科技部補助
大專學生研究計畫研究成果報告
* ********* ********************************************** *
*
*
*
計 畫 :
名 稱
第二型糖尿病對於鼷鼠三叉神經疼痛影響及治療
*
*
*
* ********* ********************************************** *
執行計畫學生: 丁哲浩
學生計畫編號: MOST 104-2815-C-040-003-B
研 究 期 間 : 104 年 07 月 01 日至 105 年 02 月 28 日止,計 8 個月 指 導 教 授 : 蕭水銀
處理方式: 本計畫可公開查詢
執 行 單 位: 中山醫學大學口腔科學研究所
中華民國 105 年 03 月 31 日
摘要 中文
疼痛是由於實質上或潛在性的組織破壞所引起的發炎反應而造成生理上和 心理上不愉快的感覺及經驗,而好發在許多中老年人的糖尿病患因為糖尿病促使 身體組織中過多的糖化終產物 (Advanced glycation end products)傷害神經,導致 糖尿病神經病變疼痛。因此我們推測糖尿病對於口腔顏面疼痛 (Orofacial pain) 可能有影響,並希望透過相關實驗動物允以證明之。
有關口腔顏面神經疼痛的評估方法,最近有突破的發展,主要是由 Neubert 等人設計儀器,利用「Reward & Punishment」原理,得以測量疼痛閾值,我們 使用 Neubert 等人的構想,經過修改後,自製一套新型的測量疼痛的儀器(Operant orofacial pain assessment device, OPAD),可獲得比較客觀的疼痛閾值改變的評估 方法。
本研究方法採用高脂飼料併用低劑量 Streptozotocin (35 mg/kg)誘發鼷鼠第 二型糖尿病(Type II Diabetes Mellitus),使用 Capsaicin 注射臉頰部位,引發三叉 神經中的頰神經 (Buccal Nerve)發炎而產生疼痛,我們將鼷鼠分成下列四組:(1) 正常鼠;(2)正常鼠注射 Capsaicin;(3)誘發 Type II Diabetes 鼷鼠;(4) 誘發 Type II Diabetes 鼷鼠加注射 Capsaicin。使用我們自製的 OPAD 測量各組疼痛閾值,並於 犧牲後使用免疫螢光染色分析位於三叉神經核的 c-fos 蛋白表現量以判斷痛覺神 經之活化狀態。
實 驗 結 果發 現, 經過 Capsaicin 誘 導 的臉 部 疼 痛的 鼷鼠 相 較於 沒 有 以 Capsaicin 誘導的鼷鼠在 OPAD 行為測量結果顯示其對於當作回饋的糖水攝取量 較少,且鼷鼠靠近電熱片裝置的時間減少,連帶地靠近水瓶的總時間以及次數亦 減少,然而在電熱片裝置的溫度提高後,其結果顯示誘發第二型糖尿病的誘發對 於 OPAD 行為表現變得較正常電熱片溫度重要,其對於平均每次碰觸電熱片的 時間、每次碰觸電熱片而貼近水瓶的時間都相對於沒有誘發第二型糖尿病的鼷鼠 少.也就是說,在溫度提高後,誘發第二型糖尿病的鼷鼠每次接受行為實驗的懲 罰而獲取的回饋降低.
另外,在免疫組織學染色實驗中也發現,Capsaicin 確實可以誘導三叉神經 核的 c-fos 蛋白表現,而經過第二型糖尿病的鼷鼠在 Capsaicin 的誘導下,會明顯 比未經過第二型糖尿病的鼷鼠來的低.從兩個實驗結果顯示,第二型糖尿病的鼷 鼠當組織或神經發生發炎反應,其疼痛表現會較正常的鼷鼠來的高.
English
Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage. And people in the mid age or in elderly are prone to suffer from neuropathic pain because of type II diabetes, which causes overproduction of advanced glycation end rodents (AGEs). Therefore, we hypothesized that type II diabetes can do effect on orofacial pain. Because more studies are still needed in this important issue, our study focused on the mechanism and the development of novel medication to prevent orofacial neuropathy in type II diabetes.
In addition, there is a revolutionary development in the evaluation of orofacial pain in experimental animals. Neubert et al designed the operant orofacial pain assessment device, OPAD, which used the theory of reward and punishment, to get the threshold of pain. We wanted to modify their concept to make our own novel one and get a more objective pain level data.
This study uses the high fat diet and low-dose streptozotocin (35 mg/kg) to induce type II diabetes (DM II). We also use capsaicin subcutaneously injection into the facial area to induce trigeminal neuralgia. We plan to divide mice into four groups:
(1) control mice; (2) control mice with capsaicin injection; (3) DM II-induced mice;
(4) DM II-induced mice with capsaicin injection. We plan to take advantage of our own designed OPAD to quantify the threshold level of orofacial pain. If time is permitted, we will further test tea polyphenols and curcumin, which has been investigated for a long period of time in our lab. Tea polyphenols and curcumin have multiple therapeutic effects, including anti-free radicals and neuroprotection. We attempt to have single-use or multiple-use to test the analgesic effect on neuropathic pain in DM II mice. OPAD, insulin tolerance test and glucose tolerance test will be conducted. After finishing these experiments, we will sacrifice mice to get trigeminal nucleus complex and brain tissue isolated to measure pain-associated lipid peroxidation and GSH level to explore the relationship between oxidative stress and the threshold of orofacial pain.
