Graduate Institute of Veterinary Clinical Sciences School of Veterinary Medicine
National Taiwan University Master Thesis
15 25
Comparison of Efficacy and Toxicity of 15-Week and 25-Week Chemotherapy Protocol in Canine Multicentric Lymphoma
Chia-Lien Kao
Advisor: Jih-Jong Lee, DVM, MS, Ph. D.
107 7
25 vincristine cyclophosphamide doxorubicin CHOP
15 CHOP [1]
15 CHOP 25
2010 2018
62
42 25 22 15
T
25 15 97.6%
100% 25 15 83.3% 95.5%
25
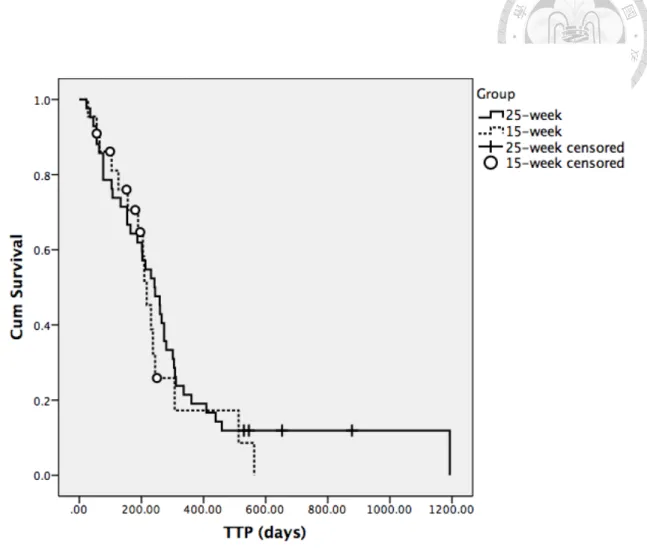
Time to progression 242 15 217
P=0.503 Overall survival time
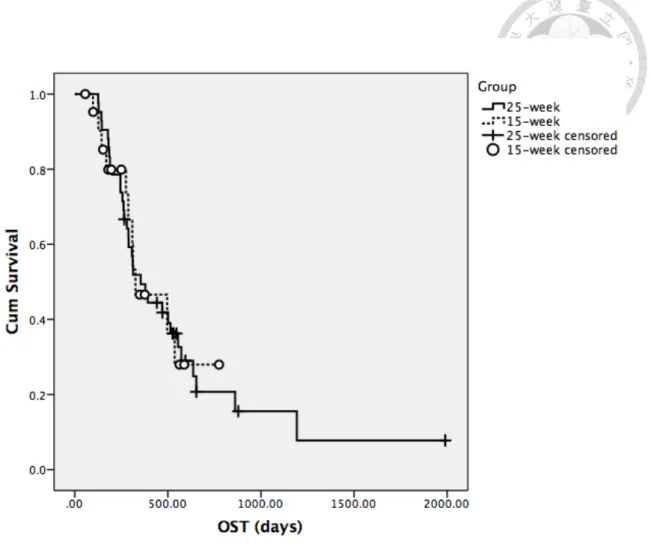
25 15 354 326 P=0.999
67.8%
50%
15 25
CHOP
ABSTRACT
Canine multicentric lymphoma is the most common lymphoproliferative disease in dogs. Multi-agent chemotherapy has been as the mainstay of treatment due to high response rate, durable remission and relatively long survival time. Among them, the modified University of Wisconsin-Madison (UW-Madison) protocol without maintenance (i.e. the 25-week protocol), which included vincristine, cyclophosphamide, doxorubicin and prednisolone (known as CHOP), has been extensively used. Although most patients generally respond well to the treatment, progressive diseases developed and the patients inevitably succumb to the disease. To improve the treatment efficacy and shorten the lengthy and costly treatment, modification of protocol in a more dose-intense fashion has been proposed, such as co-administration of chemotherapeutic agents, decreased interval of drug administration or dose escalation. A 15-week CHOP protocol was therefore reported, and comparable efficacy and well-tolerated toxicity were showed initially [1]. The purpose of this study is to further compare two protocols most commonly used in National Taiwan University Veterinary Hospital Animal Cancer Treatment Center (NTUVHACTC), the 15-week CHOP protocol and the 25-week protocol, regarding their efficacy and possibility of adverse events. Potential prognostic impact was also evaluated.
