Correlations of Anatomical and Chemical Leaf Characteristics of Eucalyptus Clones with Spontaneous Leaf Spot Disease
Severity Associated with Phaeophleospora Fungi
Sri Rahayu,
1,2)Ifert Ehrlick Tudon,
1)Eko Bhakti Hardiyanto
1)【 Summary】
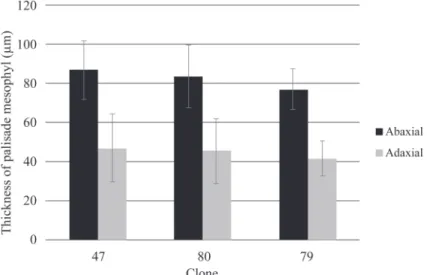
Recently, leaf spot disease caused by Phaeophleospora spp. fungi has become a severe problem in eucalyptus clonal plantations. These pathogens can kill Eucalyptus trees, and cases can be found in different stages of tree development, ranging from seedlings in nurseries to trees in the field. Before deploying a large-scale Eucalyptus clonal plantation, selecting clones resistance to disease is impor- tant in addition to selecting for optimal growth and wood properties. In this research, we explored an- atomical and chemical leaf characteristics associated with potential leaf spot disease resistance. The study was conducted on a 6-mo-old eucalypt clonal plantation at a forestry company in South Suma- tra, Indonesia. Three selected clones, i.e., clones 79 and 80 (E. pellita × E. brassiana), and clone 47 (pure E. pellita), were assessed for their growth, severity of spontaneously occurring disease, and leaf characteristics (the stomatal density, stomatal size, thickness of adaxial and abaxial palisade meso- phyll, and phenol contents). Clone 79 was susceptible, while clones 47 and 80 were more resistant to the disease. The stomatal size and density and leaf phenol contents assessed from healthy clones were not good indicators for determining resistant clones. The thickness of the abaxial palisade parenchy- ma, however, was negatively correlated with disease severity. Comparing palisade mesophyll thick- ness is suggested to be a quick, simple, and cheap approach for a preliminary assessment of potential resistance against leaf spot disease among different Eucalyptus clones.
Key words: Clonal forestry, leaf disease, palisade mesophyll, Eucalyptus pellita, Eucalyptus brassiana.
Rahayu S, Tudon IE, Hardiyanto EB. 2020. Correlations of anatomical and chemical leaf charac- teristics of Eucalyptus clones with spontaneous leaf spot disease severity associated with Phaeophleospora fungi. Taiwan J For Sci 35(3):239-49.
1)
Faculty of Forestry, Universitas Gadjah Mada, Jalan Agro No. 1, Bulaksumur, Yogyakarta 55281, Indonesia.
2)
Corresponding author, e-mail:sri.rahayu2013@ugm.ac.id 通訊作者。
Received July 2020, Accepted December 2020. 2020年7月送審 2020年12月通過。
研究報告
桉樹營養系的解剖及化學特徵與
Phaeophleospora spp.真菌
所引起的自發性葉斑病嚴重程度的關係Sri Rahayu
1,2)Ifert Ehrlick Tudon
1)Eko Bhakti Hardiyanto
1)摘 要
近年來,Phaeophleospora spp.真菌所引起的葉斑病已對桉樹(Eucalyptus)營養系人工林地造成嚴重 影響。從苗圃內的苗木到野外的樹木,均可見到病原菌造成不同生長時期桉樹死亡的案例。在進行大規 模的桉樹造林前,除了考量生長和木材性質良好的性狀外,篩選具抗病性的營養系也顯得相當重要。
本研究中,我們探討了與潛在葉斑病抗性相關的葉片解剖和化學特徵。該研究是在印度尼西亞南蘇門 答臘的一家林業公司之 6個月大桉樹營養系人工林中進行。針對挑選的3個營養系,即營養系79、80 (E.
pellita × E. brassiana)、和47 (E. pellita),分析它們的生長,發病嚴重程度和葉片特徵(氣孔密度、氣孔
大小、近軸和遠軸端柵狀葉肉厚度以及酚含量)。營養系79呈現感病,而營養系47和80則呈現較高抗病 性。健康營養系的氣孔大小,密度和葉酚含量,並不是評估營養系抗病性的良好指標。然而,遠軸端柵 狀葉肉的厚度與染病的嚴重程度呈負相關。比較柵狀葉肉厚度可做為初步評估不同桉樹營養系對葉斑病 潛在抗性一種快速、簡單且便宜的方法。
關鍵詞:營養系林業,葉病,葉柵葉肉,粗皮桉,吉桉。
短期標題:桉樹無性繁殖系的解剖與葉斑病的關係。
Sri Rahayu、Ifert Ehrlick Tudon、Eko Bhakti Hardiyanto。2020。 桉樹營養系的解剖及化學特徵與 Phaeophleospora spp.真菌所引起的自發性葉斑病嚴重程度的關係。台灣林業科學35(3):239-
49。
INTRODUCTION
Eucalyptus species are globally impor- tant planted trees due to their fast growth, valuable wood properties, and wide adapt- ability (Orwa et al. 2009). Several Eucalyptus species have long been grown in many tropi- cal regions, including Indonesia. However, in recent years, only E. pellita has been planted on a large scale across Indonesia due to its resistance or tolerance to pests and diseases (Nambiar et al. 2018). So far, E. pellita is the best alternative species to replace Acacia mangium which is very susceptible to Cera- tocystis disease and monkey and squirrel at- tacks after 3 or 4 successive rotations (Tarigan et al. 2011). Relying only on a single species of Eucalyptus is certainly a risky management
policy; thus other alternatives, i.e., different Eucalyptus species and hybrids, need to be tested for their productivity and resistance to pests and disease.