In this study, we found out that ICR mice with capsaicin-induced trigeminal neuralgia had lower sucrose drink, the reward in OPAD behavior test, uptake than the control mice. In addition, those mice with capsaicin-induced pain also had less time on heater contact, so as less bottle tip contact than the control mice. However, with the temperature of the heaters rose, DM II had more significant role, as compared to the heaters at normal temperature. DM II-induced mice had lower licking time per
licking event, facial contact duration per facial contact event and licking time per facial contact event than the mice without DM induction. That is, when the temperature rose, DM II-induced mice decreased the reward taking each time they got punishment.
Furthermore, capsaicin could induce the c-fos reaction in trigeminal nucleus caudalis in immunohistochemistry. However, DM II-induced mice getting capsaicin injection had significantly higher c-fos reaction than the normal mice also getting capsaicin injection. From the study, it indicated that DM II-induced mice had higher pain reaction than the control mice with tissue or neural inflammation.
(一) 前言
Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage. It is estimated that there are more than 10% of population suffering from chronic pain, such as arthritis and diabetic neuralgia. Among that, the unbearable severe feelings from cancer pain can only be treated with opiate drugs, such as morphine, and it associated medication currently. However, long-term usage usually cause severe side effect, such as hyperalgesia, drug tolerance and addiction. How to overcome side effect from opiate drugs is an important issue in this current medical field.
Type II diabetes, which belongs to adult chronic disease, have a very crucial role in the whole body pathophysiology. Patients mostly have unknown fatigue and uncomfortableness, and accompany with chronic symptoms, including cardiovascular, retinal, nephrotic and neurological pathophysiology. Due to the AGEs in the tissue, nervous system is in the condition of reduced threshold of pain. There is strong association between obesity and type II diabetes. Insulin resistance is one of the most important factors caused by obesity [1]. Long-term insulin resistance can cause hypertension, hyperglycemia and atherosclerosis. DM involves immune, neural, endocrine and cardiovascular interactions. Diabetic rats produced high quantity of pro-inflammatory cytokines and Prostagladin E2. [2] In peripheral nerves, high activation of NMDA receptors appeared because over secretion of glutamate [3], which make rats in hyper-algesia.
Trigeminal neuralgia is prone to happen to women over 40 years old. Based on previous studies, many patients with trigeminal neuralgia have had tooth root canal treatment or heavy oral surgery. In addition, what is important is that patients with
trigeminal neuralgia have strong inclination to commit suicide. It reveals that pain has impact on not only the physiological inflammation and neural sensory transmit, but psychological depression. However, in the current dental therapy and physical neurological treatment, only opiate drugs and some of anticonvulsant drugs, such as phenytoin, carbamazepine, and gabapentin. Thus, how to effectively alleviate the pain with lower side effects of those drugs is what I am interested.
Rossi, H, L. et al have mentioned that the trigeminal nucleus complex had stronger immune and inflammatory response with treatment of capsaicin on face than control in 2013. In this study, c-fos expression in trigeminal nucleus complex was 3.3 times in obese mice than control. It indicated the pain threshold in the trigeminal nerve is low because of the chronic inflammatory conditions by obesity. It intrigued me to focus on some of drugs with effects of acute inflammation, such as capsaicin, formalin or Freud’s adjuvants, on some of chronic diseases, including type II diabetes.
In current animal behavior models, evaluation of the level of orofacial pain is challenging. In the past, mechanical stimulation, such as von Frey filaments [4], and thermal stimulation [5] have been widely used. However, these methods can cause physiological stress in experimental animals in manipulation and withdrawal responses can only be measured. In 2005, John K. Neubert et al [6] published a paper in Pain. They used the concept of receiving a reward or escaping theory in Operant Orofacial Pain Assessment Device, OPAD, to evaluate orofacial pain. To be more objective in orofacial pain data, we used the idea from John K. Neubert and modified it. We used infrared sensors and thermistors as signal detection and input the original signal into computers for further analysis.
In current study, I only found out that OPAD is only used in capsaicin-induced neuralgia [7] and obesity [8]. However, there is no study on type II diabetic animal models. Thus, it encouraged me to study this research project.
