中 國 醫 藥 大 學 醫學研究所
碩士學位論文
Statin 的短期治療可以改善川崎症後期合併冠狀動脈異 常病童的血管內膜功能異常和動脈血管硬化
Does Statin Therapy Improve Endothelial Dysfunction and Arterial Stiffness in the Long Term Follow-up Patients of Kawasaki Disease
Complicating with Coronary Arterial Abnormality
指 導 教 授 : 張 正 成 教授 共同指導教授: 謝 凱 生 教授
研 究 生: 黃 士 銘
中 華 民 國 九十六 年 七 月
中 國 醫 藥 大 學
醫 學 研 究 所
碩 士 學 位 論 文
空 間 構 成 形 式 對 高 齡 者 居 住 行 為 及 意 識
中 文 摘 要
題目:Statin 治療是否可以改善川崎症後期合併冠狀動脈異常病童的血管內膜功
能異常和動脈血管硬化。
研究背景:川崎症是一種好發在亞洲兒童的全身性血管炎,距第一個發生案例
至今已有將近 30 年了。目前研究發現這些病人在年輕時就有早發性血管粥狀硬 化的傾向發生。一些研究也顯示在川崎症急性期併發冠狀動脈異常的病人在後期 仍有低度進行性血管發炎、血管內膜功能異常及動脈血管硬化的情況發生。此 外,血脂肪的代謝異常也被認為是川崎症多年後造成血管內膜功能異常及動脈血 管硬化的原因之一。藉由測量血液高敏感度 CRP 來評量慢性血管發炎、藉由測 量臂動脈血流擴張程度和頸動脈內膜中層厚度來評量血管內膜功能及藉由測量 臂踝脈波傳遞速度、動脈血管硬化指數和臂踝血壓指數來評量評估動脈血管硬化 程 度 , 這 些 方 法 已 經 被 用 來 當 作 衡 量 心 血 管 異 常 的 指 標 。 Statins, hydroxymethylglutaryl coenzyme A 還原酶抑制劑,已經被證實不僅可以降低 LDL-C,同時可以改善成人的血管粥狀硬化和血管內膜的功能異常。 然而,
statin 對於治療後期川崎症併發冠狀動脈異常的兒童在改善血脂肪異常、慢性血 管發炎、血管內膜功能異常和動脈血管硬化方面尚未被評估過。
研究目的:本實驗的研究目的就是為了證明 statin 治療是否可以改善併發冠狀
動脈異常川崎症後期病童的血脂肪異常、慢性血管發炎、血管內膜功能異常及動 脈血管硬化。
研究方法: 本實驗分為兩部份。第一部份乃比較11名川崎症合併冠狀動脈異常
病童與11名健康且年齡、性別相符沒有川崎症兒童的各種周邊動脈異常測量值的 差異。第二部份再比較這11名川崎症合併冠狀動脈異常病童服藥前後的周邊動脈 異常的改變。這些實驗組的兒童每日睡前口服一次simvastatin 10 mg 持續3個 月。服用藥物前及服用藥物後每個月皆要接受血脂肪、高敏感性CRP檢測及頸動
脈內膜中層厚度、右臂動脈血流擴張程度、臂踝脈波傳遞速度、動脈血管硬化指 數和臂踝血壓指數檢查,直到3個月研究期間結束。初步的藥物效果評估乃比較 川崎症兒童服用藥物前及服用藥物後每個月血中脂肪、高敏感性CRP、頸動脈內 膜中層厚度、右臂動脈血流擴張程度、臂踝脈波傳遞速度、動脈血管硬化指數和 臂踝血壓指數的變化,且和健康的對照組兒童比較是否有顯著差異和改善。至於 藥物的安全性乃藉由每個月監控肌肉酵素(CPK)、肝功能(AST/ALT)及是否有 臨床症狀產生。
實驗結果:[第一部份] 川崎症組與健康組不管在性別、年齡、身體質量指數和
血壓方面皆沒有顯著的差別。在服用藥物前,川崎症實驗組有較正常對照組有較 高的高敏感性CPR、較高的動脈血管硬化指數、較快的臂踝脈波傳遞速度和較低 的臂動脈血流擴張程度。至於在血脂肪、動脈內膜中層厚度和臂踝血壓指數則沒 有顯著的不同。[第二部份]川崎症病童經過3個月的statin治療後,血脂肪方面皆 有顯著的改善,除了TG沒有顯著地下降之外。高敏感度CRP經過3個月的治療後 也有顯著下降,但改善後仍高於正常兒童。在血管內膜功能和動脈血管硬化方 面,臂動脈血流擴張程度、動脈內膜中層厚度、臂踝脈波傳遞速度、動脈血管硬 化指數和臂踝血壓指數皆有顯著地改善。動脈內膜中層厚度、臂踝脈波傳遞速度 和臂踝血壓指數皆有改善至與正常對照組比較沒有顯著差別,但臂動脈血流擴張 程度和動脈血管硬化指數改善後仍和正常對照組有顯著的差異。在3個月的研究 療程中,在CPK、AST和ALT方面並沒有明顯的變化,而且並沒有臨床副作用症 狀產生。
研究結論:在川崎症合併有冠狀動脈異常的兒童發病多年後依然可以發現有慢
性血管發炎、血管內膜功能異常及動脈血管硬化的情況發生。在合併有冠狀動脈 異常長期追蹤的川崎症兒童,短期的statin治療可以顯著地改善且正常化血脂 肪、慢性血管發炎、血管內膜功能異常及動脈血管硬化的情況,而且沒有肌肉或 肝功能異常等副作用的產生,雖然在我們的研究中長期的安全性仍未知。我們的
研究結果也強調當血管粥狀硬化過程尚為可逆時,在合併冠狀動脈異常的川崎症 兒童早期服用statin治療或許可以避免未來早發性血管粥狀硬化傾向的發生。
Abstract
Topic: Does Statin Therapy Improve Endothelial Dysfunction and Arterial Stiffness in the Long Term Follow-up Patients of Kawasaki Disease Complicating with Coronary Arterial Abnormality
Background: Kawasaki disease (KD), a systemic vasculitis with predilection for Asian children, has been noted for almost 40 years since its first description in 1967.
Latest concern falls on the possibility of its predisposition to premature atherosclerosis in young adulthood. Several studies showed evidences of ongoing low grade inflammation, endothelia dysfunction and arterial stiffness in those KD patients complicating with coronary arterial abnormality (CAA) even late after their acute phases. Besides, their abnormalities in lipoprotein metabolism are also believed to be determinant factors for the persistent endothelial dysfunction and arterial stiffness many years later. Many tools have been used to assess the arterial dysfunction in adults, which include: chronic vascular inflammation measured by high-sensitive CRP (hs-CRP), endothelial dysfunction measured by flow-mediated dilation (FMD) of the brachial artery & intima-media thickness (IMT) and arterial stiffness measured by brachial-ankle pulse wave velocity (baPWV), arterial stiffness index (ASI) and ankle brachial pressure index (ABI). Long term medication of statin, a HMG CoA reductase inhibitor, have been proven effective in reducing LDL-C levels and improving the endothelial dysfunction in adult patients of atherosclerosis. However, its efficacy in treating late KD children complicating with CAA has not yet been evaluated.