Medical records of dogs diagnosed with multicentric lymphoma by either cytology or histopathology evaluation in NTUVHACTC from January 2010 to February 2018 were included for comparison. A total of sixty-four dogs met the inclusion criteria and were enrolled. According to the treatment protocol each patient received, forty-two dogs are assigned to 25-week group and twenty-two dogs were assigned to the 15-week group, respectively. There was no significant difference in distribution of patient
demographics and clinical characteristics including stage, substage and parameters possibly associated with poor prognosis (e.g. thrombocytopenia, T-cell immunophenotype and hypercalcemia). Objective response rate of 25-week and 15-week protocol was 97.6% and 100% respectively. Among them, 83.3% of dogs in 25-week group and 95.5% of dogs in 15-week group attained complete remission. No statistically significance was noted for either objective response rate or distribution of each response between two groups. Median time to progression was 242 days in 25-week group and 217 days in 15-week group, which was showed no significantly different (P=0.503). Median overall survival time was 354 days in 25-week group and 326 days in 15-week group, without statistical significance presented (P=0.999). For adverse events, 67.8% of episodes were gastrointestinal in nature, with anorexia and vomiting equally and both more frequently presented than diarrhea. Rest of episodes were hematological in nature, majorly consisting of neutropenia. More than 50% of episodes were grade 1 to 2 toxicity, regardless of type of adverse events. No significant differences for the adverse event were noted between two groups. In the aspect of prognostic factors, dogs with body weight higher than median value, Golden retrievers and presence of abnormal thoracic image had significantly lower rate of complete remission. Dogs attaining complete remission had significantly longer time to progression in univariate and multivariate analysis. Dogs experiencing neutropenia were associated with significantly longer overall survival time only in univariate analysis.
In conclusion, the 15-week protocol was generally comparable to the modified UW-Madison 25-week protocol in both efficacy and adverse events. Therefore, a shorter, dose-intense protocol with similar efficacy and toxicity profile can bring more clinical
Keywords: CHOP protocol, chemotherapy, canine multicentric lymphoma
CONTENTS
... #
... 1
... 2
ABSTRACT ... 4
CONTENTS ... 7
LIST OF FIGURES ... 10
LIST OF TABLES ... 11
ABBREVIATION LIST ... 13
Chapter 1 Literature review ... 14
1.1 Canine multicentric lymphoma ... 14
1.1.1 Signalment ... 14
1.1.2 Etiology ... 15
1.1.3 Clinical presentation ... 15
1.1.4 Staging ... 16
1.1.5 Immunophenotype ... 17
1.2 Chemotherapy protocol ... 18
1.3 Prognostic factors ... 20
Chapter 2 Introduction ... 22
Chapter 3 Materials and methods ... 23
3.1 Patient selection ... 23
3.4 Chemotherapy ... 24
3.4.1 Protocol ... 24
3.4.2 Monitor ... 25
3.5 Response ... 26
3.6 Toxicity ... 26
3.7 Statistical analysis ... 26
Chapter 4 Results ... 29