(二) 研究方法及步驟 1. Animals
Male mice (ICR strain, 4 weeks old, n=38) were housed in group of four or five in acrylic cages in a standard 12-h light/ dark cycle and were allowed access to food and water ad libitum when not being tested. They were divided into 4 experimental groups: control mice (C, n=9), mice with
pain induction (P, n=9), HFD/STZ-induced DM mice (D, n=9), or HFD/STZ-induced DM and pain induction mice (D+P, n=10). Mice’s weights are recorded every week to monitor general health. Animal testing procedures and general handling complied with the ethical guidelines and standards established by the Institutional Animal Care & Use Committee at Chung Shan Medical University.
2. Induction of type II DM
2.1 Development of obesity by high fat diet (HFD)[9, 10]
The mice were allocated into different dietary regimens by feeding either normal diet (ND) or HFD (58% fat, 25% protein and 17% carbohydrate, as a percentage of total kcal) as libitum, respectively for 5 weeks.
2.2 Development of obesity/ type II DM by HFD/STZ[9, 10]
As described above, the mice fed with either ND or HFD for initial 2 weeks and then the mice were fasted for 16 hours. After fasting, mice with ND were intra-peritoneally (i.p.) injection with vehicle (citrate buffer, pH=4.5) and those with HFD were i.p. with low dose of streptozotocin (STZ, 35 mg/ kg). The mice with the non-fasting plasma glucose level of >200 mg dl-1 were considered diabetic. The mice were allowed to continue to feed with their respective diets until the end of the study.
3. Operant orofacial pain assay[6, 8]
3.1 Assay description
A testing cage (4.0 cm W × 4.0 cm D × 6.0 cm H) with acrylic walls was constructed with an opening in one wall (2.0 cm × 2.0 cm).
Two 2.0 cm × 2.0 cm thin heaters (TSA(C) 0200020eR19.8, King Lung Chin Corp., Taichung City, Taiwan) were connected to a digital temperature time controller (DTC-A1045B-150, King Lung Chin Inc., Taichung City, Taiwan). The temperature range was from environmental temperature to 60℃. A standard rodent watering bottle containing water was mounted outside the cage. The room temperature was maintained at 22±1℃ for all behavioral tests. Unrestrained
animals were placed separately in a testing cage, and the data acquisition system was made by our own. The bottle was then positioned in proximity to the cage such that the animal was allowed access to the reward bottle when simultaneously contacting the heaters with its face. When the mouse drank from the water bottle, the skin on its shaved face contacted the heaters, and the animal’s tongue contacted the metal spout on the water bottle. (Figure 1(A)-(C)) We used 2 sets of infrared transmitters and receivers near the heaters and water bottle tips as signal recording for facial contact and drinking.
Data were collected at 6 Hz for the entire length of the experiment.
(Figure 2(D)-(F)) Each spout contact was recorded as a ‘licking’ event.
Each heater contacted for recording of ‘facial contact’ events. The duration of each facial contact and the total number of events (licking, facial contact) were recorded.
During offline data analysis, the threshold for detection of the facial contacts and licking contacts was set at 800. An event (licking or facial contact) was registered when the signal went beneath threshold and ended when the signal dropped below threshold. The cumulative duration and frequency of events were determined for both the licking (reward) contact data and the facial stimulus contact data. A reward/facial contact event ratio was calculated by dividing the number of licking events by the number of facial contact events and the duration per contact for the facial stimulus was also calculated. The total amount of water consumed (g) was measured and compared at each of the testing temperatures. Data analyses were achieved using custom-written routines in Matlab (v. 2014a, The MathWorks, Inc., Natick, Massachusetts, U.S.A) and Excel (Microsoft). (Figure 2) 3.2 Behavior test
Animals were first trained to drink water while contacting the heaters set at environmental temperature for baseline training (5 sessions, 10 min for each). This lead-in training period was necessary to acquaint the animals with the task of locating the reward bottle.
After training, each group of animals is then tested during a 2-week period according to a fixed but non-sequential order of stimulus temperatures (32, 45, and 57℃, 3 min for each order) for 18 min.
Before testing, hair on two vibrassal pads is cut without anesthesia.
Either capsaicin or saline is injected subcutaneously in the area of vibrassal pads before the test for 15 min.
4. Immunohistochemistry 4.1 c-fos assay[11]
The tissue with perfused with 4% formalin was post-fixed in the same fixative overnight at 4°C, transferred to 30% sucrose for cryoprotection, and then cut into 40-µm coronal sections. Every 4th section was processed for Fos immunohistochemistry. Free-floating sections were incubated with 0.3% hydrogen peroxide in 50%
methanol to block endogenous peroxidase activity, washed three times in PBS, treated with blocking buffer (3% Normal Goat Serum in 0.25% Triton X-100) to eliminate nonspecific binding, and then incubated with a primary antibody against Fos (1:20,000, EMD 4 Biosciences Ab-5, anti-c-fos polyclonal rabbit) for 40 hours at 4°C.
Fos immunohistochemistry was detected with a biotinylated secondary antibody (6 µg/mL, raised in goat), amplified with an avidin-biotin complex reaction (standard ABC kit) and visualized with a nickel enhanced peroxidase-diaminobenzidine (DAB) reaction.