Eucalyptus hybrids are essentially a
cross-breed between 2 or more eucalypt
species which possess a combination of de-
sirable characteristics. Eucalyptus species
with potential to be developed as hybrids in
Indonesia are limited, as the majority of sites
for plantation development are located in
lowland areas with high rainfall and humidity,
where many eucalypts are unsuitable to be
grown, as they are susceptible to leaf diseases
or poor growth. However, there are a number
of species with high potential for hybrid de-
velopment, namely E. pellita, E. grandis, E.
brassiana, E. camaldulensis, and E. urophyl- la. Normally, E. pellita serves as the female parent in interspecific hybridizations, combin- ing its best traits, particularly its tolerance to disease, with desirable traits such as growth and wood properties of other species (Prasetyo et al. 2018).
In forestry companies that have adopted clonal forestry techniques, vegetative propaga- tion of the best selected Eucalyptus clones is carried out to a greater or lesser degree of so- phistication to capture the full genetic potential of the clones. The yield of Eucalyptus planta- tions will continue to increase as greater num- bers of improved clones are developed and best silvicultural practices are applied (Zobel 1993).
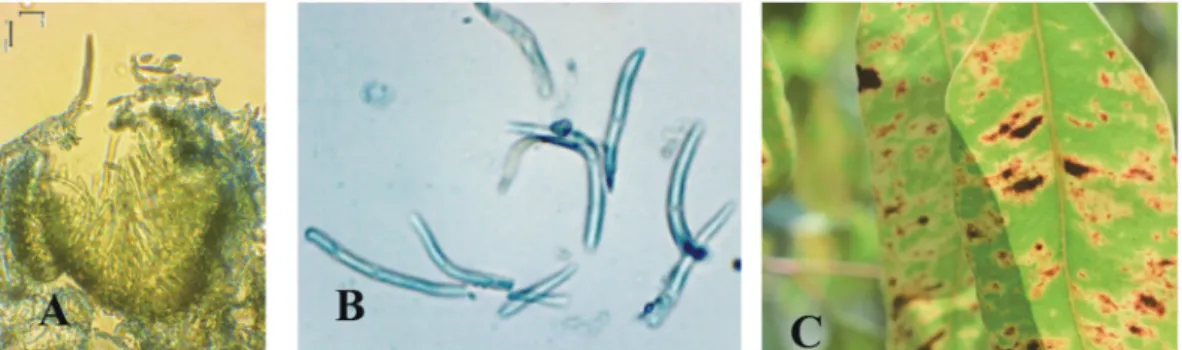
One of the virulent diseases in Euca- lyptus clonal plantations recently observed in South Sumatra is leaf spot disease caused by Phaeophleospora spp. Recently, leaf spot disease has emerged as a significant threat to eucalypt plantations in a number of South East Asian countries including Indonesia, Thailand, and Vietnam, and consequently the disease has been considered worthy of a proper management response.
Phaeophleospora spp. can cause the death of Eucalyptus trees, and they have been detected in different tree development stages, ranging from seedlings in nurseries to trees in the field with symptoms of reddish leaf patches and black spores on the leaf surface (Old et al.
2003, Videira et al. 2017). Before deploying a large-scale plantation of Eucalyptus and its hybrids, selection of clones resistant to disease is of paramount importance in addition to se- lecting for optimal growth and wood proper- ties. To date, all Phaeophleospora species are known to be associated with leaf spot diseases of plants (Taylor and Crous 1999). The asso- ciation between leaf anatomy and resistance to leaf pathogens was investigated in a number
of studies, including Mycosphaerella fijiensis M. Morelet on bananas (Craenen et al. 1997), frog-eye leaf spot (Cercospora sojina Hara) on soya beans (Yang, 2000), and Mycosphae- rella berkeleyi W.A. Jenkins on groundnuts (Jyosthna et al. 2004), as well as Terato- sphaeria leaf disease on Eucalyptus globulus (Smith et al. 2017). Stomata are located at the frontline between internal plant tissues and the surrounding environment, making them convenient gates for phytopathogens including Phaeophleospora spp. However, the infective process of Phaeophleospora spp. has not been studied in detail.
