Sea-Fue Wang,a)Yung-Fu Hsu, Tzuu-Hsing Ueng, and Chung-Chuang Chiang Department of Materials and Minerals Resources Engineering, National Taipei University of Technology, Taipei, Taiwan, Republic of China
Jinn P. Chu
Institute of Materials Engineering, National Taiwan Ocean University, Keelung, Taiwan, Republic of China
Chi-Yuen Huang
Department of Mineral and Petroleum Engineering, National Cheng Kung University, Tainan, Taiwan
(Received 16 May 2002; accepted 18 February 2003)
Preparation of dense and phase-pure Ba2Ti9O20is generally difficult to achieve using
a solid-state reaction, due to the presence of several thermodynamically stable compounds in the vicinity of the desired composition. This work investigated the effects of various additives (TiO2, MnO, and ZrO2) on the densification,
microstructural evolution, phase stability, and dielectric properties of Ba2Ti9O20.
Ceramics with theoretical density of 艌95% were achieved in all cases after sintering at 1300 °C. A pure Ba2Ti9O20phase was obtained by treating the material with TiO2
additions (艋5.6 wt.%) and sintering at temperatures ranging between 1200 and
1350 °C. Ba2Ti9O20 is a nonstoichiometric compound that can accommodate an excess
amount of TiO2. As the temperature was increased, pure Ba2Ti9O20partially
decomposed and formed a mixture of BaTi4O9and Ba2Ti9O20. The ceramic with
excess TiO2sintered at 1390 °C possessed a higher permittivity and a lower quality
factor due to the larger grain size and lower density. For ceramic with the addition of ZrO2(艋6 wt.%), pure Ba2Ti9O20phase was obtained after sintering between 1200 and
1390 °C, and the quality factor was improved. The decomposition temperature of the Ba2Ti9O20phase was greater than 1390 °C. For sintering temperatures艌1350 °C,
the extent of Ba2Ti9O20phase decreased with MnO additions. As the MnO content
reached 0.5 wt.%, only BaTi4O9and TiO2 phases existed, suggesting a decrease in the
decomposition temperature of Ba2Ti9O20with the addition of MnO. The microwave
properties of the ceramics degraded significantly at the sintering temperature of 1390 °C.
I. INTRODUCTION
Commercial wireless communication has been a rapid growth market during the past decade. This technology advancement was made possible, in part, with the recent revolution in the miniaturization of microwave circuits by using low-loss and temperature-stable dielectric reso-nators. Several ceramic materials have been developed for use as microwave resonators, such as (Zr0.8Sn0.2)TiO4,
Ba(Zn,Ta)O3, BaTi4O9, and Ba2Ti9O20. Among the
vari-ous candidates, Ba2Ti9O20, which contains 81.8 mol% of
TiO2 and 18.2 mol% of BaO, has received much
atten-tion for its desirable microwave properties.1,2 It has a good quality factor (8000 at a frequency of 4 GHz), a high dielectric constant (39.8), and a low temperature coefficient (f⳱ 2 ppm/°C).
3
The crystal structure of
Ba2Ti9O20 is layered, having a hexagonal, close-packed
arrangement of Ba and O, with Ti occupying the appro-priate octahedral sites, making up a primitive triclinic unit cell, forming six crystal-structure layers.4
For the preparation of monophasic samples of Ba2Ti9O20from BaCO3and TiO2by solid-state reaction,
the stoichiometry and fabrication parameters must be precisely controlled, since there are various thermody-namically stable compounds in the vicinity of the desired composition in the TiO2-rich BaO–TiO2 system:
Ba6Ti17O40; BaTi3O7; BaTi4O9; BaTi5O11; Ba4Ti13O30. 5,6
O’Bryan and Thomson reported that the formation of some Ba2Ti9O20 in calcination is required for
the complete transformation of mixed powders into monophase Ba2Ti9O20 during sintering.
7
Jaakola et al. noted that a reaction temperature of 1150 °C or higher is necessary for the crystallization of Ba2Ti9O20
a)
phase within a reasonable time span in a mixture of BaCO3–TiO2.
8
Negas et al.9 in studies on subsolidus phase relations in the BaTiO3–TiO2 system, reported
that the upper temperature at which Ba2Ti9O20 was
stable was near 1300 °C, while O’Bryan and Thomason reported that it was near 1400 °C and, above 1420 °C, that the phase underwent a peritectoid decomposi-tion.10
Several studies have suggested that diffusion was the rate-limiting factor in controlling the forma-tion of Ba2Ti9O20
4,10,11
The precursor transforms more easily into Ba2Ti9O20 if a shorter diffusion distance
is needed. Wu and Wang gave a phase development sequence of BaTi5O11→ BaTi4O9→ Ba2Ti9O20 and
proposed that, in addition to diffusion, the nuclea-tion barrier is another important factor responsible for the formation of Ba2Ti9O20.
12
A lower formation rate of Ba2Ti9O20, compared with those of BaTi4O9 and
BaTi5O11, was attributable to high surface and interface
energies. Yu et al. proposed that stress might arise along the crystal structure layers because of the structural fac-tor, which in turn would result in a high potential ener-gy barrier for nucleation.13 Fang et al. suggested that the difficulty in completely forming Ba2Ti9O20 in a
short time might be due to the fact that remain-ing BaTi4O9 grains were stressed by the surrounding
Ba2Ti9O20 grains. 14
On account of the above reasons, the preparation of single-phase Ba2Ti9O20by solid-state
reaction appears to be very challenging. Chemical routes such as the sol-gel processing, citrate precursor methods, and alkoxide precursor synthesis were developed by several research groups to obtain a homogenous mixing of the precursors and thus promote the formation of Ba2Ti9O20.