4.1 Demography ... 29
4.1.1 Patient characteristics ... 29
4.1.2 Clinical stage ... 29
4.1.3 Immunophenotype ... 31
4.1.4 Laboratory findings ... 31
4.2 Chemotherapy protocol ... 31
4.2.1 Treatment of the 25-week group ... 32
4.2.2 Treatment of the 15-week group ... 33
4.3 Outcome ... 34
4.3.1 Response ... 34
4.3.2 Time to progression and overall survival ... 35
4.4 Adverse events ... 36
4.5 Prognostic factors ... 39
4.5.1 Prognostic factors analysis for CR ... 39
4.5.2 Prognostic factors analysis for TTP ... 40
4.5.3 Prognostic factors analysis for OST ... 41
4.5.4 Prognostic factors analysis for large-sized population and non-GR population ... 42
Chapter 5 Discussion ... 43
5.1 Outcomes of different protocols ... 43
5.2 Adverse events ... 45
5.3 Prognostic factors ... 47
5.4 Limitations ... 55
Chapter 6 Conclusion ... 56
REFERENCES ... 92
LIST OF FIGURES
Fig. 1. The Kaplan-Meier curve for time to progression (TTP) of the two groups ... 58 Fig. 2. The Kaplan-Meier curve for overall survival time (OST) of the two groups ... 59
Fig. 3. The Kaplan-Meier curve of time to progression (TTP) for body weight (< and ≥ median body weight) from all patients ... 60
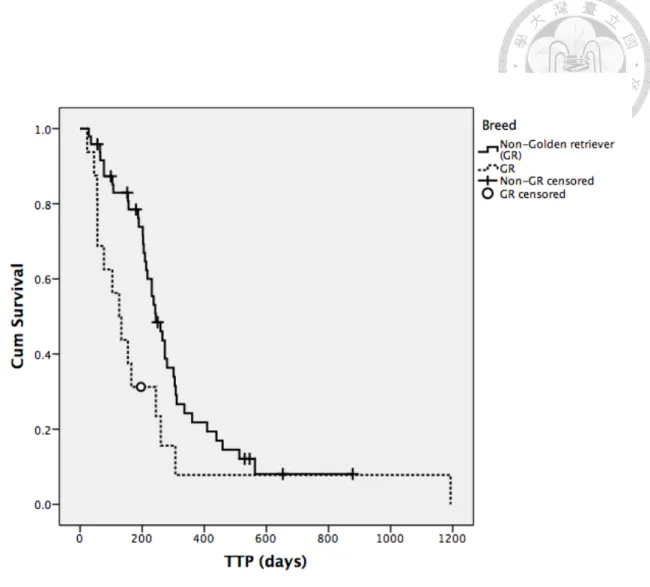
Fig. 4. The Kaplan-Meier curve of time to progression (TTP) for breed (Non-GR and GR) from all patients ... 61
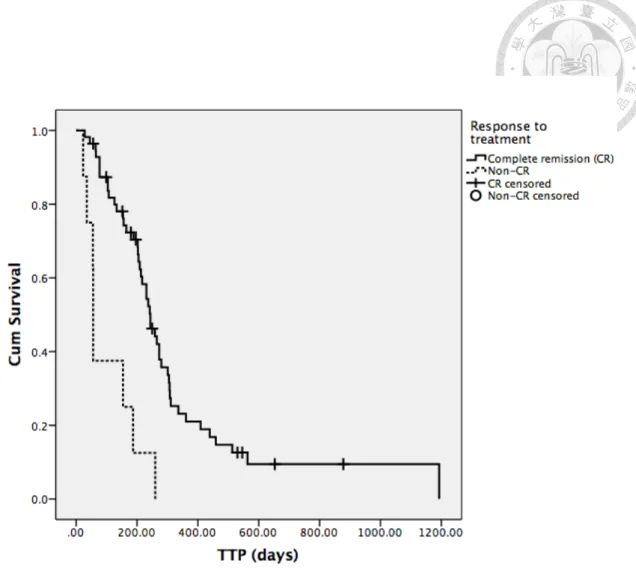
Fig. 5. The Kaplan-Meier curve of time to progression (TTP) for response to treatment (CR and Non-CR) from all patients ... 62
Fig. 6. The Kaplan-Meier curve of time to progression (TTP) for substage (a and b) from all patients ... 63
Fig. 7. The Kaplan-Meier curve of overall survival time (OST) for body weight (< and ≥ median body weight) from all patients ... 64
Fig. 8. The Kaplan-Meier curve of overall survival time (OST) for breed (Non-GR and GR) from all patients ... 65
Fig. 9. The Kaplan-Meier curve of overall survival time (OST) for the presence of neutropenia during treatment from all patients ... 66
Fig. 10. The Kaplan-Meier curve of overall survival time (OST) for response to treatment (CR and Non-CR) from all patients ... 67
LIST OF TABLES
Table 1. World Health Organization’s clinical staging system for lymphoma in domestic animals ... 69
Table 2. 15-week CHOP protocol dosing schedule ... 70 Table 3. Modified University of Wisconsin-Madison protocol without maintenance (25-week protocol dosing schedule) ... 71
Table 4. Veterinary Cooperative Oncology Group Common Terminology Criteria for Adverse Events (version 1.1) ... 72
Table 5. Comparison of characteristics between two groups ... 73 Table 6. Distribution of findings in laboratory examination at diagnosis between two groups ... 74 Table 7. Summary of response to treatment in two groups ... 75 Table 8. Summary of median time to progression (TTP) and overall survival time (OST) for two groups ... 76 Table 9. Summary of adverse events and dose adjustment for two groups ... 77 Table 10. Univariate analysis of factors associated with demographics of patients in the whole population for attaining CR or not ... 78 Table 11. Univariate analysis of factors associated with clinical findings in the whole population for attaining CR or not ... 79 Table 12. Univariate analysis of factors associated with adverse events and treatment in the whole population for attaining CR or not ... 80