Objective: This study was designed to determine whether statin therapy (simvastatin) improve lipoprotein abnormalities, chronic vascular inflammation, arterial stiffness and endothelial dysfunction in children of KD complicating with CAA at their long term follow-up.
Methods: This study is divided into two parts. Part one study included 11 KD children complicating with CAA as study group and 11 healthy age- and gender-matched children as control group. Part two study compared the arterial dysfunctional parameters on these 11 KD patients before and after they received the simvastatin therapy 10 mg single dose per day at bedtime for three months. Lipid profiles (LDL-C, HDL-C, TG, TC), hs-CRP, IMT, ASI, baPWV, ABI and FMD were performed during each visit at baseline and every month until finishing three months therapy.The primary efficacy outcome was the change from baseline in lipid profiles, hs-CRP, IMT, ASI, baPWV, ABI and FMD after three months of treatment. The principal safety was monitored every month by muscle-related enzyme (CPK), liver enzyme (AST and ALT) measurements and relevant clinical complaints, especially muscle cramps.
Results: Part one study: There are no significant difference between the KD patients and normal control group in the demographic data including gender, age, body mass index and blood pressure. However, the KD group had significantly higher hs-CPR (0.430 ± 0.225 vs. 0.045 ± 0.028 mg/L, p < 0.001) and ASI (4.82 ± 0.86 vs. 2.96 ± 0.89, p < 0.001), faster baPWV (1261 ± 117 vs 1070 ± 173 cm/sec, p = 0.006) and decreased FMD (6.12 ± 1.61 vs. 13.11 ± 1.00 %, p < 0.001) than the normal control.
There were no significant difference in lipid profiles, IMT and ABI. Part two study:
After three months of statin treatment on the KD patients, there were 9.4 % decreased in TC level, 12.4 % decreased in LDL-C level and 7.3 % increased in HDL-C level.
All were statistically significant. Serum hs-CRP level decreased from 0.430 ± 0.225 mg/L to 0. 209 ± 0.098 (p = 0.001). The final hs-CRP level were still significantly higher than that of normal control. There were also 7.5 % decreased of IMT, 10.2 % decreased of baPWV, 22.2 % decreased of ASI, 7.3 % increased of ABI, and 68.6 %
to that of normal control, but ASI and FMD values remained worse than the normal control. There were neither significant changes in ALT, AST and CK levels nor any adverse effects related to the drug.
CONCLUSIONS: Chronic vascular inflammation, endothelium dysfunction and arterial stiffness persist in the KD patients complicating with CAA at their long term follow-up. We find that a 3-month medication of statin therapy can significantly improve their lipoprotein abnormalities, chronic vascular inflammation, endothelial dysfunction and arterial stiffness. No adverse effect was found.
Acknowledgements
I want to offer thanks to all of those who made this thesis possible and make our study so enjoyable. I am very fortunate to have had the opportunity to work with such an amazing group of people.
I would like to thank my research supervisor, Dr. Jeng-Sheng Chang and co-advisor Dr. Kai-Sheng Hsieh, for providing their incredible insight and advisee to make this thesis better. I would also like to thank Kaohsiung Veterans General Hospital for providing the equipment and place to finish our study. I express my sincere gratitude for their useful help for this thesis.
Finally, I would like to thank my family members for their supports, patience and encouragement. I sincerely devote this thesis to all of my loving family members, my wife, sun and daughter.
目 錄
中文摘要 --- 一 英文摘要 --- 四 誌謝辭 --- 七 第一章 前言
第一節 研究背景 --- 1
第二節 研究目的 --- 3
第二章 研究方法 第一節 研究材料 --- 4
第二節 研究設計與進行方法 --- 5
第三節 統計方法 --- 8
第三章 研究結果 --- 9
第四章 討論 第一節 結果討論 --- 12
第二節 研究限制 --- 18
第五章 結論與建議 第一節 結論 --- 19
第二節 建議 --- 20
參考文獻 --- 21
附錄 表格 --- 24
圖表 --- 27
第一章 前 言
第一節 研究背景
Kawasaki disease (KD), a systemic vasculitis with predilection for Asian children, is the most common acquired heart disease in children of developed countries. It has now been almost 40 years since the first description of KD. Concerns have been raised regarding the existence of endothelial damage and the possibility of its predisposition to premature atherosclerosis in young adulthood [1]. Histopathologic and intravascular ultrasound studies have demonstrated extensive fibro-intimal thickening and infiltration of lymphocytes & plasma cells in the coronary arterial walls late after KD [2,3]. These findings suggest that chronic ongoing low grade arterial inflammation is possible even years after the acute phase of KD [4].
Furthermore, this low grade inflammation has a role in increasing systemic arterial stiffness and endothelial dysfunction [1,5,6].
There has been strong evidence showing that inflammation plays a key role in the cascade of atherosclerosis, from its beginning, development of instability and plaque rupture, to clinical vascular events [7]. In addition, this endothelial dysfunction and arterial stiffness are also a precursor of atherosclerosis and subsequent coronary artery damage [8]. In KD, proinflammatory cytokines and inflammatory antigens are activated during the acute phase. This inflammatory process may be one of the factors that induce atherogenesis [3]. Besides, abnormalities of lipoprotein metabolism are also believed to be a cause of endothelial dysfunction that may occur many years after KD [9].
Chronic vascular inflammation measured by high-sensitive CRP (hs-CRP), endothelial function measured by flow-mediated dilation (FMD) of the brachial artery
& intima-media thickness (IMT), and arterial stiffness measured by brachial-ankle
pulse wave velocity (baPWV), arterial stiffness index (ASI), and ankle brachial pressure index (ABI) have been used as the surrogate markers of cardiovascular disease. Statins, hydroxymethylglutaryl coenzyme A reductase inhibitors, have been proven to not only reduce LDL-C levels, but also improve these surrogate markers of atherosclerosis and cardiovascular disease [10]. Furthermore, several clinical trials even demonstrated that statins are safe agents and do not impair growth or sexual development in children [10]. However, the efficacy of statin therapy for improving lipoprotein abnormalities, chronic vascular inflammation , arterial stiffness, and endothelial dysfunction have not been evaluated in children late after KD with a history of coronary arterial abnormality (CAA).
第二節 研究目的
This study was designed to determine whether statin therapy (simvastatin) improve lipoprotein abnormalities, chronic vascular inflammation, arterial stiffness and endothelial dysfunction in children late after KD with a history of CAA.
第二章 研 究 方 法
第一節 研究材料
We studied children who were seen during the acute phase of KD and followed up at Kaohsiung Veterans General Hospital. All children met the criteria for KD previously defined [11]. These KD children with a history of documented CAA are recruited. Exclusion criteria included that children diagnosed as having KD within 12 months of the study, infectious diseases, chronic inflammatory disease, malignancy, and clinical evidence of heart failure. We comprised 11 KD children with CAA referred to as the study group ( 8 males and 3 females) with a mean age of 12.90 ± 2.50 years (age range, 9.25 to 16.67 years), 6.25 to 15.75 years after acute KD (mean 10.77 ± 3.01 years), which had occurred at 0.67 to 6.33 years of age (mean 2.13 ± 1.53 years)(Table 1). Five patients had mild coronary aneurysms, four had moderate aneurysms and two had giant aneurysms during the acute stage. Four patients still had persistent CAA at the time of the present study. We also studied 11 healthy age- and gender-matched children referred to as the normal control group with a mean age of 12.97 ± 2.45 years (age range, 9.33 to 16.67 years)(Table 1). These healthy children were previously discharged from our clinic with a diagnosis of a functional heart murmur and their healthy siblings. This study was approved by the ethics committee of Kaohsiung Veterans General Hospital. Informed consent for the research protocol was obtained from their parents of all subjects and themselves.