15–18
The crystallization temperature was shown to be lowered to 1100 °C using a novel wet chemical route.15
Though the chemistry of Ba2Ti9O20 seems to be
complex, efforts have been made toward the improve-ment of the dielectric properties using suitable addi-tives. It was found that the addition of a small quantity of Mn would lead to a large increase in the Q value.19,20 Nd2O3 addition was found to increase the dielectric
constant and temperature coefficient but reduced the quality factor.8 Using CeO2 as a sintering aid did
not change the dielectric constant, whereas Qudecreased
and f increased with CeO2 content. Some
investiga-tions have shown that addiinvestiga-tions of solid solution addi-tives, such as SnO2, ZrO2, SrO, Al2O3, or Bi2O3,
are essential for the formation of a pure Ba2Ti9O20
phase.3,4,13,21,22 Nevertheless, contradicting results as well as detrimental effects due to dopant addi-tion were also addressed in the literature.4 There-fore, uncovering the dependence of the crystallization behavior, microstructure, and properties of Ba2Ti9O20
upon the dopant additions is of great interest to develop high-performance microwave dielectrics.
To clarify the role of additives on the stabilization of the Ba2Ti9O20 phase, a systematic investigation on the
effects of additives on the densification, microstructural evolution, phase stability, and microwave dielectric properties of Ba2Ti9O20 was performed in this study.
Three additives, including TiO2, MnO, and ZrO2, were
used in the present investigation. TiO2 was selected to
explore the effect of nonstoichiometry on the Ba2Ti9O20
formation. MnO and ZrO2were chosen on account of the
differences in their solubility and chemical nature.18,23,24 MnO has little solubility in Ba2Ti9O20. However, the
solubility of ZrO2can reach as much as 5 mol% due to
the substitution of Ti4+by Zr4+.24
II. EXPERIMENTAL
The detailed experimental flow chart for the study is shown in Fig. 1. A host material, containing 81.8 mol% TiO2 and 18.2 mol% BaO was used throughout this
work. The powders were mixed in methyl alcohol solu-tion using polyethylene jars and zirconia media for 8 h. After drying, the powders were then calcined/preheated at 1000 °C for 6 h. The powders were then mixed with different amounts of TiO2, MnO, or ZrO2, using the
con-ventional solid-state mixing process for 8 h in methyl alcohol. Subsequently, a second calcination/milling was performed to ensure a more homogeneous distribution of the various ingredients. The calcination was carried out at 1000 °C for 6 h, and subsequent milling was in methyl alcohol for 8 h. The powders had an average mean par-ticle size of 0.73m, measured by light scattering (Zeta 1000) and an average surface area of approximately 7.60 mm2
/g from Brunauer–Emmett–Teller analysis. Phase identification on the calcined powders was per-formed using x-ray diffraction (XRD, Rigaku D/max-B, Tokyo, Japan). Differential thermal analysis (DTA) was performed at a heating rate of 10 °C/min, using a Perkin-Elmer calorimeter, Series 1700 DTA (Shelton, CT), on mixtures to evaluate melting reactions.
After calcination, the powders were combined with a 3.5 wt.% of 15 wt.% poly(vinyl alcohol) (PVA) solution and then pelletized into disc shapes using a uniaxial pres-sure of 5 tons/cm2. The samples were then heat treated at 550 °C for 2 h to eliminate the PVA, followed by sintering at 1200, 1250, 1300, 1350, or 1390 °C for 6 h (heating rate⳱ 10 °C/min). The densities of specimens were measured using the liquid displacement method. XRD, scanning electron microscopy (SEM, JOEL JSM-T330A, Tokyo, Japan) and energy dispersive spectros-copy (EDS) on the sintered surfaces were used to confirm the formation of phases and characterize the microstruc-tures. For a semiquantitative analysis of the phase concentration of BaTi4O9 and Ba2Ti9O20, the
of BaTi4O9 (2 ⳱ 29.92°) and the (421) reflection of
Ba2Ti9O20 (2 ⳱ 28.46°) were recorded by
step-scanning around the Bragg peaks.
To understand the effect of additives on sintering, di-latometric analyses were performed to characterize the shrinkage of the ceramics with respect to temperature. Experiments were performed using a DIL 402C dilatom-eter (NETZSCH, Geratebau GmbH, Selb) in air and at a heating rate of 3 °C/min. Microwave dielectric properties were measured using the Kobayashi method25
with a network analyzer (Agilent 8722).
III. RESULTS
The results of the effects of excess TiO2, MnO, and
ZrO2on the Ba2Ti9O20ceramic are shown with respect
to densification, microstructural evolution, phase stabil-ity, and dielectric properties.