Table 14. Univariate analysis of factors associated with clinical findings in the whole population for time to progression (TTP) ... 82 Table 15. Univariate analysis of factors associated with adverse events and treatment in the whole population for time to progression (TTP) ... 83 Table 16. Multivariate analysis of univariate factors with significance in the whole population for time to progression (TTP) ... 84 Table 17. Univariate analysis of factors associated with demographics of patients in the whole population for overall survival time (OST) ... 85 Table 18. Univariate analysis of factors associated with clinical findings in the whole population for overall survival time (OST) ... 86 Table 19. Univariate analysis of factors associated with adverse events and treatment in the whole population for overall survival time (OST) ... 87 Table 20. Multivariate analysis of univariate factors with significance for overall survival time (OST) in all patients ... 88 Table 21. Body weight distribution in large-sized dogs (≥ 20 kg) ... 89 Table 22. Comparison of rate of complete remission, time to progression (TTP) and overall survival time (OST) in three subgroups ... 90
ABBREVIATION LIST
Abbreviations Description Abbreviations Description
ATLS Acute tumor lysis
syndrome ORR Objective response rate
AE Adverse event OST Overall survival time
BAL Bronchoalveolar lavage PARR Polymerase chain reaction for antigen receptor rearrangement
BSA Body surface area PCR Polymerase chain
reaction
CR Complete remission PCV Packed cell volume
CBC Complete blood count PD Progressive disease
CHOP Cyclophosphamide, Hydroxy-daunorubicin, Oncovin, Prednisolone
PD-1 Programmed cell death-1
CI Confidence interval PD-L1 Programmed cell
death-ligand 1 CMOP
Cyclophosphamide, Mitoxantrone, Oncovin,
Prednisolone PR Partial remission
DNA Deoxyribonucleic acid QBC Quantitative blood count
FC Flow cytometry SD Stable disease
G-CSF Granulocyte
colony-stimulating factor SHC Sterile hemorrhagic cystitis
GR Golden retriever TTP Time to progression
IHC Immunohistochemistry VCOG Veterinary Cooperative Oncology Group
ICC Immunocytochemistry WHO World Health
Organization HR Hazards ratio
LR Labrador retriever MCP-1 Monocyte chemotactic
protein-1
MRD Minimal residual disease
Chapter 1 Literature review
1.1 Canine multicentric lymphoma
Lymphoma is one of the most common neoplastic diseases in pet dog population, with an annual incidence estimated to be 13-114 per 100, 000 dogs [2]. It comprises 7 to 24% of all canine neoplasia and 83% of all canine hematopoietic tumor [3], making it a significant disease in this species. Lymphoma consists of diverse groups of neoplasia, possessing heterogeneity regarding etiology, clinical and histopathological presentation, treatment response and prognosis. Among all the dogs diagnosed with lymphoproliferative diseases, 84% of the population develop multicentric lymphoma [3].