Slide-mounted sections were dehydrated in a graded series of alcohol, cleared with xylene or xylene substitute (Electron Microscopy Sciences), and coverslipped with Permount (Fisher Scientific).
The number of Fos-immunoreactive (Fos-IR) neurons and their distribution pattern were quantified directly from the slides using an upright microscope with 20x objective, viewed through a video camera, with the aid of a computer-controlled stage driven by Neurolucida (v7, MBF Biosciences). A Fos positive cell was defined as a cell with a dark grey or black nucleus, visible above the background level of staining. Anatomic divisions were determined using the Franklin and Paxinos Compact Mouse Brain Atlas [12]. Fos-IR cells were counted for every hemi-section in each sample (ipsilateral and contralateral to the injection). Fifteen to 17 sections per mouse were counted. The sections were aligned across samples to ensure that comparison between samples occurred at equivalent anatomical levels through the brainstem and upper cervical spinal cord. We use the pyramidal decussation as a landmark to align the samples. The experimenter
counting Fos-IR neurons was blinded to the treatment and diet group.
Exemplar images were obtained with a BX-51 microscope using MicroSuite digital imaging software (Olympus) using the 20x objective.
5. Statistical analysis
Data were presented as means±SD. Statistical significance was set at P
< 0.05. Statistically significant difference was determined by the analysis of variance in SPSS statistical software (SPSS, Inc., Chicago, IL).
We also looked at the effects of capsaicin and HFD/ STZ, which used 2-way ANOVA to analyze the interaction of both factors.
(三) 結果
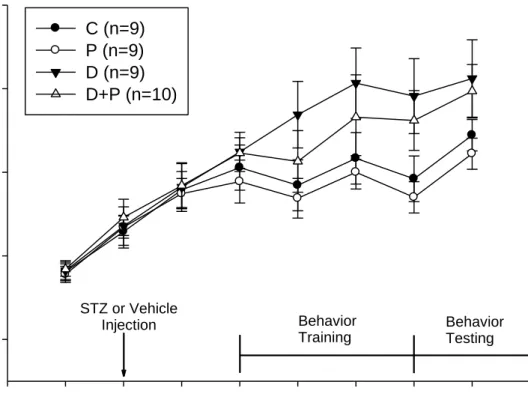
1. Effects of HFD/ STZ and capsaicin on body weights
During the experimental period of 8 weeks, a trend of higher body weights in HFD/ STZ-induced DM mice was observed after 5-week feeding with different diet (Table 1). With the capsaicin-induction, slight loss of body weight could be observed but without statistical difference (p>0.05).
The body weights were plotted in Figure 3.
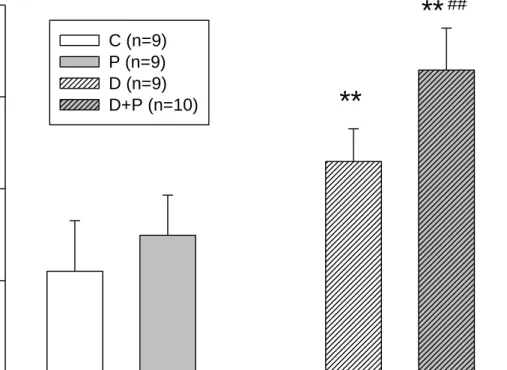
2. Serum glucose level
The blood glucose level in HFD/ STZ-induced DM group of mice were significantly higher than contrl group. Besides, with capsaicin-induced pain, the glucose level were even higher (D, 230±36; D+P, 329±46; C, 110±55) (Figure 4).
3. Operant orofacial pain assay in behavior training
From the fourth to sixth week of experiments, the mice were in the operant orofacial pain assay for eight times as behavior training. Each time took 30 minutes, and the heaters were settled at 25℃. We found out that mice needed more times for acquisition of device than the rats written in papers [6]. During the whole training period, we could observe that all groups of mice had more intake of reward, more facial contact events and duration and more licking events and time duration of the bottle tips compared to the first time of behavior training. However, less reward intake
and lower licking time duration was observed in HFD/ STZ-induced DM mice than mice with normal diet. Except that, no specific statistical differences were found in the data (Figure 5).
