Effect of Temperature and Extrusion Pass on the Consolidation
of Magnesium Powders Using Equal Channel Angular Extrusion
Hsiao-Chien Lee, Chuen-Guang Chao
+, Tzeng-Feng Liu, Che-Yi Lin and Hsiao-Chung Wang
Department of Materials Science and Engineering, National Chiao Tung University, Hsinchu, Taiwan 30049, Republic of China
The microstructure and characteristics of bulk magnesium consolidated from Mg powder by equal channel angular extrusion (ECAE) were investigated. Cu cansfilled with Mg powder, of about 74 µm in diameter, were ECAE processed for one, two and four passes via the Bc route at 473, 523 and 573 K. The microstructure of ECAE-processed samples was observed by OM and SEM. The density of each sample was determined using Archimedes’ principle. Microhardness and compression tests were conducted to investigate the mechanical properties of each ECAE-processed sample. The best consolidated condition between powders was achieved after four passes of ECAE at 573 K. Density at 98.4% of the ideal density of bulk Mg was achieved, microhardness was about 49 Hv, and compressive yield stress was about 100 MPa.
[doi:10.2320/matertrans.M2013006]
(Received January 7, 2013; Accepted February 4, 2013; Published March 15, 2013) Keywords: magnesium, powder metallurgy, equal channel angular extrusion, consolidation
1. Introduction
Recently, magnesium-based alloys have become attractive materials because of their low density, good machinability, high specific strength and high stiffness. However, the hexagonal closed-packed (HCP) structure of Mg alloys leads to poor formability and ductility at room temperature. Experiments show that by reducing the grain size of Mg alloy to submicron, good ductility at room temperature1) and superplasticity at low temperature2)are evident. Several techniques can be used to obtain ultrafine-grain Mg alloys, such as equal channel angular extrusion (ECAE),3,4) accumulative roll bonding (ARB),5) high pressure torsion (HPT),6) and powder metallurgy.7) For Mg alloys, a submicron grain size can be achieved by numerous passes of ECAE,3,4) ARB,5) or HPT,6) but not nanometer grain. To obtain nanometer-grain bulk Mg alloys, ECAE processing by the powder-in-tube technique, associated with severe strain,810)is effective.
Powder-in-tube ECAE under different temperatures has been used to consolidate a variety of powders, such as those of copper,810)aluminum,1114)intermetallic compounds,15,16) and even magnesium.17,18) The research in these areas has demonstrated that it is possible to achieve bulk materials of nanometer grain size. Karman’s research9) shows that bulk copper with the smallest grain size of <100 nm has been obtained by ECAE. Other experiments show that magnesium powder can be consolidated into bulk with nanometer grain by powder-in-tube ECAE.17,18)In studies on consolidation of magnesium powder by ECAE, only the feasibility of deriving fully dense bulk magnesium from the ECAE processing of magnesium powders has been discussed. No results have been published on the factors, such as temperature and/or number of ECAE passes, that influence the consolidated condition of magnesium powders. Senkov’s research14) shows that temperature influences the consolidation of powders because the ductility of powders changes under different temperatures. There is also no research on the
mechanical properties of bulk magnesium consolidated from Mg powder.
In this study, ¹200 mesh grade pure magnesium powder is consolidated by ECAE for one, two and four passes (N) under different elevated temperatures (473, 523 and 573 K). We discuss the influence of these parameters on the consolidated condition of the powders along with the mechanical properties of the bulk magnesium consolidated from the powder.
2. Materials and Methods
The powder used in this study was magnesium with a purity of 99.8% and ¹200 mesh grade (diameter of ³74 µm) in size. For the ECAE consolidation experiments, a copper can was sufficiently filled with magnesium powder and then compacted by mechanical force. After filling, the can was sealed with a copper cylinder measuring³11 mm in diameter and 10 mm in height. The can, illustrated in Fig. 1, had outer dimensions of 18© 18 © 65 mm3 and inner chamber with dimensions of 12 mm in diameter and 50 mm in depth (the space filled with magnesium powder). The density of the as-compacted powder was determined using Archimedes’ principle. To consolidate the powder by ECAE, the sample was inserted into a preheated ECAE die with a 18© 18 © 70 mm3channel having a 120° corner and held for 15 min to ensure that the temperature of the can equilibrated with that of the ECAE die. Then the samples were extruded for one, two and four passes in route Bc at 473, 523 and 573 K, and at an extrusion rate of 2 mm/min. The microstructure and chemical composition of the powder and ECAE-processed samples were observed by SEM (JOEL-6500) and EDX. X-ray was used to calculate grain size of samples. Grain
Fig. 1 Illustration of copper can tofill powder.