A. Densification
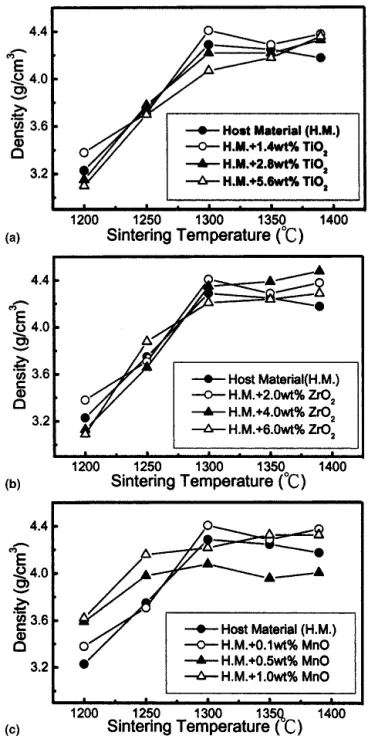
Figures 2(a)–2(c) show the densities of the calcined host material (BaO/TiO2⳱ 18.18/81.82) with different
amounts of TiO2, ZrO2, or MnO sintered at various
temperatures, respectively. For the host material with TiO2 additions, sintered densities generally decreased
with increasing TiO2content. The densification profiles
for the ceramics with ZrO2additions are similar to those
of TiO2 additions. Density increases considerably with
the sintering temperature up to 1300 °C and then levels off above this temperature. With the addition of MnO, the results indicate that densification was significantly enhanced at sintering temperatures less than 1300 °C.
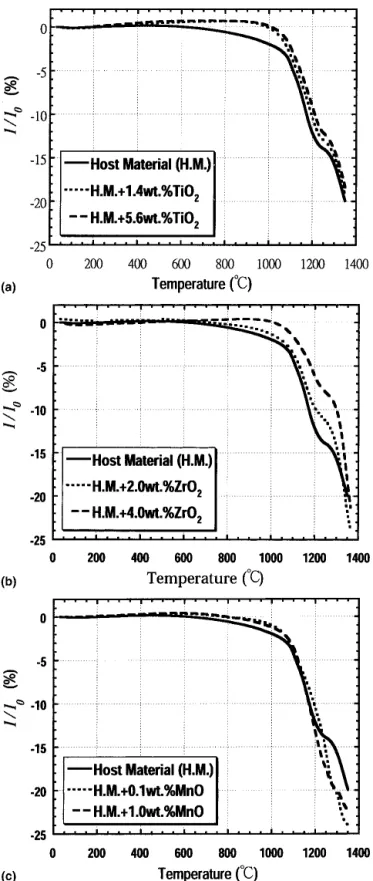
Sintering shrinkage of the pure host material and those with various amounts of TiO2, ZrO2, or MnO additions
were measured using dilatometric analyses. The results FIG. 1. Detailed experimental flow chart for this study.
FIG. 2. Sintered densities of pure host material (HM) with (a) TiO2, (b) ZrO2, and (c) MnO, additions at different sintering temperatures.
are shown in Figs. 3(a), 3(b), and 3(c) for the addi-tions of TiO2, ZrO2, or MnO, respectively. In general,
the shrinkage profiles of these samples are consistent with the density results shown in Fig. 2. It is evident
that there is a shoulder located on the contraction trend around 1250 °C, in all cases. A quench study was performed at temperatures between 1200 and 1300 °C during the dilatometric experiment, and phases were then identified by XRD. It appears that the shoulder likely corresponds to the following reaction: BaTi5O11
→ BaTi4O9+ TiO2. The shrinkage curves of ceramics
with ZrO2 and TiO2 additions were shifted to higher
temperatures compared with those of the pure host ma-terial. These curves eventually merged together at tem-peratures above 1300 °C.
To understand the possible melting reactions during heating, DTA analysis was performed on the host mate-rials mixed with 5.6 wt.% TiO2, 1.0 wt.% MnO, and
6.0 wt.% ZrO2. The results are shown in Fig. 4. Similar
to the pure host material, materials with the TiO2or ZrO2
additions show noticeable peak corresponding to a melt-ing reaction. However, for the material containmelt-ing MnO, there are two endothermic reactions occurring at tem-peratures of 1285 and 1325 °C, which could likely cor-respond to eutectic liquid formations.
B. Phase stability
Host materials and with various additives after calci-nation at 1000 °C were phase identified using XRD analysis. The XRD patterns, which are nearly identical, showed that they consist of BaTi4O9 and BaTi5O11
phases. No other detectable phases were found. This il-lustrates that the addition of TiO2, ZrO2, or MnO has no
effect on the phase ratio of BaTi5O11 and BaTi4O11.