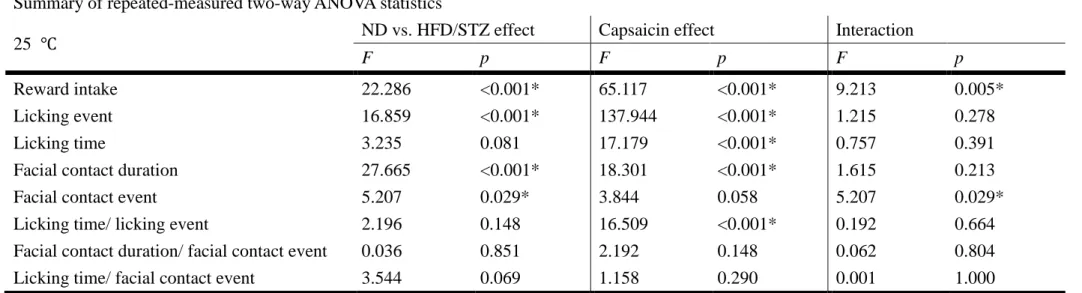
4. Effect of capsaicin and HFD/STZ on operant orofacial pain assay
For the studies using sucrose solution as reward, we could find out that in the first testing, the mice with capsaicin injection on the vibrasssal pads had significant decline of reward intake. Capsaicin also had effects on licking event, licking time and facial contact duration when the heaters were set on normal environmental temperature (Table 2). With the temperature of heaters rose up, the reward intake of all groups of mice decreased, which revealed that temperature had effects on the performance to all groups of mice. However, when the heaters were set on 53℃, the effect of HFD/ STZ and capsaicin and their interaction became dominant role on the behavior (Table 3). Furthermore, we could also found out that when the heaters were set above 37℃, mice with HFD/STZ and capsaicin had great negative effect on the performance compared to the mice with HFD/STZ but without capsaicin except facial contact events (Figure 6).
5. C-fos immunohistochemistry on trigeminal nucleus caudualis
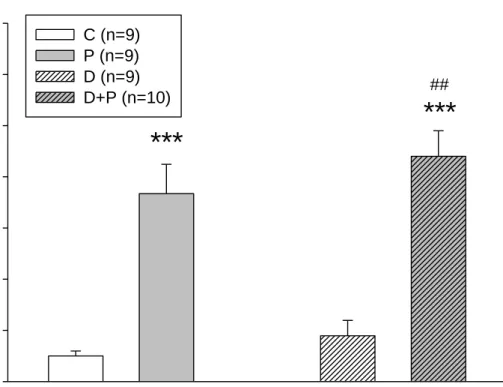
With the capsaicin induction, there were more c-fos immunohistoreactive cells than those without capsaicin induction. Slight increase of c-fos in group D mice, as compared to group C mice was observed. However, the c-fos reactive cells were much more in group D+P mice than the group C mice; in addition, there was statistical significant difference between group P and group D+P (Figure 7 & 8).
From number of c-fos immunohistoreactive cells, it was indicated that capsaicin-injection on mice’s vibrassal pads induced the pain reaction and neural body inflammation in trigeminal nucleus caudualis. Besides, with interaction of HFD and STZ injection, capsaicin-induced pain reaction could be aggravated.
(四) 討論
This study combined the behavior performance and the tissue bio-marker reaction. In the past time, pain is really difficult to quantify, so as the animal study. Neubert’s creative idea stimulated me to remake the new OPAD model,
which used infra-red light as the recording material. We also used the immunohistochemistry as support for our behavior data.
Type II DM is a widespread disease with a common, chronic condition with serious health implications. Consistent with the evidence in humans, a number of animal studies have demonstrated that diabetes may increase the inflammatory response to bacteria. DM mice were shown to stimulate an exaggerated cytokine expression and inflammatory infiltrate compared to the response observed in non-diabetic mice [13]. Long-term hyperglycemia prompts the production of advanced glycation end products, which increase the cell stress and then, as well, aggravate the inflammation [14]. However, the relationship between DM and neuralgia had not been proven. We assumed that when the mice with DM were subject to capsaicin-induced neuralgia had exacerbated tissue inflammation and then cause more nociception.
Long-term HFD could increase insulin resistance [14], which increase the possibility of successful DM-induction. Over 5 weeks of different diets, mice with HFD had higher body weights than those with ND. With streptozotocin injection, it reduced the secretion of insulin and cause hyperglycemia. From the blood glucose test after sacrifice, mice with HFD/ STZ had higher blood glucose than those with ND, which showed the success of DM induction.
In OPAD test, we observed that capsaicin had effect on reward intake, licking event, licking time, facial contact duration and facial contact event while the heaters were set at environmental temperature. It is indicated that capsaicin caused the unwillingness of taking reward in mice. However, when the temperature of heaters rose, the effect of DM condition became more significant.
It revealed that mice with DM not only had lower reward intake, licking event, facial contact duration, but also lower licking time per licking event, facial contact duration per facial contact event, and licking time per facial contact event.
It indicated that when the pain reaction exacerbated when DM-induced mice had capsaicin-injection when the face contacted with stress.
In addition, we also found out that mice with capsaicin-induction had higher c-fos protein reaction in trigeminal nucleus caudalis than mice without capsaicin-induction. However, DM-induction exacerbates c-fos reaction, which supported the OPAD data.
Future study would be focused on the detailed mechanism of interaction between the local tissue inflammation and nociceptors in DM mice. We would
also try to use some of combination medication to test the effects on reduction of pain reaction in DM.