第二節 研究設計與進行方法
Study protocol
From the medical records, the following patient data were retrieved: interval from disease onset to time of study, coronary complications, cardiac symptoms, and medications at the time of study. Coronary aneurysms were documented by serial two-dimensional echocardiography. The participants were categorized into two groups for comparisons. Study group comprised KD patients with a history of CAA, and control group comprised healthy age- and gender-matched subjects. The study group received oral simvastatin 10 mg single dose per day at bedtime for three months.
Lipid profiles (LDL-C, HDL-C, TG, TC), hs-CRP, IMT, ASI, baPWV, ABI and FMD were performed during each visit at baseline and every month until finishing three months therapy. The baseline cardiovascular disorder in study group will be revealed after compare with the control group by these evaluating parameters. The primary efficacy outcome of simvastatin is the change from the baseline value in lipid profiles, hs-CRP, IMT, ASI, baPWV, ABI and FMD. The principal safety was monitored every month by muscle-related enzyme (CPK) and liver enzyme (AST and ALT) measurements and relevant clinical complaints, especially muscle cramps.
Discontinuation criteria included persistent increase of >3-fold the upper limit of normal level in ALT or AST, or >10-fold increase the upper limit of normal level for CK or 5- to 10-fold increases in CK with symptoms.
All subjects participate the study will come for a special visit after an overnight fast.
Assessments of endothelial function, arterial stiffness and blood withdrawal were then performed sequentially, as described subsequently. The assessments of IMT, ASI, baPWV, ABI and FMD were performed as published previously. In summary, all measurements were performed during the morning in a fasting state. All children will
be refrained from alcohol & caffeine-containing beverages and against performing heavy exercise on the day before the examination. All subjects were studied with the supine position in a temperature-controlled room (25 ℃). All measurements were performed by the same observer, unaware of clinical details and the stage of the experiment.
Measurement of IMT and ASI
For IMT, the right carotid artery was similarly imaged using the 7 ~ 15 MHz linear array transducer (Vivid 7; GE Medical Systems, USA) at about 1 cm proximal to the carotid bifurcation. The IMT of the common carotid artery far wall was measured using the electronic calipers of the ultrasound machine as described previously [12].
The average of three measurements was used for subsequent analyses.
The carotid artery stiffness was assessed by calculating the stiffness index as reported previously [4,13]. Briefly, a 7~15 MHz linear array transducer was used to image the right carotid artery at about 1 cm proximal to the carotid bifurcation. The systolic and diastolic diameters were measured between the intima of the near and far walls. The onset of the electrocardiogram R-wave is used to identify end diastole, and the peak of the T-wave reproducibly identifies end systole. Three measurements each of systolic and diastolic diameters were averaged for the calculation of stiffness index according to the formula:
ln (SBP/DBP)/(dD/D),
where ln denotes natural logarithm, SBP systolic blood pressure, DBP diastolic blood pressure, dD the difference between systolic and diastolic diameters, and D the diastolic diameter.
Measurement of FMD
After a 10 to 15 min rest, the brachial artery in the right antecubital fossa was visualized using a 7 ~15 MHz transducer. After an optimal longitudinal image of the
brachial artery wall was obtained, two baseline vessel diameters were measured.
Reactive hyperemia was induced by inflating the blood pressure cuff to 200 mm Hg or at least 50 mm Hg above systolic pressure on the forearm distal to the location of transducer for 5 minutes, and then deflating the cuff. End-diastolic images, concurrent with the onset of the QRS complex on electrocardiogram, were acquired at baseline and 1 minute after cuff deflation. Baseline vessel diameter was calculated as the average of two measurements. The percentage change from the baseline diameter to the value during reactive hyperaemia was calculated to determine flow-mediated dilatation (% FMD) [14].
Measurement of baPWV and ABI
We measured the baPWV and ABI by a previously described noninvasive volume plethysmographic technique (form PWV/ABI, Colin Co., Komaki, Japan) [15,16].
Briefly, the occlusion and monitoring cuffs were wrapped around the brachia and ankles, electrocardiogram electrodes were placed on both wrists, and a microphone was placed on the left edge of the sternum. Pulse wave contours in the four extremities were then simultaneously recorded, and baPWV was determined from the pulse transit time and the distance between the brachial and ankle regions. ABI, which is calculated by dividing ankle SBP by brachial SBP, was evaluated simultaneously.
第三節 統計方法
Analyses were performed using SPSS 11.00 for Windows software. The baseline characteristics of the normal control versus the KD study group were compared by using Student t test for continuous data. Mean values before and after statin therapy in KD group was compared by using the paired sample t test. The mean values after three months treatment versus the normal control was compared by using Student t test. A p value <0.05 was considered statistically significant.
第三章 研究結果
Baseline characteristics
11 KD children with a history of CAA as study group and 11 healthy age- and gender-matched children as control group (referents) were included in the study analyses. Baseline characteristics showed no significant difference with regard to gender, age, BMI, and blood pressure between the KD study group and normal control group. Their baseline characteristics are summarized in Table 1.
Serum lipid profiles and marker of chronic vascular inflammation before treatment
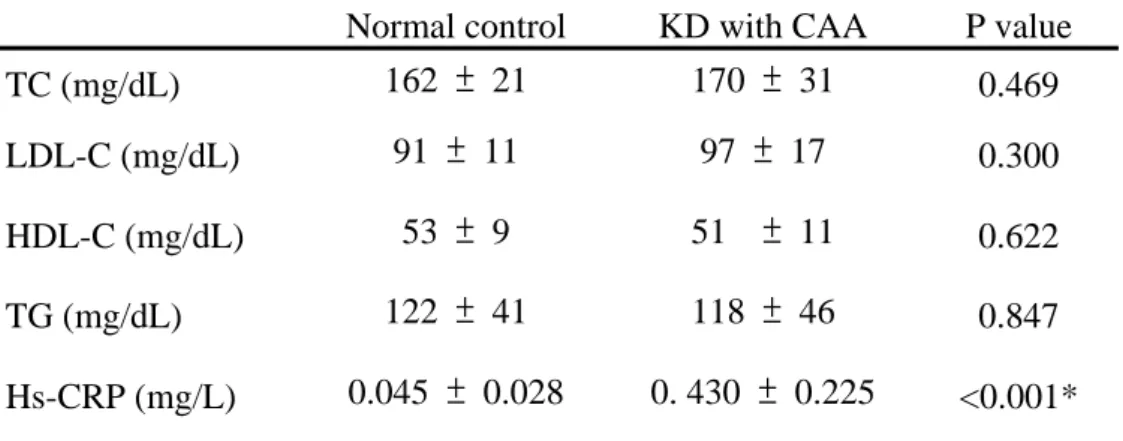
The lipid profiles of the cohort are summarized in Table 2.Lipid profiles were not significantly different between these two groups. Hs-CRP was significantly higher in KD with CAA children compared with the normal control (0.430 ± 0.225 mg/L vs.