1.1.1 Signalment
For canine multicentric lymphoma, some species are well-known for increased risks for developing, including Bullmastiff, Rottweiler, Scottish terrier, Boxer, Cocker spaniel, German shepherd, Golden retriever (GR), Labrador retriever (LR) [2-5]. Even though any age dogs can be diagnosed with lymphoma, middle-aged or older dogs with the median age of 6 to 9 years are more likely to be affected [6]. Based on a large-scaled investigation for canine lymphoma in France where 608 cases were studied, the age at occurrence ranged from 1-16 years, with a mean of 8.3 years and a median of 8 years [5]. There is no such evidence of sex predisposition, although intact females associated with lower risk was once reported [6].
1.1.2 Etiology
The etiology of multicentric lymphoma remains unclear and is thought to be multifactorial. Investigated factors include exposure to chemicals, waste incinerators, polluted sites and radioactive waste [3, 5]. DNA repair deficiency is also suspected, based on the finding of significantly increased chromosomal damage following ionizing radiation exposure and bleomycin exposure in lymphocytes of Golden retrievers diagnosed with lymphoma compared to which of normal Golden retrievers and mixed breed dogs [7].
1.1.3 Clinical presentation
Generalized, non-painful lymphadenopathy is the most common symptom in affected dogs. Clinically, most dogs show no signs. However, the swollen lymph nodes, especially those of mandibular or prescapular region, sometimes can still result in precaval syndrome, characterized by edema of head, neck and forelimbs, when the sizes become large enough to compress the circulation of local lymphovascular system (e.g.
cranial vena cava). Dogs with precaval syndrome may suffer from dyspnea, noisy breathing sound, choking or difficulty of swallowing. Other nonspecific signs, such as anorexia, vomiting, depression, fever, or polydipsia and polyuria, may be seen as well and usually depend on the location and severity of organs involved.
Laboratory abnormalities associated with multicentric lymphoma included anemia, thrombocytopenia, neutrophilia, or circulating atypical lymphocytes which may indicate bone marrow involvement. Hypercalcemia may be noted as a paraneoplastic syndrome in approximately 10-40% of the clinical cases [8] and tends to associated with the
[8, 9].
Internal organ involvement can also result in abnormal imaging findings. In thoracic radiographs, abnormal pulmonary parenchymal patterns and enlargement intrathoracic (sternal, tracheobronchial and cranial mediastinal) lymph nodes are noted in 37% and 26 to 40% of dogs with multicentric lymphoma, respectively [10]. Pleural effusion may be presented as well. There are still other differentials accounting for the similar image changes, such as bronchopneumonia or chronic bronchial changes.
However, so far, the information of the correlation or consistency between the abnormal imaging changes mentioned above and the cytological or histopathological findings from other diagnostic methods (i.e., bronchoalveolar lavage or lung biopsy) regarding determining pulmonary infiltration by multicentric lymphoma is scarce. Only one study in 1993 showed cytology finding of bronchoalveolar lavage (BAL) was more sensitive compared with radiographic evaluation of lungs, with 66% and 34% positive result, respectively [11]. In abdominal radiographs or ultrasonography, sublumbar lymphadenopathy, hepatomegaly and splenomegaly with echogenicity changes are three most common findings, ranging from 46 to 75% in dogs affected [10].