In conclusion, this study highlights the correlation between the DM induction and capsaicin effects in ICR mice. Capsaicin could induce trigeminal pain reaction, but the extent magnified with the interaction of DM.
(五) 參考文獻
1. Kahn, B.B. and J.S. Flier, Obesity and insulin resistance. The Journal of clinical investigation, 2000. 106(4): p. 473-481.
2. Ahlgren, S.C. and J.D. Levine, Mechanical hyperalgesia in streptozotocin-diabetic rats. Neuroscience, 1993. 52(4): p. 1049-1055.
3. Malcangio, M. and D.R. Tomlinson, A pharmacologic analysis of mechanical hyperalgesia in streptozotocin/diabetic rats. Pain, 1998. 76(1–
2): p. 151-157.
4. Vos, B.P., A.M. Strassman, and R.J. Maciewicz, Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat's infraorbital nerve. J Neurosci, 1994. 14(5 Pt 1): p. 2708-23.
5. Imamura, Y., H. Kawamoto, and O. Nakanishi, Characterization of heat-hyperalgesia in an experimental trigeminal neuropathy in rats. Exp Brain Res, 1997. 116(1): p. 97-103.
6. Neubert, J.K., et al., Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain, 2005. 116(3): p.
386-395.
7. Neubert, J.K., et al., Differentiation between capsaicin-induced allodynia and hyperalgesia using a thermal operant assay. Behavioural Brain Research, 2006. 170(2): p. 308-315.
8. Rossi, H.L., et al., Effects of diet-induced obesity on motivation and pain behavior in an operant assay. Neuroscience, 2013. 235(0): p. 87-95.
9. Srinivasan, K., et al., Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacological Research, 2005. 52(4): p. 313-320.
10. Reed, M., et al., A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism, 2000. 49(11): p. 1390-1394.
11. Rossi, H.L., et al., Obesity increases nociceptive activation of the trigeminal system. European journal of pain (London, England), 2013. 17(5): p.
649-653.
12. Franklin, K.B.J. and G. Paxinos, The mouse brain in stereotaxic coordinates.
3rd ed. 2008, Boston: Elsevier/Academic Press.
13. Schmitz, J., et al., IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity, 2005. 23(5): p. 479-490.
14. Wautier, M.-P., et al., Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. American Journal of Physiology-Endocrinology And Metabolism, 2001. 280(5): p. E685-E694.
(A) (B) (C)
(D) (E)
(F)
Figure 1. The manipulation principles of operant orofacial pain assay. (A)When the experimental animals moved forward to have the access to the water, vibrassal pads must touch bilateral thin heaters. (B) Rats got the rewarding milk when touching bilateral thermodes. (Reference from J.K. Neubert et al/ Pain 116(2005) p. 388). (C) Rats got the rewarding 30% sucrose solution when touching bilateral thin heaters in our self-made operant orofacial pain assessment device. (D) The data acquisition chip and the digital temperature controller. (E) The operant orofacial pain assessment device with smaller size of cage for mice. (F) The whole view of the operant orofacial pain assessment device. (The cage is for rats.)
*
#
Figure 2. Offline data analysis is conducted by Matlab. The first row is the temperature of thin heaters, and the second and the third is the signal detection from the infrared sensors.
*When the infrared sensor does not detect the signal, it means that the experimental animals contact the thin heaters.
#When the infrared sensor does not detect the signal, it means that the experimental animals touch the bottle tip.
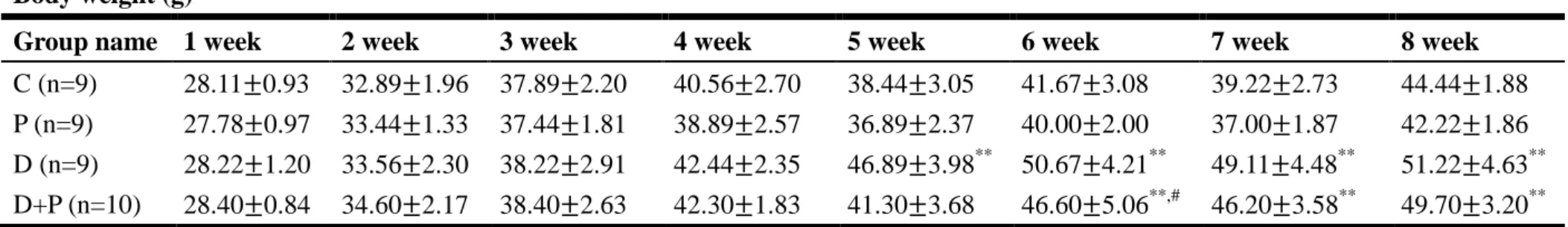
Body weight (g)
Group name 1 week 2 week 3 week 4 week 5 week 6 week 7 week 8 week
C (n=9) 28.11±0.93 32.89±1.96 37.89±2.20 40.56±2.70 38.44±3.05 41.67±3.08 39.22±2.73 44.44±1.88 P (n=9) 27.78±0.97 33.44±1.33 37.44±1.81 38.89±2.57 36.89±2.37 40.00±2.00 37.00±1.87 42.22±1.86 D (n=9) 28.22±1.20 33.56±2.30 38.22±2.91 42.44±2.35 46.89±3.98** 50.67±4.21** 49.11±4.48** 51.22±4.63**
D+P (n=10) 28.40±0.84 34.60±2.17 38.40±2.63 42.30±1.83 41.30±3.68 46.60±5.06**,# 46.20±3.58** 49.70±3.20**
Table 1. Comparison of body weight between different groups of ICR mice during 8-week experiment. Group D and D+P mice were i.p. with streptozotocin on the third week. Note the increase of body weight on HFD/STZ-induced DM groups and decrease of body weight on capsaicin-induced pain groups of mice.
