngör N, Schins RP, Borm PJ, Van Schooten FJ (2006). Neutrophils and respiratory tract DNA damage and mutagenesis: a review. Mutagenesis 21, 225–236.
Landolfi R, Mower RL, Steiner M (1984). Modification of plate-let function and archidonic acid metabolism by bioflavo-noids. Structure-activity relations. Biochem. Pharmacol. 33, 1525–1530.
Lin MC., Cheng, YW, Tsai, YC, Liao PL, Cheng HW (2007). Podophyllin, but not the constituents of quercetin and kaempferol, induced genotoxicity in vitro and in vivo through ROS production. The XXIV Joint Annual Conference of Biomedical Sciences, Taipei, Taiwan. p. 707.
Maron M, Ames BN (1983). Revised methods for the Salmonella mutagenicity tests. Mutat. Res. 113, 173–215.
Matsuoka A, Hayashi M, Ishidate M Jr. (1979). Chromosomal aberration tests on 29 chemicals combined with S9 mix in vitro. Mutat. Res. 66, 277–290.
Mertens-Talcott SU, Bomser JA, Romero C, Talcott ST, Percival SS (2005). Ellagic acid potentiates the effect of quercetin on p21waf1/cip1, p53, and MAP-kinases without affecting intracellular generation of reactive oxygen species in vitro. J. Nutr. 135, 609–614.
Monari M, Trinchero A, Calabrese C, Cattani O, Serrazanetti GP, Foschi J et al. (2006). Superoxide dismutase in gastric adenocarcinoma: is it a clinical biomarker in the development of cancer? Biomarkers 11, 574–584.
Nagao M, Morita N, Yahagi T, Shimizu M, Kuroyanagi M, Fukuoka M et al. (1981). Mutagenicities of 61 flavonoids and 11 related compounds. Environ. Mutagen. 3, 401–419.
Drug and Chemical Toxicology Downloaded from informahealthcare.com by Taipei Medical University on 08/08/11
Okamoto T (2005). Safety of quercetin for clinical application (review). Int. J. Mol. Med. 16, 275–278.
Oliveira NG, Rodrigues AS, Chaveca T, Rueff J (1997). Induction of an adaptive response to quercetin, mitomycin C, and hydrogen peroxide by low doses of quercetin in V79 Chinese hamster cells. Mutagenesis 12, 457–462.
OECD: Organization for Economic Cooperation and Development Test Guideline 471 (1997). Bacterial reverse mutation test. In, OECD Guideline for Testing of Chemicals. Paris, France, Organization for Economic Cooperation and Development.
Petersen CS, Weisman K (1995). Quercetin and kaempherol: an argument against the use of podophyllin? Genitourin. Med. 71, 92–93.
Reynold JEF, Prasad AB (1996). Podophyllum. In, Martindale, The Extra Pharmacopoeia, 31st ed (p. 1092). London, UK, Royal Pharmaceutical Society.
Rice-Evans C (2001). Flavonoid antioxidants. Curr. Med. Chem. 8, 797–807.
Rivera A, Tyring SK (2004). Therapy of cutaneous human Papillomavirus infections. Dermatol. Ther. 17, 441–448. Rothe G, Valet G (1990). Flow cytometric analysis of respiratory
burst activity in phagocytes with hydroethidine and 2’,7’-dichlorofluorescin. J. Leuk. Biol. 47, 440–448.
Sahu RK, Basu R, Sharma A (1981). Genetic toxicological of some plant flavonoids by the micronucleus test. Mutat. Res. 89, 69–74. Saito A, Sugisawa A, Umegaki K, Sunagawa H (2004). Protective
effects of quercetin and its metabolites on H2O2-induced chromosomal damage to WIL2-NS cells. Biosci. Biotechnol.,
Biochem. 68, 271–276.
Samhan-Arias AK, Martín-Romero FJ, Gutiérrez-Merino C (2004). Kaempferol blocks oxidative stress in cerebellar granule cells and reveals a key role for reactive oxygen species production at the plasma membrane in the commitment to apoptosis. Free Rad. Biol. Med. 37, 48–61.
Silva ID, Rodrigues A, Gaspar J, Maia R, Laires A, Rueff J (1996). Mutagenicity of kaempferol in V79 cells: the role
of cytochromes P450. Teratogen. Carcinogen. Mutagen. 16, 229–241.
Silva ID, Rodrigues AS, Gaspar J, Maia R, Laires A, Rueff J (1997). Involvement of rat cytochrome 1A1 in the biotrans-formation of kaempferol to quercetin: relevance to the genotoxicity of kaempferol. Mutagenesis 12, 383–390. Suter W, Hartmann A, Poetter F, Sagelsdorff P, Hoffmann P, Martus
HJ (2002). Genotoxicity assessment of the antiepileptic drug AMP397, an Ames-positive aromatic nitro compound. Mutat. Res. 518, 181–194.
ten Kate M, van der Wal JB, Sluiter W, Hofland LJ, Jeekel J et al. (2006). The role of superoxide anions in the development of distant tumour recurrence. Br. J. Cancer 95, 1497–1503. van Duursen MB, Sanderson JT, de Jong PC, Kraaij M,
van den Berg M (2004). Phytochemicals inhibit cate-chol-O-methyltransferase activity in cytosolic fractions from healthy human mammary tissues: implications for cate-chol estrogen-induced DNA damage. Toxicol. Sci. 81, 316–324. Vanherweghem JL, Depierreux M, Tielemans C, Abramowicz D,
Dratwa M, Jadoul M et al. (1993). Rapidly progressive inter-stitial renal fibrosis in young women: associated with sliming regimen including Chinese herbs. Lancet 341, 387–391. Wang IK, Lin-Shiau SY, Lin JK (1999). Induction of apoptosis
by apigenin and related flavonoids through cytochrome C release and activation of caspase-9 and caspase-3 in leukae-mia HL-60 cells. Eur. J. Cancer 35, 1517–1525.
Wang H Joseph JA (1999). Structure-activity relationships of quercetin in antagonizing hydrogen peroxide-induced calcium dysregulation in PC12 cells. Free Rad. Biol. Med. 27, 683–694. West WM, Ridgeway NY, Morris AJ, Sides PJ (1982). Fatal
podo-phyllin ingestion. S. Med. J. 75, 1269–1270.
Xiao PG, Li DP, Yang SL(eds) (2002). Modern Chinese Material Medica Volume I (pp. 13–16), Volume II (pp. 70–74). Beijing, China, Chemical Industry Press.
Yamashita N, Tanemura H, Kawanishi S (1999). Mechanism of oxidative DNA damage induced by quercetin in the pres-ence of Cu (II). Mutat. Res. 425, 107–115.
Drug and Chemical Toxicology Downloaded from informahealthcare.com by Taipei Medical University on 08/08/11