1.1.4 Staging
Evaluation of clinical staging is essential once the diagnosis of multicentric lymphoma confirmed on a dog. To provide the disease extent and tailor the treatment plan. The most widely adopted staging system is World Health Organization’s clinical staging system for lymphoma in domestic animals proposed in 2011 by Valli et al. [3]
(Table 1).
To define a clinical stage, information, such as hematology data, image details for liver and spleen, or bone marrow evaluation, is required. Among numerous diagnostic
tools, application of more sensitive staging tests can detect previously unnoted lesions, especially those that are asymptomatic, and sometimes leads to stage migration.
Substitution of abdominal radiography with ultrasonography and quantitative blood count (QBC) with complete blood count (CBC) both result in significant stage migration from less advanced to advanced stages (i.e. stage IV & V) [12]. Addition of splenic and hepatic cytology may help to distinguish between stage IV and III [13];
however, the addition of bone marrow cytology will not have a significant impact on staging [12]. Interestingly, even though the variable degree of stage migration is commonly noted among different studies, these changes of diagnostic methods exert no significant influence on either remission rate, remission duration, or survival in these studies [12, 13]. A possible explanation that disease extent may not be an essential determinant was proposed [13]. Therefore, application of additional diagnostic methods, especially more invasive ones, may be limited by the prognostic value in practice.
1.1.5 Immunophenotype
Immunophenotyping provides information of whether clonality exists and what type of cells comprising the disease. The ways of immunophenotyping include flow cytometry (FC), immunohistochemistry (IHC), immunocytochemistry (ICC) and polymerase chain reaction for antigen receptor rearrangement (PARR), according to the sample submitted [14-19]. For canine lymphoma, the distribution of B-cell, T-cell and unclassified subtype was estimated about 51.2 to 74%, 26 to 36% and 19.5%, respectively [18, 20, 21]. In the ability of correctly predicting immunophenotype (with IHC serving as a gold standard), FC was showed to be superior to PARR, with the
70%, respectively [18]. However, if a fresh sample is not available or the result of flow cytometry is confounding, PARR is still highly suggested for immunophenotyping due to its high specificity of 98% [18].
1.2 Chemotherapy protocol
Chemotherapy is the mainstay for treatment of canine multicentric lymphoma.
Significantly longer median survival time of 8 to 12 months can be achieved, compared to survival time of 1 to 7.5 months using steroid only and 4 to 6 weeks without treatment [3, 22]. Since lymphoma is chemosensitive, the goals of chemotherapy in these patients are aiming at a high response rate, a soon-achieved first complete remission and a durable response (> 6 months) [3].
Numerous chemotherapy protocols have been proposed and can be classified as single-agent or multi-agent therapy. Multi-agent protocol is the treatment of choice due to difficulty maintaining durable remission duration solely with one antitumor mechanism provided by a single agent (median survival time of 5.7 to 9 months for doxorubicin alone [23]). Multi-agent protocol is usually doxorubicin-based or refers to as CHOP protocol with drug combination of vincristine (Oncovin), cyclophosphamide, doxorubicin (Hydroxy-daunorubicin) and prednisolone.
For the past 20 years, modification of protocol while maintaining the same efficacy without further toxicity has been an important topic of multi-agent chemotherapy.
Although high remission rate of more than 85%, traditional UM-Madison protocol with maintenance phase is thought to be lengthy and therefore money and time-consuming.
Also, there is no inclusion of a maintenance phase in most human non-Hodgkin’s lymphoma protocol [24]. Whether maintenance phase is necessary for protocol has been
questioned. Based on several studies, a maintenance-free protocol can achieve similar response rate, remission duration and survival time without change of toxicity profile [25-29]. Therefore, a discontinuous protocol is more widely accepted now.