C, control mice; P, mice with pain induction (P, n=9); D, HFD/STZ-induced DM mice; D+P, HFD/STZ-induced DM and pain induction mice.
All of the data were displayed in mean±SD.
** P < 0.01 compared to the group C mice and # P < 0.05 compared to the group D mice.
Age (weeks)
3 4 5 6 7 8 9 10 11 12
Weight (g)
20 30 40 50 60
C (n=9) P (n=9) D (n=9) D+P (n=10)
Behavior Training
Behavior Testing STZ or Vehicle
Injection
Figure 3. Effect of HFD/ STZ and capsaicin on weight gain in ICR mice during 8 weeks of experiments
C, control mice; P, mice with pain induction (P, n=9); D, HFD/STZ-induced DM mice;
D+P, HFD/STZ-induced DM and pain induction mice.
Glucose (mg/dL)
0 100 200 300 400
C (n=9) P (n=9) D (n=9)
D+P (n=10)
**
**
##Figure 4. Effect of HFD/ STZ and capsaicin on blood sucrose level in ICR mice after 8 weeks of experiments.
C, control mice; P, mice with pain induction (P, n=9); D, HFD/STZ-induced DM mice;
D+P, HFD/STZ-induced DM and pain induction mice.
** p<0.01 compared to the group C mice.
## p<0.01 compared to the group P mice.
(A)
Training Order
1 2 3 4 5 6 7 8
Intake (g)
0 1 2 3 4 5
C (n=9) P (n=9) D (n=9) D+P (n=10)
* *
(B)
Training Order
1 2 3 4 5 6 7 8
Licking Events
0 5 10 15 20 25
(C)
Training Order
1 2 3 4 5 6 7 8
Licking Total Duration (s)
0 20 40 60 80 100 120 140 160 180
* *
(D)
(E)
(F)
(G)
(H)
Figure 5. Behavior training for 8 times on operant outcomes measures. (A) Reward intake, (B) licking events, (C) total licking duration, (E) total facial contact duration, and (F) facial contact events were recorded by self-made operant orofacial pain device with the heaters settled at 25℃. Each training time took half an hour. With further calculation, (D) licking duration/ events and (G) facial contacts duration/ events and (H) licking duration/ facial contacts event could be further accessible.
C, control mice; P, mice with pain induction (P, n=9); D, HFD/STZ-induced DM mice;
D+P, HFD/STZ-induced DM and pain induction mice.
All of the data were displayed in mean±SD.
** P < 0.01 compared to the group C mice.
Summary of repeated-measured two-way ANOVA statistics
25 ℃ ND vs. HFD/STZ effect Capsaicin effect Interaction
F p F p F p
Reward intake 22.286 <0.001* 65.117 <0.001* 9.213 0.005*
Licking event 16.859 <0.001* 137.944 <0.001* 1.215 0.278
Licking time 3.235 0.081 17.179 <0.001* 0.757 0.391
Facial contact duration 27.665 <0.001* 18.301 <0.001* 1.615 0.213
Facial contact event 5.207 0.029* 3.844 0.058 5.207 0.029*
Licking time/ licking event 2.196 0.148 16.509 <0.001* 0.192 0.664
Facial contact duration/ facial contact event 0.036 0.851 2.192 0.148 0.062 0.804
Licking time/ facial contact event 3.544 0.069 1.158 0.290 0.001 1.000
Table 2. Summary of statistical comparisons of reward intake, licking event, licking time, facial contact duration, facial contact event, licking time/ licking event, facial contact duration/ facial contact event, and, licking time/ facial contact event while the heaters were set at 25 ℃.
* Indicates a significant difference, p<0.05. Note the significant effects of capsaicin on the behavior.