0.045 ± 0.028 mg/L, p < 0.001)(Table 2).
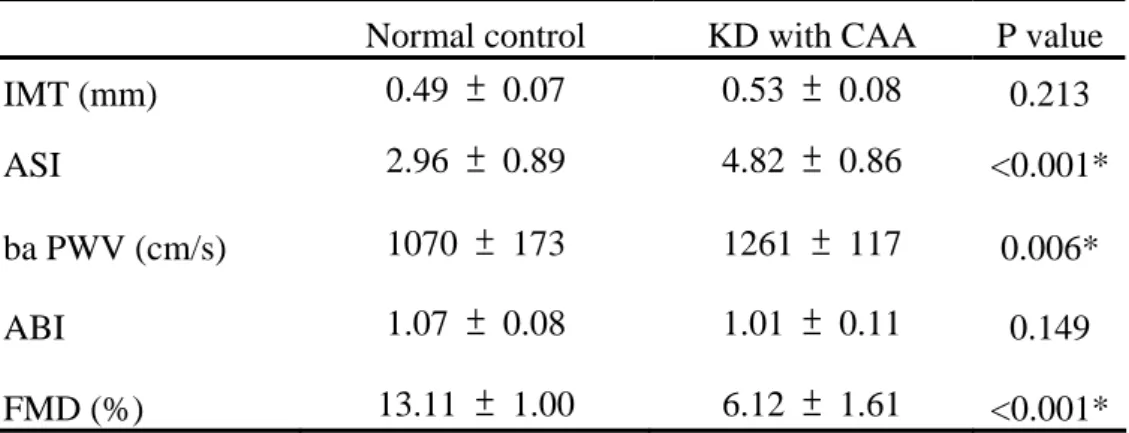
Surrogates for endothelial function and arterial stiffness before treatment With regard to endothelial function, FMD was significantly reduced in KD children versus the normal control (6.12 ± 1.61 % vs. 13.11 ± 1.00 %, p < 0.001). However, there was no significant difference with regard to IMT between the KD and normal control (Table 3).
With regard to arterial stiffness, ASI and baPWV were significantly higher in KD with CAA children compared with the normal control (4.82 ± 0.86 vs. 2.96 ± 0.89, p < 0.001; 1261 ± 117 cm/sec vs 1070 ± 173 cm/sec, p = 0.006). As for ABI, there was no significant difference between the KD group and normal control (Table 2).
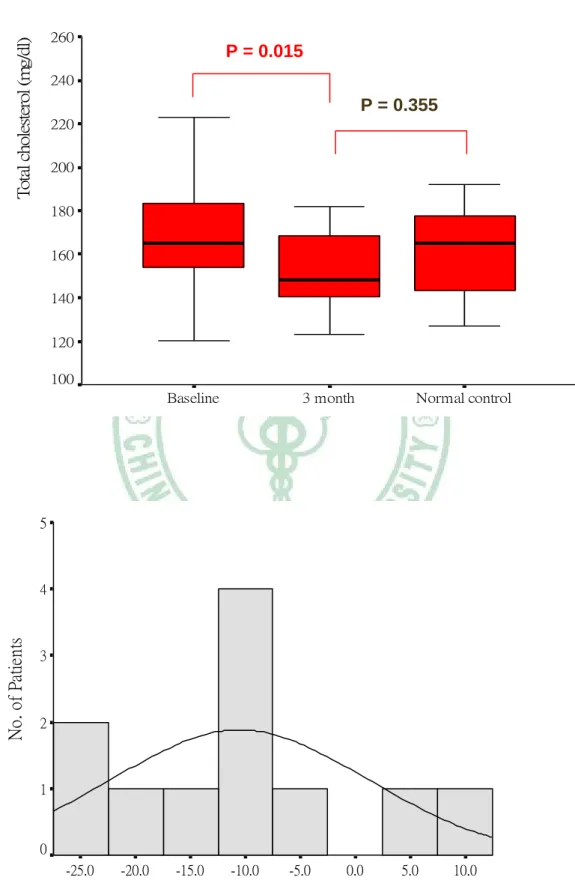
Changes in Serum Lipid profiles after three months treatment
TC level decreased 9.4 % from a mean (SD) of 170 (31) mg/dL at baseline to 154 (20) mg/dL after 3 months treatment (p = 0.015). LDL-C level decreased 12.4 % from
a mean (SD) of 97 (17) mg/dL at baseline to 85 (14) mg/dL after 3 months treatment (p < 0.001). HDL-C level increased 7.3 % from a mean (SD) of 51 (11) mg/dL at baseline to 55 (13) mg/dL after 3 months treatment (p = 0.050). However, there was not statistically significant in the change of TG level from a mean (SD) of 118 (46) mg/dL at baseline to 117 (34) mg/dL after three months treatment. After statin therapy, the lipid profiles in KD children including TC, LDL-C and HDL-C were all improved;
however, they remained no statistical difference from the control. The changes in serum lipid profiles are illustrated in Table 4 and Figure 1.
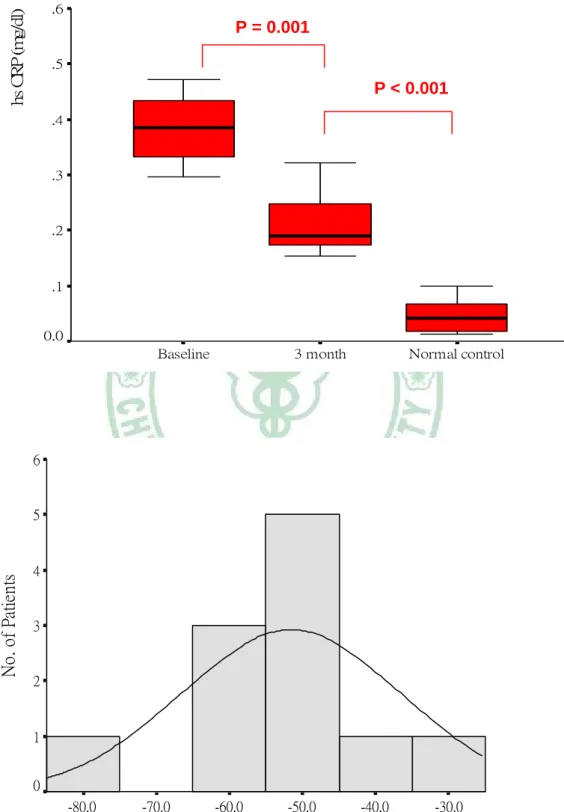
Changes in Serum hs-CRP level after three months treatment
Serum hs-CRP level decreased significantly from a mean (SD) of 0.430 (0.225) mg/L at baseline to 0. 209 (0.098) mg/L after 3 months treatment (p = 0.001);
however, this post-treatment level was still significantly higher than that of normal control (0.209 ± 0.098 mg/L vs. 0.045 ± 0.028 mg/L, p < 0.001). The change in serum hs-CRP is illustrated in Table 4 and Figure 2.