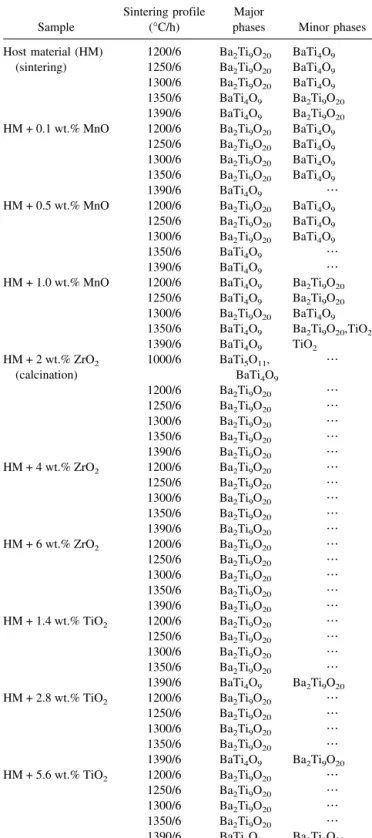
Table I summarizes the phases identified from the XRD patterns, for pure host materials and with various amounts of TiO2, ZrO2, or MnO additives sintered at
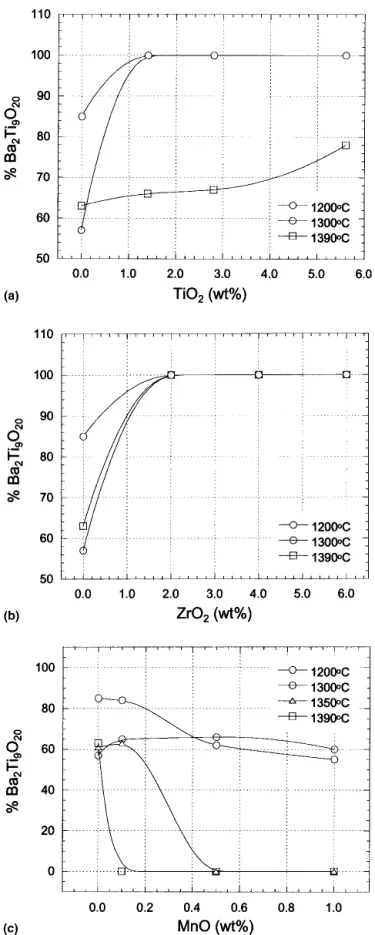
different temperatures. The percentages of Ba2Ti9O20in
each case, derived from semiquantitative analysis of XRD peaks, are plotted in Fig. 5. Pure host material sin-tered above 1200 °C produced a mixture of BaTi4O9and
Ba2Ti9O20(Table I). This is generally in agreement with
FIG. 3. Dilatometric shrinkage curves of pure calcined host material with different amounts of (a) TiO2, (b) ZrO2, and (c) MnO additions.
FIG. 4. DTA curves of host materials mixed with 5.6 wt.% TiO2, 6.0 wt.% ZrO2, and 1.0 wt.% MnO.
the results shown in the literature,26 even though few studies have reported the presence of BaTi5O11.
12,27
De-pending on the precursors and thermal history of the powders, BaTi5O11 was reported to decompose into
BaTi4O9 and TiO2 or Ba2Ti9O20 as the soaking time
proceeds at 1200 °C.28 For the materials with 1.4 wt.% TiO2(i.e., BaO/TiO2⳱ 17.56/82.44) and 5.6 wt.% TiO2
(i.e., BaO/TiO2⳱ 16.94/83.06), it is interesting to note
that sintering of these materials between 1200 and 1350 °C yielded a pure Ba2Ti9O20phase. As the sintering
temperature reached 1390 °C, it resulted in a mixture of BaTi4O9and a minor Ba2Ti9O20phase. For material with
2 and 6 wt.% ZrO2addition, sintered at various
tempera-tures, pure Ba2Ti9O20was obtained in all sintering
tem-peratures. It is evident that Ba2Ti9O20could be stabilized
by the additions of ZrO2[Fig. 5(b)]. None of TiO2, ZrO2,
or ZrTiO4was detectable.
The effect of Mn doping on the phase stability of Ba2Ti9O20 is shown in Fig. 5(c). As the sintering
tem-perature reached 1200 °C, a mixture of Ba2Ti9O20 and
BaTi4O9with Ba2Ti9O20as a major phase was observed.
At sintering temperatures above 1350 °C, the relative concentration of Ba2Ti9O20 phase decreased with
in-creasing MnO content. For ceramics containing 0.1 wt.% MnO, only BaTi4O9was found when the sintering
tem-perature reached 1390 °C. However, as MnO content ex-ceeded 0.5 wt.%, the disappearance of Ba2Ti9O20phase
lowered to 1350 °C. Results of this study apparently in-dicate that MnO addition inhibits the formation of Ba2Ti9O20.