Summary of repeated-measured two-way ANOVA statistics
53 ℃ ND vs. HFD/STZ effect Capsaicin effect Interaction
F p F p F p
Reward intake 785.082 <0.001* 712.615 <0.001* 30.433 <0.001*
Licking event 4.268 0.047* 83.065 <0.001* 0.724 0.401
Licking time 3.199 0.083 167.841 <0.001* 87.947 <0.001*
Facial contact duration 43.144 <0.001* 217.459 <0.001* 0.581 0.451
Facial contact event 0.062 0.806 139.327 <0.001* 1.424 0.241
Licking time/ licking event 22.792 <0.001* 2.267 0.142 38.487 <0.001*
Facial contact duration/ facial contact event 34.935 <0.001* 1.154 0.290 19.145 <0.001*
Licking time/ facial contact event 16.017 <0.001* 1.751 0.195 32.944 <0.001*
Table 3. Summary of statistical comparisons of reward intake, licking event, licking time, facial contact duration, facial contact event, licking time/ licking event, facial contact duration/ facial contact event, and, licking time/ facial contact event while the heaters were set at 53 ℃.
* Indicates a significant difference, p<0.05. Note the significant effects of diet and STZ injection on the behavior.
(A)
The l ast
beha vior traini
ng
Intake (g)
0 1 2 3 4 5
25 37 46 53
Temperature (C)
* *
### ###
###
C (n=9) P (n=9) D (n=9) D+P (n=10)
(B)
Licking Events
0 5 10 15 20 25
The last
behav
ior training 25 37 46 53
Temperature (C)
#
###
(C)
Licking Total Duration (s)
0 20 40 60 80 100 120 140 160
The last
behavior training 25 37 46 53
Temperature (C)
* *
### ###
###
(D)
Facial Contact Duration (s)
0 20 40 60 80 100 120 140 160 180
The last
behavior training 25 37 46 53
Temperature (C)
###
###
##
###
(E)
Facial Contact Event
0 5 10 15 20 25
The last
behav
ior training 25 37 46 53
Temperature (C)
##
(F)
Lcking Duration/ Licking Event (s)
0 10 20 30 40 50
The l ast
beh
avior training 25 37 46 53
Temperature (C)
### ###
###
(G)
Facial Contact Duration/ Facial Contact Event (s)
0 10 20 30 40
The l ast
beh
avior training 25 37 46 53
Temperature (C)
###
##
###
(H)
Licking Duration/ Facial Contact Event (s)
0 10 20 30 40
The last
behav
ior training 25 37 46 53
Temperature (C)
### ### ###
Figure 6. The last time of behavior training (without capsaicin injection) and behavior testing with different temperature setting on operant outcomes measures. (A) Reward intake, (B) licking events, (C) total licking duration, (E) total facial contact duration, and (F) facial contact events were recorded by self-made operant orofacial pain device.
Each training time took half an hour. With further calculation, (D) licking duration/
events and (G) facial contacts duration/ events and (H) licking duration/ facial contacts event could be further accessible.
C, control mice; P, mice with pain induction (P, n=9); D, HFD/STZ-induced DM mice;
D+P, HFD/STZ-induced DM and pain induction mice.
All of the data were displayed in mean±SD.
** P < 0.01 compared to the group C mice.
# P < 0.05 compared to the group D mice.
## P < 0.01 compared to the group D mice.
### P < 0.001 compared to the group D mice.
(A) (B)
(C) (D)
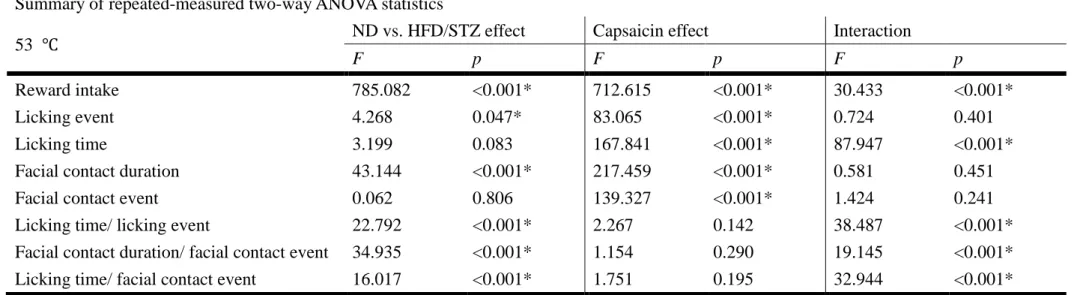
Figure 7. Photomicrographs of c-fos immunohistochemical staining on trigeminal nucleus caudualis in ICR mice under 40 times magnification. Representatives of (A) group C mice, (B) group P mice, (C) group D mice and (D) group D+P mice.
C, control mice; P, mice with pain induction (P, n=9); D, HFD/STZ-induced DM mice;
D+P, HFD/STZ-induced DM and pain induction mice.
Number of c-fos immunoreactive cells
0 20 40 60 80 100 120
140 C (n=9)
P (n=9) D (n=9) D+P (n=10)
***
##
***
Figure 8. Number of c-fos immunohistoreactive cells in different groups of ICR mice.
*** p<0.001 compared to the group C mice.
## p<0.01 compared to the group P mice.