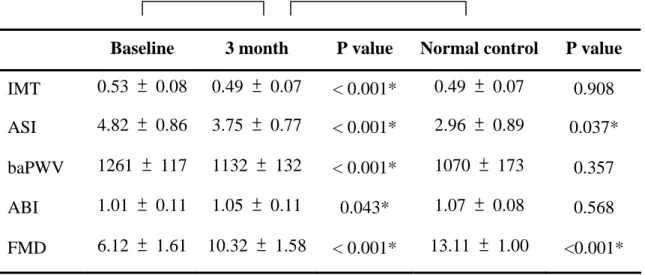
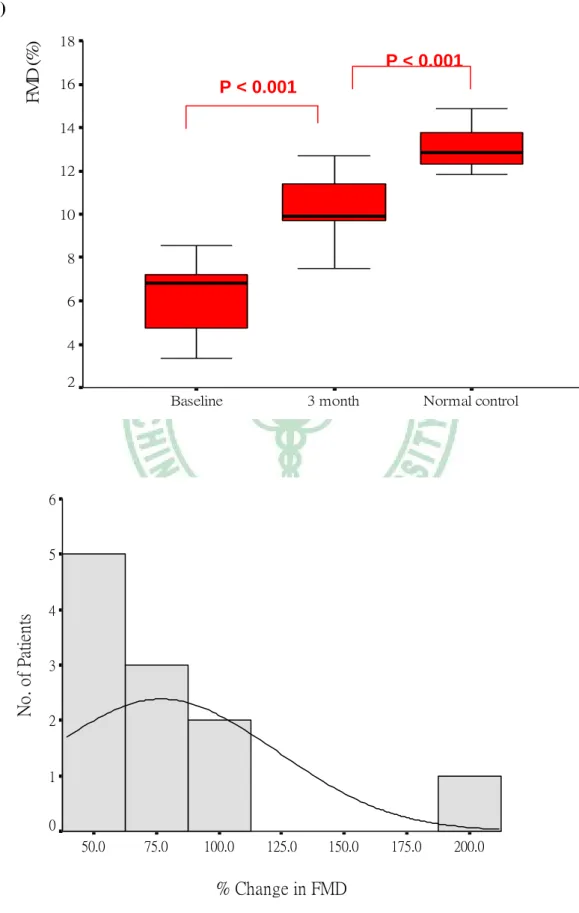
Changes in IMT, endothelial functional surrogate and arterial stiffness after 3 months treatment
IMT decreased 7.5 % from a mean (SD) of 0.53 (0.08) mm at baseline to 0.49 (0.07) mm after 3 months treatment (p < 0.001). BaPWV decreased 10.2 % from a mean (SD) of 1261 (117) m/sec at baseline to 1132 (132) m/sec after 3 months treatment (p
< 0.001). ABI increased 7.3 % from a mean (SD) of 1.01 (0.11) at baseline to 1.05 (0.11) after 3 months treatment (p = 0.043). ASI decreased 22.2 % from a mean (SD) of 4.82 (0.86) at baseline to 3.75 (0.77) after 3 months treatment (p< 0.001), and FMD increased 68.6 % from a mean (SD) of 6.12 (1.61) % at baseline to 10.32 (1.58) % after 3 months treatment (p < 0.001). Both indicated significant improvement of arterial dilatability by only 3 months of statin therapy. Comparing with the normal control, ASI and FMD improved to a level closing to that of normal control, though
still significantly different (3.75 ± 0.77 vs. 2.96 ± 0.89, p = 0.037; 10.32 ± 1.58 % vs 13.11 ± 1.00 %, p < 0.001). The change in IMT, endothelial functional surrogate, and arterial stiffness are illustrated in Table 5 & Figure 3.
Safety and adverse events within 3 months statin treatment
There were no significant differences with regard to safety measurements (ALT, AST, and CK levels) before and after 3 months therapy and no adverse events were also reported. The changes in ALT, AST, and CK are illustrated in Figure 4.
第 四 章 討 論
第一節 結果討論
Our study reports that ongoing chronic vascular inflammation, endothelial dysfunction and arterial stiffness abidingly exist in the late KD children with a history of CAA, since the results revealed reduced FMD and increased hs-CRP, ASI and baPWV in these patients. More importantly, we also demonstrate that statin therapy (simvastatin) significantly improves lipoprotein abnormalities, chronic vascular inflammation, endothelial dysfunction and arterial stiffness in KD children with CAA after short-term (3 months) statin treatment, without adverse effects on liver or muscle tissue. To the best of our knowledge, this is the first clinical trial that assessed the effects of statin therapy on lipoprotein, vascular inflammation, endothelial dysfunction and arterial stiffness in the late KD children.
Lipid abnormalities in the acute phase of KD, with decreased TC and HDL-C, are reminiscent of those in acute inflammation, although such changes are transient as reported by several studies [1]. Newburger et al. have reported that HDL-C level remain low as long as three years after the initial illness [17]. Cheung et al. also reported that lower HDL-C and higher LDL-L occurred in children after KD, performed even at a mean of 7.1 years after the initial illness [1]. They also further clarify that low HDL-C level is confined to patients with a history of CAA [1]. In our study, we did not find any significant differences in these lipid profiles between KD patients and normal control group, performed at a mean of 10.77 years after the initial illness. We speculate that the control population is not big enough to reveal the difference. In addition, we also review several studies about the lipid profile changes late after the onset of KD and find that not all of these studies demonstrated the conditions of lower HDL-C and higher LDL-C, like Newburger and Cheung et al.
Maybe, we speculate that all of these current studies, including our study, are cross-sectional studies and do not really represent all the long-term lipid profile changes as their studies.
Hs-CRP was deemed as a powerful marker for chronic vascular wall inflammation and endothelial dysfunction in patients late after the onset of KD [18]. It is well known that inflammatory processes play an important role in the pathogenesis of atherosclerosis. In a meta-analysis of seven prospective studies, elevated hs-CRP was shown to predict future risk of ischaemic stroke, peripheral arterial disease and coronary heart disease [19]. In our study, agreeing with other late KD studies, the hs-CRP value in KD children with CAA was significantly higher than those in normal control. This observation means that chronic low-grade inflammation could persist late after the onset of KD, particularly in those with a history of CAA and would be related to endothelial dysfunction, not only in the vascular wall of coronary arteries but also in the vascular wall of systemic arteries.
Endothelial dysfunction and increased IMT have both been validated as predictors for further cardiovascular disease. Endothelium-dependent FMD of the brachial artery has been used to assess the endothelial function, mainly due to its nitric oxide (NO) releasing function by the endothelium, which is also well correlated with coronary endothelial function. Several other reports have shown that there were systemic endothelial dysfunction late after the onset of KD, as reflected in the FMD
dysfunction of brachial artery, particularly in those children with a history of CAA [5,9,24]. In our study, we also showed significantly decreased FMD of brachial artery as compared to normal control. Increase of IMT is also a strong risk factor for early atherosclerosis and premature onset of cardiovascular disease. KD children with CAA have been proved to manifest an increase of IMT [12, 25]. This phenomenon was not
so that our data can not reveal the significant difference in IMT when compared with normal control.