C. Microstructural evolution
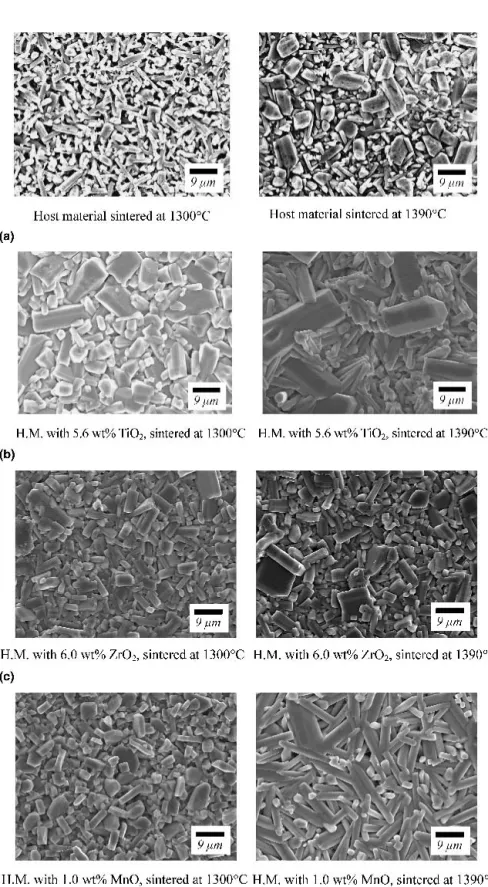
SEM micrographs of various well-densified specimens including pure host material and those with 5.6 wt.% TiO2, 6.0 wt.% ZrO2, or 1.0 wt.% MnO additives
sin-tered at 1300 and 1390 °C are shown in Figs. 6(a)–6(d), respectively. EDS was performed to characterize the compositions of the grains with different morphologies. The microstructural evolution confirms the observations of phases identified in the XRD patterns. In Fig. 6(a), a mixture of elongated, needlelike BaTi4O9 and angular
Ba2Ti9O20was seen in the host material. It is noted that
the growth of Ba2Ti9O20grains with temperature is
ap-parent. The ceramic with excess TiO2 after sintering at
1300 °C exhibited an angular Ba2Ti9O20microstructure,
as indicated in Fig. 6(b). The grain sizes increase with TiO2content as well as the temperature, particularly
the size of the angular Ba2Ti9O20 phase. However, in
Fig. 6(c), almost all the grains were angular Ba2Ti9O20
phase for the host material with ZrO2additions at 1300
and 1390 °C. It appears that the average grain size is not sensitive to the ZrO2 content as well as the sintering
temperature. In the case of ceramic with MnO addition and sintered at 1300 °C, the microstructures contain some small, granular, or columnar BaTi4O9distributed in
the matrix of angular Ba2Ti9O20grains. As sintering
tem-perature was raised to 1390 °C, only needle-shaped grains of BaTi4O9 were observed.
TABLE I. The phases identified from the XRD patterns for pure host materials and with various amounts of TiO2, ZrO2, or MnO additives sintered at temperatures between 1200 and 1390 °C for 6 h.
Sample
Sintering profile (°C/h)
Major
phases Minor phases Host material (HM) 1200/6 Ba2Ti9O20 BaTi4O9
(sintering) 1250/6 Ba2Ti9O20 BaTi4O9 1300/6 Ba2Ti9O20 BaTi4O9 1350/6 BaTi4O9 Ba2Ti9O20 1390/6 BaTi4O9 Ba2Ti9O20 HM + 0.1 wt.% MnO 1200/6 Ba2Ti9O20 BaTi4O9 1250/6 Ba2Ti9O20 BaTi4O9 1300/6 Ba2Ti9O20 BaTi4O9 1350/6 Ba2Ti9O20 BaTi4O9 1390/6 BaTi4O9 ⭈⭈⭈ HM + 0.5 wt.% MnO 1200/6 Ba2Ti9O20 BaTi4O9 1250/6 Ba2Ti9O20 BaTi4O9 1300/6 Ba2Ti9O20 BaTi4O9 1350/6 BaTi4O9 ⭈⭈⭈ 1390/6 BaTi4O9 ⭈⭈⭈ HM + 1.0 wt.% MnO 1200/6 BaTi4O9 Ba2Ti9O20 1250/6 BaTi4O9 Ba2Ti9O20 1300/6 Ba2Ti9O20 BaTi4O9 1350/6 BaTi4O9 Ba2Ti9O20,TiO2 1390/6 BaTi4O9 TiO2 HM + 2 wt.% ZrO2 (calcination) 1000/6 BaTi5O11, BaTi4O9 ⭈⭈⭈ 1200/6 Ba2Ti9O20 ⭈⭈⭈ 1250/6 Ba2Ti9O20 ⭈⭈⭈ 1300/6 Ba2Ti9O20 ⭈⭈⭈ 1350/6 Ba2Ti9O20 ⭈⭈⭈ 1390/6 Ba2Ti9O20 ⭈⭈⭈ HM + 4 wt.% ZrO2 1200/6 Ba2Ti9O20 ⭈⭈⭈ 1250/6 Ba2Ti9O20 ⭈⭈⭈ 1300/6 Ba2Ti9O20 ⭈⭈⭈ 1350/6 Ba2Ti9O20 ⭈⭈⭈ 1390/6 Ba2Ti9O20 ⭈⭈⭈ HM + 6 wt.% ZrO2 1200/6 Ba2Ti9O20 ⭈⭈⭈ 1250/6 Ba2Ti9O20 ⭈⭈⭈ 1300/6 Ba2Ti9O20 ⭈⭈⭈ 1350/6 Ba2Ti9O20 ⭈⭈⭈ 1390/6 Ba2Ti9O20 ⭈⭈⭈ HM + 1.4 wt.% TiO2 1200/6 Ba2Ti9O20 ⭈⭈⭈ 1250/6 Ba2Ti9O20 ⭈⭈⭈ 1300/6 Ba2Ti9O20 ⭈⭈⭈ 1350/6 Ba2Ti9O20 ⭈⭈⭈ 1390/6 BaTi4O9 Ba2Ti9O20 HM + 2.8 wt.% TiO2 1200/6 Ba2Ti9O20 ⭈⭈⭈ 1250/6 Ba2Ti9O20 ⭈⭈⭈ 1300/6 Ba2Ti9O20 ⭈⭈⭈ 1350/6 Ba2Ti9O20 ⭈⭈⭈ 1390/6 BaTi4O9 Ba2Ti9O20 HM + 5.6 wt.% TiO2 1200/6 Ba2Ti9O20 ⭈⭈⭈ 1250/6 Ba2Ti9O20 ⭈⭈⭈ 1300/6 Ba2Ti9O20 ⭈⭈⭈ 1350/6 Ba2Ti9O20 ⭈⭈⭈ 1390/6 BaTi4O9 Ba2Ti9O20
D. Dielectric properties
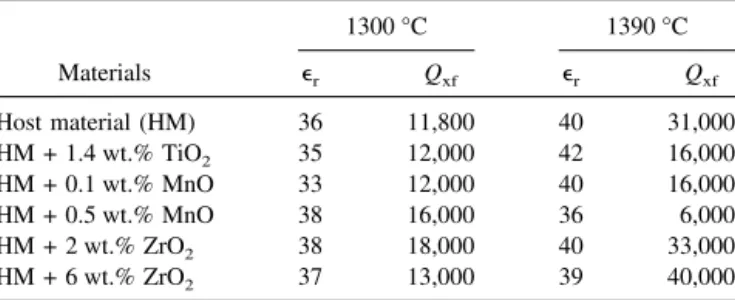
The effects of additives on the permittivity and Qxfof
the ceramics sintered at 1300 and 1390 °C are summa-rized in Table II. For the host materials, the dielectric properties are quite consistent with those reported in the literature.28
The permittivity and Qxf are larger for
the ceramic sintered at 1390 °C compared with those sintered at 1300 °C. Compared with the stoichiometric composition, the ceramic with excess 1.4 wt.% TiO2
sin-tered at 1390 °C possessed a larger permittivity and a lower quality factor. Similar values were observed for ceramics with TiO2contents of 2.8 and 5.6 wt.%. In the