Stomata play an important role as a natu- ral entry point for pathogens; thus leaves with higher numbers of stomata per unit area will have greater chances that fungal mycelia can penetrate (Smith 2006). On the other hand, it was proposed that the first stage of the de- fense mechanism of plants involves a rapid accumulation of phenols at the infection site, which inhibits the growth of pathogens that penetrate into plant cells. Several phenols are regarded as a pre-infection inhibitors, pro- viding plants with a certain degree of basic resistance against pathogenic microorganisms (Satisha et al. 2008). A simple indicator of leaf properties can possibly be used to iden- tify susceptible clones to Phaeophleospora spp. before selected clones are released into the field as operational plantations. The aim of this study was to identify leaf properties (including leaf stomatal properties, the pro- portion of palisade mesophyll, and phenol leaf contents) potentially associated with leaf spot disease resistance of eucalypt clones of E.
pellita and its hybrids.
MATERIALS AND METHODS
Based on a previous preliminary study,
specific leaf spot symptoms caused by Pha-
eophleospora spp. were clearly observable on 6-mo-old Eucalyptus trees in the field.
Subsequently the study was conducted at a 6-mo-old eucalypt plantation belonging to PT Musi Hutan Persada, a forestry company growing eucalyptus in South Sumatra, Indo- nesia. Three clones, viz., clones 47, 79, and 80, were used in this research. A preliminary study on clones 79 and 80, both E. pellita × E. brassiana hybrids, indicated that they were respectively susceptible and resistant to Pha- eophleospora spp., while clone 47, a pure E.
pellita, was resistant.
The following characteristics were as- sessed: 1) leaf spot disease severity caused by Phaeophleospora spp.; 2) leaf stomatal attri- butes; 3) thickness of the palisade mesophyll;
and 4) constitutive total leaf phenols.
Disease severity of leaf spot caused by Phaeophleospora spp.
In order to assess the severity of spon- taneously occurring leaf spot disease caused by Phaeophleospora spp., an investigation was conducted on 6-mo-old trees in the field.
Trees were planted at a 3 × 2-m spacing. Ob- served traits were disease severity (DS) based on index scores as described in Table 1. Each clone was observed in 3 blocks, and in each block 40 trees were systematically selected, with 4 trees in every row. Leaf samples were systematically taken from 30% of the branch- es of every sample tree (from lower, middle,
and upper stems), with 10 leaves from every branch. Tree height was also measured in order to assess the effect of the disease on the growth of young eucalypt trees.
Disease severity was calculated as fol- lows (modified from Chester 1959):
where z1, z2, … z5 are index scores of leaf spot, n is the number leaves with scores of 1 to 5, N is the total number of leaves, and Z is the highest index score (5).
With regard to DS, the level of tree resis- tance was categorized as follows: resistant (R;
DS = 0~20%), somewhat resistant (SR; DS 21~40%), tolerant (T; DS = 41~60%), some- what susceptible (SS; DS = 61~80%), and susceptible (S; DS = 81~100%).
Leaf stomata
Leaf stomatal density, stomatal size, and stomatal proportion were assessed for clones 47, 80, and 79 at the age of 6 mo. Stomata were observed on 5 parts of each leaf lamina:
the tip, left, right, middle, and base. A thin layer of clear nail polish was spread on the bottom side of the leaf surface and then al- lowed to dry. Clear sticky tape was applied to the top of the nail polish and pressed down to make a good connection with the nail pol- ish and slowly released. The sticky tape was placed on a microscope slide. Slides were ob- Table 1. Index scores of leaf spot symptoms caused by Phaeophleospora spp. on 6-mo-old Eucalyptus clones in the field
No. Stage of symptomatic leaf Index score
1 Necrosis spots 1
2 Necrosis spots with chlorosis 2
3 Necrosis spots with chlorosis and spores emerging 3
4 Necrosis spots with chlorosis, blight, and spores emerging 4
5 Necrosis spots with chlorosis, blight, and spores emerging, followed 5
by leaf curling
served under an Olympus CX33 light micro- scope (Olympus, Tokyo, Japan) at 1000x and 400x magnifications. Images were taken with an OptiLab camera (Miconos Inc) to calculate and measure the number, size, and density of stomata in each clone.
Measurement of palisade mesophyll Leaves were sampled from the 4th leaf pair from the shoot tip to assure that all leaf samples were of the same age. Five healthy leaves were sampled from each healthy 6-mo- old seedling. Leaf samples were prepared by standard free-hand sectioning. Sections were cut with smooth strokes and placed on a Petri plate with water to keep the sample fresh. The sample was then mounted on a microscope slide for observation. Slides were viewed under an Olympus CX33 light microscope.
Images were taken with an OptiLab camera to calculate the adaxial and abaxial palisade mesophyll lengths.
Constitutive total leaf phenols
One pair of healthy Eucalyptus leaves from the 1st 3 nodes was sampled per tree, with 3 trees from each clone. Leaf samples were prepared and extracted following the method outlined by Hagerman (1995).
Freeze-dried, ground leaf material (0.5 g) was placed in a glass test tube with 5 mL of 70% acetone. Samples were sonicated for 30 min at 4℃, and centrifuged at 2800 g min
-1