+Corresponding author, E-mail: cgchao@gmail.com
Materials Transactions, Vol. 54, No. 5 (2013) pp. 765 to 768
size was calculated by Scherrer’s function according to X-ray result. The density of each sample is defined using Archimedes’ principle. For microstructure observation, samples were polished and etched with a solution of 10% HNO3and 90% alcohol. A microhardness (Hv) test, with a load of 100 g and a dwell time of 10 s, was performed to establish the microhardness of the bulk magnesium con-solidated by ECAE from powders. A compression test of each specimen was conducted under a constant strain rate of 10¹3·s¹1.
3. Results and Discussion
Figure 2 shows that the magnesium powder size was ³74 µm in diameter. EDX results indicated that the magnesium powder contained only magnesium and oxygen. The average percentage of oxygen in the magnesium powder was about 4.7 mass%, which corresponds to 7.5 mass% of magnesium oxide in the magnesium powder. The density of magnesium and magnesium oxide is 1738 and 3580 kg/m3, respectively. Therefore, the ideal density of this magnesium powder should be 1880 kg/m3, which should also be ideal density of ideally consolidated bulk magnesium.
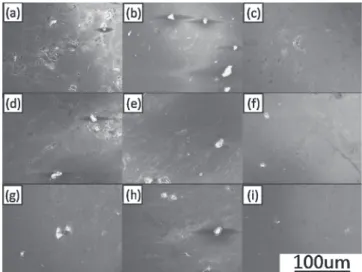
Microstructures of the as-ECAE-processed samples are shown in Fig. 3. Deformed magnesium particles, pores and oxides can be seen. First, we observe the change in pores under different numbers of ECAE passes and at different
temperatures. After one pass of ECAE, coarse pores are obvious. With increasing passes of ECAE, pores become eliminated. After four passes, pores are barely visible. The quantity of pores also decreases with increasing ECAE temperature. In Fig. 3(i), which shows samples ECAE processed for four passes at 573 K, the fewest pores can be seen. As for oxides, severly aggregated oxides appear on the boundary of particles after one pass of ECAE. After four passes, aggregation of oxides becomes finer and oxides are distributed more uniformly on the boundary of particles. The aggregation effect of oxides is also alleviated for samples ECAE processed at higher temperature. After four passes of ECAE at 473 K, the diameter of oxide aggregates on the particle boundary is about 29.4 µm on average, as shown in Fig. 4(a). However, after four passes of ECAE at 573 K, the diameter of oxide aggregates decreases to an average of 14.5 µm, as shown in Fig. 4(b). The amount of oxides after each pass of ECAE at each temperature was also calculated from a great number of SEM images. It is worth noting that the amount of oxides does not increase with increasing passes and temperature of ECAE. From the EDX results, the oxides in Fig. 3 are confirmed to be magnesium oxide, as shown in Fig. 5. Grain size of samples were observed by SEM under higher magnification. Figure 6 stands for grain size of sample after four passes of ECAE at 573 K and shows grain size is about 90 nm in average. Figure 7 is a typical X-ray result of sample after four passes of ECAE at 573 K. The peak with highest intensity in Fig. 7 is used to calculate grain size. Grain size of samples after four passes of ECAE at 473, 523 and 573 K was calculated to be 90.7, 91.9 and 92.8 nm, respectively. ECAE process which is able tofine grains into smaller size leads grain size of samples ECAEed at different temperature to have similar grain size. Due to the high temperature during ECAE, samples undergo recrystallization which reduces texture of samples. Therefore, no texture was observed in Fig. 6.
Fig. 2 (a) SEM image and (b) EDX result of magnesium powder.
Fig. 3 (a)(b)(c) Samples ECAEed at 473 K for 1, 2, 4 passes, respectively. (d)(e)(f ) Samples ECAEed at 523 K for 1, 2, 4 passes, respectively. (g)(h)(i) Samples ECAEed at 573 K for 1, 2, 4 passes, respectively.
Fig. 4 SEM images of oxides aggregation for sample after 4th pass of ECAE at (a) 473 K and (b) 573 K.
Fig. 5 (a) Oxidation in sample after 1st pass of ECAE at 473 K (b) EDX result of Fig. 5(a).