Impaired endothelial function has been demonstrated in KD patients with CAA in previous and our studies [9,20]. Inflammatory responses observed in our patients late after KD are also consistent with previously documented endothelial dysfunction in such patients. This residual endothelial dysfunction in the chronic stage of KD is supposed to be induced by the strong systemic inflammation during the acute stage of KD [26]. Because endothelium-derived nitric oxide (NO) inhibits the expression of adhesion molecules, decreased NO release may upregulate the expression of these molecules. In addition, because NO has antioxidant properties, the decreased NO production may unmask local inflammatory responses [21]. Moreover, CRP directly reduces NO production from endothelial cells and increases its expression of adhesion molecules [22]. The exposure of endothelial cells to proinflammatory cytokines impairs the endothelium-dependent vasodilating function [23]. Therefore, elevated CRP levels may be associated with chronic endothelial dysfunction in KD patients.
Echocardiography or coronary angiography accurately evaluates coronary aneurysms and stenotic lesions, but they can not assess the early progression of systemic atherosclerosis. PWV and ABI have been reported as useful and simple methods to evaluate the degree of atherosclerosis in adults, and are considered as powerful markers and predictors for cardiovascular disease [27].PWV measurement as a surrogate for arterial stiffness was first reported in 1922, but only recently were automated devices available. It is non-invasive and has been validated repeatedly.
PWV increases as arteries become more damaged and stiffer. In our study, we showed that the KD patients with a history of CAA had significantly higher ASI and faster baPWV than normal control. This might be suggesting that the systemic arteritis from the acute stage of KD has been resulted in a structural change in the arterial wall,
which manifests as an age-related increase of arterial stiffness in the long term follow up [27]. Our study revealed that the KD children with a history of CAA had systemic vascular endothelial dysfunction and arterial stiffness, and also suggested that the KD complicated with CAA is likely to prompt early development of atherosclerosis.
Although there was a significant difference in another markers and index, ABI showed no significant difference between the KD and the normal control. ABI reflects arteriosclerotic angiopathy, and ABI ≦0.9 was regarded as an independent risk factor for cardiovascular disease. We speculate that it may be due to our KD children are still in the early reversible stage of endothelial dysfunction and atherosclerosis, not yet severe to reveal abnormal arteriosclerotic angiopathy. Besides, no patient in our study had symptoms of clinical sclerotic arteriopathy [27].
Statins, hydroxymethylglutaryl coenzyme A reductase inhibitors, have been proven to not only reduce LDL-C level, but also improve surrogate markers of atherosclerosis and endothelial dysfunction [28]. Statins also have several important pleiotropic properties, such as improvement of endothelial dysfunction, inhibition of inflammatory responses, stabilization of atherosclerotic plaques, and modulation of procoagulant activity and platelet function [29]. In fact, clinical studies have suggested that the beneficial effects of statin treatment begin earlier than the cholesterol-lowering effect [29].
More recently, several prospective studies have shown that statin therapy reduces hs-CRP, and that the lower the hs-CRP the lower the event rate and the slower the progression of atherosclerosis, independent of LDL-C level [7]. A possible mechanism for this action of statins is that inhibition of cholesterol synthesis interferes with the formation of lipid rafts on the surface of lymphocytes. This in turn interferes with lymphocyte function and thereby reduces inflammation [30]. Like
hs-CRP in the late KD children with CAA after a short-term statin treatment.
However, a long-term and large sample prospective studies using hs-CRP as a guide of therapy are needed.
Arambepola et al. conducted a systematic review and meta-analyses to assess the evidence for efficacy and safety of statin therapy in children and adolescents with heterozygous familial hypercholesterolaemia (FH) [31]. They showed that statins lowered LDL-C 32.5% (95% CI 24.3, 40.7), increased HDL-C 3.4% (0.8, 6.0), insignificantly lowered TG 3.0% (-11.6, 17.6), attenuated progression of carotid IMT, and improved endothelial function (increased % FMD of brachial artery). None reported a significant difference in growth, sexual maturation and any significant increases of ALT, AST and CK values [31]. In our study for KD patients, the improvement of TC, LDL-C, TG and HDL-C levels are also compatible to their studies of statin therapy in children of FH. In agree with Arambepola’s study, our study also revealed attenuation of IMT progression in the carotid artery, and improvement of endothelial dysfunction and arterial stiffness after only 3 months of statin treatment in the late KD children complicated with CAA.
Statin therapy significantly improved endothelial dysfunction (increased % FMD of brachial artery) in children with FH after 28 weeks of therapy [28]. Remarkable lipid lowering by statin was also accompanied by carotid IMT regression in FH children [32]. These findings suggested that initiation of statin therapy in children may inhibit the progression of atherosclerosis, even leading to its regression [10,32]. Recent numerous studies have shown that the statins' cholesterol-independent vascular effects may be directly improving the endothelial function by increasing its NO production (by upregulating endothelial cell NO synthase through stabilization of messenger ribonucleic acid levels), promoting re-endothelialization after arterial injury and inhibiting inflammatory responses within the vessel wall [29]. The study of statin
therapy in FH has proved that the peripheral endothelial dysfunction can be completely reversedafter only short-term of statin therapy [33]. However, in patients with chronic coronary artery disease, the statin therapy can only attenuate the acetylcholine-induced “paradoxical” vasoconstriction, but it has no effect on the endothelium-dependent vasodilation [34]. These findings emphasize that in order to achieve normalization of endothelial function in “atherogenic” vascular beds, such as the coronary arteries, the statin therapy well need to be initiated at an early stage, before the onset of severe macrovascular structural abnormalities [28]. We believe that early statin therapy is helpful in restoring endothelial dysfunction and arterial stiffness in their reversible stage and prevent the future cardiovascular events [5].
However, what is the long term end point of statin therapy to protect the pediatric patients of KD from later cardiovascular events remains unsettled. Does delaying initiation of statin therapy until adulthood benefit the KD patients as much as the early therapy since childhood? Nevertheless, the demonstrations of early developing of atherosclerosis in many follow up studies of KD and the disability of late statin therapy to revert endothelial dysfunction in atherogenic vessels have made the early initiation of statin therapy an important issue.
In the present cohort study, no toxicity or serious adverse effects were found throughout the study. However, the follow up period of the present study is too short to draw conclusions on the safety of long-term use of simvastatin in KD children. The series of Wiegman et al have reported that two years of pravastatin therapy in children of FH did not cause any adverse effects on their growth, sexual maturation, hormone levels, or liver or muscles tissue [32].
第二節 研究限制
This study has several limitations, including a small sample size, short duration and lack of a group of KD patients without using statin for comparison. Lifestyle changes were also not actively monitored or controlled. Besides, our study is a cross-sectional and short-term clinical trial, and the cardiovascular improvement by statin therapy in this study is not able to predict a long term benefit in preventing future cardiovascular events.
第五章 結 論 與 建 議
第一節 結論
Chronic vascular inflammation, endothelium dysfunction and arterial stiffness persist in the KD patients complicated with CAA even late after their onsets of KD.