case of ceramics with MnO additions and sintered at 1300 °C, the K and Qxf do not show much difference
compared with those of host materials sintered at 1300 °C. However, as the sintering temperature was in-creased to 1390 °C, the microwave properties of the ce-ramics degraded with the MnO level.
IV. DISCUSSION
In this study, densification was investigated using both dilatometry (continuous-heating process) as well as sin-tering (isothermal process) techniques. For a single-phase material, the densification processes obtained from both experiments are expected to be similar in nature. However, for a multiphase material, the densification would be dependent on the ratio of each phase present during the sintering and thus sensitive to the heating profile. From the sintering study, it was found that sig-nificant densification does not occur until 1300 °C, as reported in the literature.13,27,29 The theoretical density of the material is dependent on the volume fractions of various phases in the sintered ceramic, and theoretical densities of BaTi4O9, Ba2Ti9O20, BaTi5O11, and TiO2
(rutile) are 4.55, 4.61, 4.58, and 4.26 g/cm3
, respectively. Thus, it is reasonable to estimate that ceramics with theo-retical density of 艌95% were achieved in most cases after sintering at 1300 °C, based on the density curves (Fig. 2), SEM microstructures (Fig. 6), and phase struc-tures (Table I). As shown in Fig. 2(a), the sintered den-sity of ceramics with TiO2additions decreasing with the
extent of the additives is due to the formation of TiO2
(rutile) and BaTi4O9 after sintering. Their theoretical
densities (4.27 and 4.55 g/cm3, respectively) are lower compared with that of Ba2Ti9O20(4.61 g/cm
3
).
The shrinkage curves of materials with ZrO2and TiO2
additions [Figs. 3(a) and 3(b)] were shifted to higher temperatures compared with those of the pure host ma-terial. This is due to the larger volume fraction of Ba2Ti9O20 phase present, which inhibited the
densifica-tion during sintering. The shoulder appearing at approxi-mately 1250 °C (in all cases) corresponds to the following reaction which was verified by a quench study: BaTi5O11→ BaTi4O9+ TiO2. Associated with it is a
FIG. 5. Semiquantitative analysis of the extent of Ba2Ti9O20in the host material with various amounts of (a) TiO2, (b) ZrO2, and (c) MnO additives, determined by the integrated intensities of the (130) reflec-tion of BaTi4O9(2 ⳱ 29.92°) and the (421) reflection of Ba2Ti9O20 (2 ⳱ 28.46°).
1.65% volume expansion, based on a density calculation. Subsequently, BaTi4O9 reacts with TiO2 to form
Ba2Ti9O20. The sintering behavior of the ceramics with
MnO additions is different from the other ones. On the
basis of the results shown in Figs. 2(c) and 3(c), more densification was observed at temperatures below 1300 °C and the shoulder in the shrinkage curves was less obvious. Also, they appear to shrink more totally in FIG. 6. SEM micrographs for (a) pure host material and those with different amounts of (b) TiO2, (c) ZrO2, and (d) MnO additives sintered at 1300 and 1390 °C.
Fig. 3(c) and be of lower density in Fig. 2(c). Two pos-sibilities may contribute to these phenomena. One possibility is the presence of the liquid phase during sintering, which enhanced the densification. Also, the formation of liquid phase usually results in a larger shrinkage in dilatometric analyses, due to the presence of a small pressure exerted by the pushhead, compared with that of free sintering. The other possibility may be due to the addition of MnO altering the densification mecha-nism. The exact densification mechanism with the MnO addition remains unclear and thus requires further investigations.