H.-C. Lee, C.-G. Chao, T.-F. Liu, C.-Y. Lin and H.-C. Wang 766
Figure 8 demonstrates the densities of the samples after each pass of ECAE at three different temperatures. According to the previous result, the ideal density of consolidated bulk magnesium is assumed to be the same as that of the initial magnesium powder, i.e., 1880 kg/m3. The density of as-compacted bulk magnesium is about 1000 kg/m3, which means a great quantity of pores remains in the bulk magnesium after the cold press process. It also indicates that cold pressing does not deform magnesium powder tofill the pores between the magnesium powder particles. After 1, 2 and 4 passes of ECAE at 473 K, the density is 1650, 1730 and 1820 kg/m3, respectively. At 523 K, the density is 1720, 1740 and 1850 kg/m3, respectively. At 573 K, the density is 1740, 1780 and 1850 kg/m3, respectively. Porosity was determined by comparing the density with the ideal density, and the results (shown in Table 1) show that the porosity decreases obviously after one pass of ECAE then decreases slightly after 2 and 4 passes. During first pass, magnesium powder is deformed servely and most pores between particles are filled, which leads to the significat decrease in porosity. After four passes, most of the residual pores are eliminated and the lowest porosity is attained. Results also show that temperature greatly affects porosity. Magnesium particles are softer at higher temperature; therefore, they deform more easily and their pores fill. This characteristic makes the decrease in porosity more evident under the same number of passes but at higher temperature.
Figure 9 shows the microhardness of consolidated bulk magnesium. At each ECAE temperature, the hardness values of the samples after one pass are lower than those after two passes; similarly, the hardness values after two passes are lower than those after four pass. The increase in microhard-ness with number of passes is due to the elimination of pores and the more uniform magnesium oxide dispersion in samples after ECAE processing. The higher the ECAE temperature, the greater is the hardness that can be achieved. As mentioned above, higher temperature exerts the best effect on eliminating pores and distributing magnesium oxide. Therefore, hardness increases with fewer pores at higher temperature. Consequently, the highest hardness is achieved in samples after four passes of ECAE at 573 K.
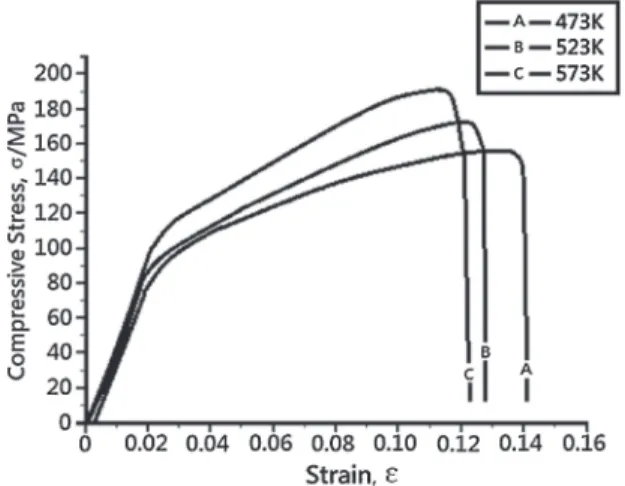
Figure 10 shows the results of the compression test for samples after four passes of ECAE at 473, 523 and 573 K. After four passes at 473 K, ultimate compressive stress (UCS) and compressive yield stress (CYS) of the consolidated bulk Mg achieve 155 and 80 MPa, respectively. By contrast, UCS and CYS of samples after four passes at 573 K achieve 193 and 100 MPa, respectively. Grain size is an important factor
Fig. 7 X-ray result of sample after 4 passes of ECAE at 573 K.
Fig. 8 Density results of each consolidation condition.
Fig. 9 Microhardness (Hv) results of each consolidation condition. Fig. 6 Higher magnification SEM image of sample after four passes of
ECAE at 573 K.
Table 1 Porosity of sample after each consolidation condition. As pressed N= 1 N= 2 N= 4 473 K 46.8% 12.22% 8.0% 3.19% 523 K 46.8% 8.5% 7.4% 1.6% 573 K 46.8% 7.4% 5.3% 1.6% Effect of Temperature and Extrusion Pass on the Consolidation of Magnesium Powders Using Equal Channel Angular Extrusion 767
effect on compressive stress of samples. However, grain size of samples ECAEed for four passes at 473, 523 or 573 K are almost the same. Therefore, we should focus on other aspects that influence the compressive stress of samples. Li’s research has demonstrated that for AZ91/SiC composite, the particle size of SiC has a great effect on the tensile properties. The yield strength and ultimate yield strength of AZ91/SiC increase when the particle size of SiC decreases; however, elongation of AZ91/SiC composite decreases slightly.19) In this study, we have similar results. Increases in UCS and CYS for samples ECAE processed at 573 K occur because of the decreasing size of the oxide aggregates. The previous result shows that the diameter of oxide aggregates decreases from about 29.4 to 14.5 µm on average. This reduction in size will lead to better UCS and CYS for consolidated bulk magnesium. A slight reduction in elongation of samples ECAE processed at higher temperature is also observed in this study, due to the smaller size of oxide aggregates. 4. Conclusion
This experiment assessed consolidation of magnesium powder in a copper can at different numbers of equal channel angular extrusion passes at elevated temperature. The consolidated condition of the magnesium powder after one, two and four passes of ECAE at 473, 523 and 573 K can be summarized as follows:
(1) Four passes of ECAE were needed to consolidated magnesium powder at 473, 523 and 573 K. The highest density of consolidated bulk magnesium was achieved after four passes of ECAE at 523 and 573 K. That density was 1850 g/m3, which is 98.4% of ideal density.