Because the outcomes of multicentric lymphoma patients did not improve much since the use of doxorubicin-based protocols starting in the1990s, and most patients still succumb to the disease and only very few long-term survivors encountered, another attempt of protocol modification focusing on dose intensity recently. To increase dose intensity, either increasing the frequency of administration by shortening the length of protocol and increasing the dose of specific chemotherapeutic agents meanwhile, or concurrent administration of two chemotherapeutic agents (i.e., vincristine and cyclophosphamide) in the same week were attempted [24, 28, 30]. The efficacy seemed to be similar to that of previous protocols and was not affected by the use of a shorter protocol, but the incidence of toxicity increased, and more treatment-related toxicities were noted [24, 28, 30]. Although these studies failed to demonstrate a better outcome as expected, administration of a short-term protocol without losing therapeutic benefit seemed to be possible.
To avoid the profound toxicity noted in other dose-intense settings, short-term, maintenance-free protocols without co-administration of chemotherapeutic agents, such as a 12 or 15-week protocol, were proposed [1, 24, 27]. The efficacy of these protocols is acceptable and comparable to that of other maintenance-free protocols. However, only one arm of the group was evaluated in those studies, and the comparison of response and survival data with historical reports concluded. It should result in certain limitations of reliability, such as bias due to an ununiform distribution of advanced stage
extended treatment-free period during remission which represented a higher quality of life in patients and may increase owner compliance to some extent [27].
1.3 Prognostic factors
Many factors have been investigated for canine multicentric lymphoma to see if any prognostic value exists. Throughout most studies, factors can generally categorize into two types. The first type has identifiable findings from examinations at diagnosis, including T-cell immunophenotype, the presence of hypercalcemia, thrombocytopenia, anemia and bone marrow infiltration [31-37]. For bone marrow infiltration, both neoplastic lymphoid cells in peripheral blood and bone marrow are discovered by flow cytometry or PCR analysis. Although the degree of peripheral blood infiltration has directly correlated with that of bone marrow infiltration [35], it is the number of tumor cells in peripheral blood at diagnosis assessed by either quantitative PCR, real-time PCR or flow cytometry that is showed no significant correlation with prognosis [38, 39].
This phenomenon implies that the status of peripheral blood may not be able to sensitively and accurately reflect that of bone marrow every time or on every patient.
However, the status of minimal residual disease (MRD), where the sequence of immunoglobulin from lymphoma cells was examined, in peripheral blood during the early phases of chemotherapy has directly associated with progression-free interval and overall survival [38]. This finding serves MRD in peripheral blood as a potential marker for early identifying patients who are more likely to relapse during the treatment and therefore aids in early initiation of a more dose-intense treatment before disease progression in these patients.
The second type is related to the clinical stage of a patient, with advanced stage
and substage (i.e., stage V and substage b) being inferior factors [33, 35, 38, 40]. These factors both indicate possibly a more extent disease distribution in patients and subsequently poor clinical performance before treatment, limiting the use of an aggressive and dose-intense chemotherapy regimen considering patient’s tolerability of toxicity.
The remaining factors more have something more to do with treatment, such as the history of steroid usage in pretreatment and response to treatment [33, 36, 40]. Use of steroid before chemotherapy leads to overexpression of drug efflux transporters (e.g., P-glycoprotein, multidrug resistance protein 1 and lung-resistance protein) on tumor cells and thus usually associates with drug resistance during treatment [41-43]. Other potential factors not routinely examined at the clinic but also showed prognostic value include overexpression of the chemokine monocyte chemotactic protein-1 (MCP-1) and hypermethylation of the DAPK CpG island [44, 45].
Recently, the role of checkpoint molecule expression (i.e., programmed cell death 1 and its ligand) has also been studied in canine lymphoma patient through examining the samples from fine needle aspiration by flow cytometry. It showed higher expression of programmed cell death 1 (PD-1) and programmed cell death-ligand 1 (PD-L1) in diseased dogs than in healthy animal [46]. Although the prognostic value of PD-L1 expression has not been established yet in canine lymphoma, the correlation between human diffuse large B-cell lymphoma and higher PD-L1 expression has been validated and carries a poor prognosis, with shorter overall survival in high expressers [47].