The host material with additions of TiO2 or ZrO2
shows no sign of liquid-phase formation, which indicates that these materials are densified simply through a solid-state sintering mechanism. This is in contrast to that ob-served by Lin et al.4 They showed that the incongruent melting temperature of BaTi4O9dropped below 1390 °C
when ZrO2 was added. Also, there is no endothermic/
exothermic peak associated with the various phase trans-formations during sintering, which suggests that they are a diffusion-controlled process, as proposed by Jonker et al.30 and Yu et al.13 In most cases, sintered density tended to be lower with sintering temperature above 1300 °C. This is probably due to the formation of trapped porosity accompanying the growth of elongated grains.
It is generally accepted in the literature that the nomi-nal composition for Ba2Ti9O20 is 81.82% TiO2.
6
How-ever, it is difficult to obtain pure Ba2Ti9O20phase using
solid-state reaction from BaCO3 and TiO2 precursors,
due to the lack of homogeneity.14,29,31In this study, the concentration of Ba2Ti9O20 phase for ceramics sintered
at temperatures greater than 1300 °C was less than that sintered at 1200 °C (Fig. 5). This confirms the difficulty in obtaining pure Ba2Ti9O20 at temperatures above
1300 °C. Wu et al. reported that the extent of Ba2Ti9O20
formation during sintering is dependent on the amount of Ba2Ti9O20 present in the calcined powder.
12
However, our results show that the phase stability of Ba2Ti9O20
depends on the sintering temperature as well as the extent of TiO2, ZrO2, or MnO additives. Formation of
Ba2Ti9O20 is apparently enhanced by a small excess
of TiO2, as shown in Fig. 5(a). There is no detectable
TiO2 phase in the XRD pattern or corresponding SEM
microstructure [Fig. 6(a)]. It appears that excess TiO2
can be accommodated in the form of nonstoichiometric Ba2Ti9O20. This is similar to the observation of O’Bryan
and Thomson in their quenching experiments from the melts.10
Yet, the results are in contradiction to the report of Lin et al.,4
who suggested that excessive titanium does not influence the stability of Ba2Ti9O20. For material
with 2 and 6 wt.% ZrO2 addition, sintered at various
temperatures, pure Ba2Ti9O20was obtained in all
sinter-ing temperatures. None of TiO2, ZrO2, or ZrTiO4 was
detectable. The results are consistent with several studies reported in the literature.5,22,27,32,33 Ba2Ti9O20 could be
stabilized by the additions of ZrO2[Fig. 5(b)], though the
mechanism by which this occurs is still not clear. On the basis of XRD results, it is apparent that the upper limit temperature at which the Ba2Ti9O20 was
stable varied with the additives. Two mechanisms caus-ing the decomposition of the Ba2Ti9O20 phase were
reported in the literature. One is the peritectoid decom-position at 1393 °C, as reported by Kirby et al.28 (at 1420 °C by O’Bryan et al.10). The other is due to the existence of the curvature at the phase boundary of Ba2Ti9O20 at high temperatures. In this study, the
de-composition temperature of Ba2Ti9O20phase for the host
material with ZrO2 addition is greater than 1390 °C,
which is consistent with the reports available in the lit-erature. However, for host material with MnO addition, the decomposition temperature was reduced to a lower temperature. The decomposition of Ba2Ti9O20 is less
than 1350 °C when 0.5 wt.% MnO is added, which cor-responds to the endothermic liquid formation shown in Fig. 4. With excess TiO2 addition, pure Ba2Ti9O20
par-tially decomposed into BaTi4O9and TiO2, as indicated in
Fig. 5(a).
The permittivity and Qxf are generally larger for
ce-ramics sintered at 1390 °C compared with those sintered at 1300 °C (Fig. 6). Compared with the stoichiometric composition, the ceramic with 1.4 wt.% excess TiO2
sin-tered at 1390 °C possessed a larger permittivity and a lower quality factor due to the greater grain size and the lower density. For the ceramics with MnO added, the microwave properties of the ceramics degraded with increasing MnO level, as the sintering temperature in-creased to 1390 °C. This might have been the result of MnO segregation to the grain boundary due to the limited solubility. For the ceramics with the addition of ZrO2, the
quality factor was improved but had no appreciable ef-fect on the value of⑀, as reported in the literature.4
V. SUMMARY
The effects of TiO2, MnO, and ZrO2 on the
densifi-cation, microstructural evolution, phase stability, and di-electric properties of Ba2Ti9O20 were investigated. A
TABLE II. Dielectric properties of the host material with various amounts of additives sintered at 1300 and 1390 °C.
Materials 1300 °C 1390 °C ⑀r Qxf ⑀r Qxf Host material (HM) 36 11,800 40 31,000 HM + 1.4 wt.% TiO2 35 12,000 42 16,000 HM + 0.1 wt.% MnO 33 12,000 40 16,000 HM + 0.5 wt.% MnO 38 16,000 36 6,000 HM + 2 wt.% ZrO2 38 18,000 40 33,000 HM + 6 wt.% ZrO2 37 13,000 39 40,000
maximum density of 艌95% theoretical density was achieved in most cases after sintering at 1300 °C. For the host material with TiO2 additions (<5.6 wt.%), pure
Ba2Ti9O20 phase was observed from 1200 to 1350 °C,
which indicates that Ba2Ti9O20 is a nonstoichiometric
compound. Comparing with the stoichiometric com-position, the ceramic with excess TiO2 sintered at
1390 °C possesses a larger permittivity and a lower qual-ity factor due to the greater grain size and the lower sintered density.