Short-term statin therapy could significantly improve lipoprotein abnormalities, chronic vascular inflammation, endothelial dysfunction and arterial stiffness in these patients. No adverse effects on liver or muscle tissue were found. Early initiation of statin therapy to prevent the predisposition of premature atherosclerosis may be warranted
第二節 建議
Our results revealed that chronic vascular inflammation, endothelium dysfunction and arterial stiffness were still noted even in patients late after the onset of KD with a history of CAA. However, further long-term follow-up study is required to reveal whether these systemic endothelial dysfunction and arterial stiffness could lead to an early onset of atherosclerosis and even future cardiovascular events in the chronic stage of KD, because impaired endothelial function and arterial stiffness might be the initial stage of atherosclerosis.
In our present cohort, no toxicity, serious adverse or side effects were reported by the children during the course of this study. However, the duration of the present study is too short to draw conclusions with regard to the safety of long-term use of simvastatin in KD children. The long-term safety and efficacy of statins in KD children are needed to be continuously evaluated in our ongoing trial.
參 考 文 獻
1. Cheung YF, Yung TC, Tam SC,et al. Novel and traditional cardiovascular risk factors in children after Kawasaki disease: implications for premature atherosclerosis. J Am Coll Cardiol. 2004 Jan 7;43(1):120-4
2. Tanaka N, Naoe S, Masuda H, Ueno T. Pathological study of sequelae of Kawasaki disease (MCLS): with special reference to the heart and coronary arterial lesions.
Acta Pathol Jpn 1986;36:1513–27.
3. Sugimura T, Kato H, Inoue O, et al. Intravascular ultrasound of coronary arteries in children: assessment of the wall morphology and the lumen after Kawasaki disease.
Circulation 1994;89:258–65.
4.Y F Cheung, M H K Ho, S C F Tam, et al. Increased high sensitivity C reactive protein concentrations and increased arterial stiffness in children with a history of Kawasaki disease Heart 2004;90:1281–1285.
5. Ikemoto Y, Ogino H, Teraguchi M, Kobayashi Y. Evaluation of preclinical atherosclerosis by flow-mediated dilatation of the brachial artery and carotid artery analysis in patients with a history of Kawasaki disease. Pediatr Cardiol.
2005;26:782-6
6. Suzuki A, Yamagishi M, Kimura K, et al. Functional behavior and morphology of the coronary artery wall in patients with Kawasaki disease assessed by intravascular ultrasound. J Am Coll Cardiol 1996;27:291–298
7. Wilson AM, Ryan MC, Boyle AJ The novel role of C-reactive protein in cardiovascular disease: risk marker or pathogen. Int J Cardiol. 2006;106:291-7.
8. Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr., Lerman A.
Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 2000;101:948–54.
9. Dhillon R, Clarkson P, Donald AE, et al. Endothelial dysfunction late after Kawasaki disease. Circulation 1996;94: 2103-6.
10. Rodenburg J, Vissers MN, Wiegman A, Trip MD, Bakker HD, Kastelein JJ.
Familial hypercholesterolemia in children. Curr Opin Lipidol. 2004;15:405-11.
11. Dajani AS, Taubert KA, Gerber MA, et al. Diagnosis and therapy of Kawasaki disease in children. Circulation 1993;87:1776–80.
12. Noto N, Okada T, Yamasuge M, et al. Noninvasive assessment of the early progression of atherosclerosis in adolescents with Kawasaki disease and coronary artery lesion. Pediatrics 2001;107:1095–9.
13. Hirai T, Sasayama S, Kawasaki T, et al. Stiffness of systemic arteries in patients with myocardial infarction: a noninvasive method to predict severity of coronary atherosclerosis. Circulation 1989;80:78–86.
14. Celermajer DS, Sorensen KE, Gooch VM, et al. Noninvasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992;340:1111–1115
15. Munakata M, Ito N, Nunokawa T, Yoshinaga K. Utility of automated brachial ankle pulse wave velocity measurements in hypertensive patients. AJH 2003;16:653–657
16. Tomiyama H, Yamashina A, Arai T, et al. Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement—a survey of 12517 subjects. Atherosclerosis 2003;30:303–309
17. Newburger JW, Burns JC, Beiser AS, Loscalzo J. Altered lipid profile after Kawasaki syndrome. Circulation 1991;84:625–31.
18. Blake GJ, Ridker PM. Novel clinical markers of vascular wall inflammation. Circ Res 2001;89:763–771
19. Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA 1998;279:1477–82.
20. Mitani Y, Okuda Y, Shimpo H, et al. Impaired endothelial function in epicardial coronary arteries after Kawasaki disease. Circulation. 1997;96:454–461.
21. Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323:27–36.
22. Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–2168.
23. Bhagat K, Moss R, Collier J, et al. Endothelial “stunning” following a brief exposure to endotoxin: a mechanism to link infection and infarction? Cardiovasc Res. 1996;32:822– 829.
24. Kadono T, Sugiyama H, Hoshiai M, et al. Endothelial function evaluated by flow-mediated dilatation in pediatric vascular disease. Pediatr Cardiol 2005;26:385–390
25. Cheung YF, Wong SJ, Ho MH. Relationship between carotid intima-media thickness and arterial stiffness in children after Kawasaki disease. Arch Dis Child.
2007;92:43-7.
26. Niboshi A, Hamaoka K, Sakata K, Yamaguchi N. Endothelial dysfunction in adult patients with a history of Kawasaki disease. Eur J Pediatr. 2007; [Epub ahead of print]
27. Ooyanagi R, Fuse S, Tomita H, et al. Pulse wave velocity and ankle brachial index in patients with Kawasaki disease. Pediatr Int. 2004;46:398-402
28. de Jongh S, Lilien MR, op't Roodt J, et al. Early statin therapy restores endothelial function in children with familial hypercholesterolemia J Am Coll Cardiol
2002;40:2117-21
29. Ii M, Losordo DW. Statins and the endothelium Vascul Pharmacol. 2007 Jan;46(1):1-9. Epub 2006 Jun 21.
30. Ehrenstein MR, Jury EC, Mauri C. Statins for atherosclerosis—as good as it gets?
N Engl J Med 2005;352:73–5.
31. Arambepola C, Farmer AJ, Perera R, Neil HA. Statin treatment for children and adolescents with heterozygous familial hypercholesterolaemia: A systematic review and meta-analysis. Atherosclerosis. 2006 Nov 9; [Epub ahead of print]
32. Wiegman A, Hutten BA, de Groot E, et al. Statin therapy in hypercholesterolemic children long-term efficacy and safety. JAMA 2004; 292 :331-337.
33. Stroes ES, Koomans HA, de Bruin TW, Rabelink TJ. Vascular function in the forearm of hypercholesterolaemic patients off and on lipid-lowering medication.
Lancet 1995;346:467–71.
34. Treasure CB, Klein JL, Weintraub WS, et al. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med 1995;332:481–7.
附 錄
Table 1. Baseline characteristics of study population
Data are presented as the mean ± SD
Table 2. Serum lipid profile and marker of chronic vascular inflammation
TC = total cholesterol; LDL-C = low-density lipoprotein cholesterol; HDL-C = high-density lipoprotein cholesterol; TG = triglycerides; hs-CRP = high-sensitivity C-reactive protein. Data are presented as the mean ± SD. *P < 0.001.