(2) Grain size of samples after four passes of ECAE at 473, 523 and 573 K are 90.7, 91.9 and 92.8 nm. Although grain size is an important effect on compressive properties, the small difference in grain size doesn’t lead to evidence difference in compressive properties. Therefore the size of oxide aggregates may influence compressive properties.
(3) Four passes of ECAE at elevated temperature
deformed magnesium powder to fill pores and break oxide aggregates into smaller sizes, leading to a more uniform dispersion of magnesium oxide. The samples after four passes of ECAE at 573 K possessed the best mechanical properties; microhardness, UCS, CYS and Young’s modulus were 49 Hv, 193 MPa, 100 Mpa and 42.5 GPa, respectively.
(4) The optimal condition for consolidating magnesium powder was four passes of ECAE at 573 K.
REFERENCES
1) W. J. Kim, S. I. Hong, Y. S. Kim, S. H. Min, H. T. Jeong and J. D. Lee:
Acta Mater. 51 (2003) 32933307.
2) M. Mabuchi, K. Ameyama, H. Iwasaki and K. Higashi:Acta Mater. 47 (1999) 20472057.
3) J. Li, L. Dongliang, M. Dali, Z. Xiaoqing, C. Bin and D. Wenjiang:
Mater. Sci. Eng. A 423 (2006) 247252.
4) Y. Li, D. Zhang, W. Chen, Y. Liu and G. Guo:J. Mater. Sci. 39 (2004) 37593761.
5) M. T. Pérez-Prado, J. A. del Valle and O. A. Ruano:Scr. Mater. 51 (2004) 10931097.
6) M. Kai, Z. Horita and T. G. Langdon:Mater. Sci. Eng. A 488 (2008) 117124.
7) W. Fang, W.-b. Fang and H.-f. Sun:J. Alloy. Compd. 509 (2011) 4887 4890.
8) J. Robertson, J.-T. Im, I. Karaman, K. T. Hartwig and I. E. Anderson:
J. Non Cryst. Solids 317 (2003) 144151.
9) I. Karaman, M. Haouaoui and H. J. Maier:J. Mater. Sci. 42 (2007) 15611576.
10) M. Haouaoui, I. Karaman, H. J. Maier and K. T. Hartwig: Metall. Mater. Trans. A 35 (2004) 29352949.
11) O. N. Senkov, D. B. Miracle, J. M. Scott and S. V. Senkova:J. Alloy. Compd. 365 (2004) 126133.
12) R. Derakhshandeh. H and A. Jenabali Jahromi:Mater. Des. 32 (2011) 33773388.
13) H. Asgharzadeh, A. Simchi and H. S. Kim:Metall. Mater. Trans. A 42 (2011) 816824.
14) O. N. Senkov, S. V. Senkova, J. M. Scott and D. B. Miracle:Mater. Sci. Eng. A 393 (2005) 1221.
15) A. V. Nagasekhar, Y. Tick-Hon and K. S. Ramakanth:Appl. Phys. A 85 (2006) 185194.
16) A. V. Nagasekhar, Y. Tick-Hon, R. K. Guduru and K. S. Ramakanth:
Phys. C 466 (2007) 174180.
17) M. Moss, R. Lapovok and C. J. Bettles:J. Miner. Met. Mater. Soc. 59 (2007) 5457.
18) G. Çakmak and T. Öztürk:J. Mater. Sci. 46 (2011) 55595567.
19) L. Li, M. O. Lai, M. Gupta, B. W. Chua and A. Osman:J. Mater. Sci. 35 (2000) 55535561.
Fig. 10 Results of compression test for samples after 4th pass of ECAE at 473, 523 and 573 K.
H.-C. Lee, C.-G. Chao, T.-F. Liu, C.-Y. Lin and H.-C. Wang 768