With the addition of ZrO2(<6 wt.%), pure Ba2Ti9O20
was obtained between 1200 and 1390 °C. The quality factor of the dense ceramics with ZrO2 additions was
improved. For host material with MnO additions, the densification was enhanced by the presence of liquid phase below 1300 °C and the relative concentration of Ba2Ti9O20 phase decreased with MnO content at
tem-peratures higher than 1350 °C. As the MnO concentra-tion reached 0.5 wt.%, only BaTi4O9 and TiO2 phases
existed. The microwave properties of the ceramics de-grade significantly with the addition of MnO for ceramic sintered at 1390 °C. SEM observations are consistent with the results of XRD patterns.
REFERENCES
1. H.M. O’Bryan, Jr. J.K. Plourde, and J. Thomson, Jr., U.S. Patent No. 4 563 661 (1986).
2. H.M. O’Bryan, Jr., J.K. Plourde, and J. Thomson, Jr., U.S. Patent No. 3 938 064 (1976).
3. C. Chatterjee, A.N. Virkar, and A. Paul, J. Mater. Sci. 9, 1049 (1990).
4. W.Y. Lin, R.F. Speyer, W.S. Hackenberger, and T.R. Shrout, J. Am. Ceram. Soc. 82, 1207 (1999).
5. C.M. Cheng, C.F. Yang, S.H. Lo, and T.Y. Tseng, J. Eur. Ceram. Soc. 20, 1061 (2000).
6. H.M. O’Bryan and J. Thomson, J. Am. Ceram. Soc. 66, 66 (1983). 7. H.M. O’Bryan and J. Thomson, J. Am. Ceram. Soc. 57, 450
(1974).
8. T. Jaakola, J. Mottonen, A. Unsimaki, R. Rautioaho, and S. Leppavuori, Ceram. Int. 13, 151 (1987).
9. T. Negas, R.S. Roth, H.S. Parker, and D. Minor, J. Solid State Chem. 9, 297 (1974).
10. H.M. O’Bryan and J. Thomson, J. Am. Ceram. 57, 522 (1974) 11. T. Jaakola, A. Uusimaki, and S. Leppavuori, Int. J. High Technol.
Ceram. 2, 195 (1980).
12. J.M. Wu and H.W. Wang, J. Am. Ceram. Soc. 71, 869 (1988). 13. J. Yu, H. Zhao, J. Wang, and F. Xia, J. Am. Ceram. Soc. 77, 1052
(1994).
14. T.T. Fang, J.T. Shiue, and S.C. Liou, J. Eur. Ceram. Soc. 22, 79 (2002).
15. H.C. Lu, L.E. Burkhart, and G.L. Schrader, J. Am. Ceram. Soc. 74,968 (1991).
16. J.J. Ritter, R.S. Roth, and J.E. Blendell, J. Am. Ceram. Soc. 69, 155 (1986).
17. G. Pfaff, J. Mater. Sci. Lett. 12, 32 (1993).
18. J.H. Choy, Y.S. Han, J.H. Sohn, and M. Itoh, J. Am. Ceram. Soc. 78,1169 (1995).
19. S. Nomura, K. Tomaya, and K. Kaneta, Jpn. J. Appl. Phys. 22, 1125 (1983).
20. M.J. Lee, C.Y. Kim, B.D. You, and D.S. Kang, J. Mater. Sci. 6, 173 (1995).
21. K.H. Yoon, J.B. Kim, W.S. Kim, J. Mater. Res. 11, 1996 (1996). 22. H. Sreemoolanadhan, J. Isaac, P. Koshy, M.T. Sebastian, K.A. Jose,
and P. Mohanan, Br. Ceram. Trans. 94, 157 (1995).
23. W.Y. Lin and R.F. Speyer, J. Am. Ceram. Soc. 82, 325 (1999). 2 4 . T . T a k a d a , S . F . W a n g , S . Y o s h i k a w a , S . J . J a n g , a n d
R.E. Newnham, J. Am. Ceram. Soc. 77, 1909 (1994).
25. Y. Kobayashi and M. Minegishi, IEEE Trans. Microwave Theory Tech. 36, 1727 (1988).
26. J. Javadpour and N.G. Eror, J. Am. Ceram. Soc. 71, 206 (1988). 27. D.E. Rase and R. Roy, J. Am. Ceram. Soc. 38, 102 (1958). 28. K.W. Kirby and B.A. Wechsler, J. Am. Ceram. Soc. 74, 1841
(1991).
29. J.K. Plourde, D.F. Linn, H.M. O’Bryan, Jr., and J. Thomson, Jr. J. Am. Ceram. Soc. 58, 418 (1975).
30. J.H. Jonker and W. Kwestroo, J. Am. Ceram. Soc. 41, 1390 (1958).
31. W.Y. Lin, R.A. Gerhardt, R.F. Speyer, and J.Y. Hsu, J. Mater. Sci. 34, 3021 (1999).
32. C. Chatterjee, A.N. Virkar, and A. Paul, J. Mater. Sci. Lett. 9, 1049 (1990).
33. T. Noguchi, Y. Kajiwara, M. Suzuki, and H. Takamizawa, U.S. Patent No. 4 353 047 (1982).