Normal Control KD with CAA P value
Number 11 11
Age (years) 12.97 ± 2.42 12.90 ± 2.50 0.950
(9.33 – 16.67) (9.25 – 16.67)
Gender (M:F) 8:3 8:3
BMI (kg/m2) 20. 02 ± 6.00 23.18 ± 9.10 0.347
(14.2 – 32.4) (14.6 – 36.1)
SBP (mm Hg) 114 ± 15 107 ± 16 0.250
DBP (mm Hg) 56 ± 6 57 ± 7 0.793
Onset age (yrs) 2.13 ± 1.53
( 0.67 - 6.33 )
Interval (yrs) 10.77 ± 3.01
( 6.25 - 15.75 )
Normal control KD with CAA P value
TC (mg/dL) 162 ± 21 170 ± 31 0.469
LDL-C (mg/dL) 91 ± 11 97 ± 17 0.300
HDL-C (mg/dL) 53 ± 9 51 ± 11 0.622
TG (mg/dL) 122 ± 41 118 ± 46 0.847
Hs-CRP (mg/L) 0.045 ± 0.028 0. 430 ± 0.225 <0.001*
Table 3. Surrogates for endothelial function and arterial stiffness before treatment
Normal control KD with CAA P value
IMT (mm) 0.49 ± 0.07 0.53 ± 0.08 0.213
ASI 2.96 ± 0.89 4.82 ± 0.86 <0.001*
ba PWV (cm/s) 1070 ± 173 1261 ± 117 0.006*
ABI 1.07 ± 0.08 1.01 ± 0.11 0.149
FMD (%) 13.11 ± 1.00 6.12 ± 1.61 <0.001*
IMT, intima-media thickness; ASI, arterial stiffness index; baPWV, brachial-ankle pulse wave velocity; FMD, flow-mediated dilatation; ABI, ankle brachial pressure index. Data are presented as the mean ± SD. * Statistically significant.
Table 4. Changes in Serum Lipid profile and hs CRP after 3 months treatment
Baseline 3 month P value Normal control P value TC 170 ± 31 154 ± 20 0.015* 162 ± 21 0.355 TG 118 ± 46 117 ± 34 0.755 122 ± 41 0.744 LDL-C 97 ± 17 85 ± 14 <0.001* 91 ± 11 0.304
HDL-C 51 ± 11 55 ± 13 0.05* 53 ± 9 0.769
Hs-CRP 0. 430 ± 0.225 0. 209 ± 0.098 0.001* 0.045 ± 0.028 < 0.001*
Data are presented as the mean ± SD. * Statistically significant.
Table 5. Changes in IMT, endothelial function surrogate and arterial stiffness after 3 months treatment
Baseline 3 month P value Normal control P value IMT 0.53 ± 0.08 0.49 ± 0.07 < 0.001* 0.49 ± 0.07 0.908 ASI 4.82 ± 0.86 3.75 ± 0.77 < 0.001* 2.96 ± 0.89 0.037*
baPWV 1261 ± 117 1132 ± 132 < 0.001* 1070 ± 173 0.357 ABI 1.01 ± 0.11 1.05 ± 0.11 0.043* 1.07 ± 0.08 0.568 FMD 6.12 ± 1.61 10.32 ± 1.58 < 0.001* 13.11 ± 1.00 <0.001*
Data are presented as the mean ± SD. * Statistically significant.
Figure 1. Box plot of changes and histogram analysis of percent changes in serum lipid profiles after 3 months of simvastatin treatment.
(a)
% Change in serum TC concentration
10.0 5.0
0.0 -5.0
-10.0 -15.0
-20.0 -25.0
No. of Patients
5
4
3
2
1
0
Normal control 3 month
Baseline
Total cholesterol (mg/dl)
260
240
220
200
180
160
140
120 100
P = 0.015
P = 0.355
(b)
% Change in serum LDL concentration
-8.0 -10.0
-12.0 -14.0
-16.0 -18.0
-20.0
No. of Patients
4.0
3.0
2.0
1.0
0.0
Normal control 3 month
Baseline
LDL (mg/dl) 140
120
100
80
60
40
P < 0.001
P = 0.304
(c)
% Change in serum HDL concentrations
25.0 20.0
15.0 10.0
5.0 0.0
-5.0 -10.0
No. of Patients
4.0
3.0
2.0
1.0
0.0
Normal control 3 month
Baseline
HDL (mg/dl) 90
80
70
60
50
40
30 20
P = 0.050 P = 0.769
(d)
% Change in TG
60.0 50.0 40.0 30.0 20.0 10.0 0.0
-10.0 -20.0
No. of Patients
5
4
3
2
1
0
Normal control 3 month
Baseline
Triglycerides (md/dl) 250
200
150
100
50
0
P = 0.755
P = 0.744
Figure 2. Box plot of change and histogram analysis of percent change in serum high-sensitivity C-reactive protein (hs-CRP) levels after 3 months of simvastatin treatment.
% Change in serum hs CRP concentration
-30.0 -40.0
-50.0 -60.0
-70.0 -80.0
No. of Patients
6
5
4
3
2
1
0
Normal control 3 month
Baseline
hs CRP (mg/dl) .6
.5
.4
.3
.2
.1
0.0
P = 0.001
P < 0.001
Figure 3. Box plot of changes and histogram analysis of percent changes in IMT, endothelial function surrogate and arterial stiffness after 3 months of simvastatin treatment.
(a)
% Change in FMD
200.0 175.0
150.0 125.0
100.0 75.0
50.0
No. of Patients
6
5
4
3
2
1
0
Normal control 3 month
Baseline
FMD (%) 18
16
14
12
10
8
6
4 2
P < 0.001
P < 0.001
(b)
% Change in ba PWV
-4.0 -6.0
-8.0 -10.0 -12.0
-14.0 -16.0
-18.0
No. of Patients
4.0
3.0
2.0
1.0
0.0
Normal control 3 month
Baseline
ba PWV (cm/sec) 1600
1500 1400 1300 1200 1100 1000 900
800 700
P < 0.001
P = 0.357
(c)
% Change in ASI
-15.0 -20.0
-25.0 -30.0
-35.0
No. of Patients
5
4
3
2
1
0
Normal control 3 month
Baseline
Artery stiffness index 8
7
6
5
4
3
2
1
P < 0.001
P = 0.037
(d)
% Change in IMT
-5.0 -6.0
-7.0 -8.0
-9.0 -10.0 -11.0
-12.0
No. of Patients
4.0
3.0
2.0
1.0
0.0
Normal control 3 month
Baseline
IMT (mm) .8
.7
.6
.5
.4
.3
P < 0. 001
P = 0.908
(e)
% Change in ABI
17.5 15.0
12.5 10.0
7.5 5.0
2.5 0.0
No. of Patients
6
5
4
3
2
1
0
Normal control 3 month
Baseline
ABI
1.6 1.5 1.4 1.3 1.2 1.1 1.0 .9 .8 .7 .6
P = 0. 043
P = 0. 568