Chapter 2 Introduction
Lymphoma is the most common hematopoietic tumor in dogs, comprising 7-24%
of all canine neoplasia. Multicentric lymphoma serves 84% of dogs diagnosed with lymphoproliferative disease [3]. Chemotherapy has been the treatment of choice for its high response rate and most extended remission duration and survival time among all the treatment choices. Especially, multi-agent chemotherapy protocols have been showed to be more effective than single-agent use due to multiple different cytotoxic mechanisms in these protocols [3, 23, 48]. In traditional modified UW-Madison protocol without maintenance (i.e. 25-week CHOP protocol), the median remission duration was 220 days and median survival time was 303 days [25]. More dose-intense protocols such as 12-week or 15-week CHOP protocol were proposed to see if efficacy can remain with a shorter length of protocol, also fewer costs of time and money at the same time.
From one study where the 15-week CHOP protocol was first proposed [1], the response rate and overall survival time of the protocol were showed to be comparable with previous data, although shorter progression-free survival was noted. Application of this shorter, more dose-intense protocol was potentially feasible. However, comparison with other protocols through a second arm or control group has been lacking so far.
Since 15-week CHOP protocol has been used more in NTUVH for the recent years, the difference between the traditional 25-week protocol in actual efficacy and toxicity profile is also not extensively evaluated. Therefore, the purpose of this study was to compare the efficacy and toxicity of 15-week CHOP protocol and 25-week protocol in dogs diagnosed with canine multicentric lymphoma. Also, prognostic factors evaluated meanwhile. Comparable or even better outcome for 15-week protocol was expected.
Chapter 3 Materials and methods
3.1 Patient selection
Dogs diagnosed with multicentric lymphoma and treated with a 15-week CHOP or 25-week CHOP/CMOP protocol were enrolled in this study. Medical records from January 2010 to February 2018 in National Taiwan University Veterinary Hospital (NTUVH) animal cancer treatment center were reviewed retrospectively. Patients eligible for inclusion in this study included dogs with a histological or cytological diagnosis of multicentric lymphoma, no previous therapy for tumor control except prednisolone and administration of either a 15-week or 25- week protocol as their initial chemotherapy. Criteria for cytology diagnosis included the predominance of medium to large lymphocytes constituting at least 50% of the total cell population. Use of L-asparaginase at induction of their CHOP protocol was acceptable. Dogs treated with any other chemotherapy agents or radiation therapy for lymphoma before the protocols begin were excluded from this study.
3.2 Data collection
The following information of cases, including breed, sex, weight, and age at diagnosis, stage, methods of staging, substage and status of steroid pretreatment, was collected. Immunophenotype and the results of complete blood count (CBC) and biochemistry panel at diagnosis were evaluated if available. Immunophenotyping was performed by either flow cytometry or immunohistochemistry.
3.3 Stage
Cases were staged according to World Health Organization (WHO) clinical staging system for lymphoma in domestic animals [3] (Table 1).
Not every case had hepatic or splenic cytology and bone marrow biopsy evaluated routinely. For stage 4, either abdominal radiography or ultrasonography was used to evaluate liver/spleen involvement, requiring the finding of hepatomegaly and/or splenomegaly, with or without abnormal echogenicity in both organs. For stage 5, the following situations are suggestive of bone marrow involvement: 1. Presence of lymphocytosis in peripheral blood with the morphology of medium to large lymphocytes predominates in the blood smear. 2. Thrombocytopenia and anemia lack of presence of Ehrlichia canis and/or Babesia (i.e., Babesia canis and Babesia gibsoni) in blood smear, along with negative result in Ehrlichia antibody (IDEXX SNAP®
4Dx® Plus Test) or PCR test for Ehrlichia canis and Babesia (i.e., Babesia canis and Babesia gibsoni).
3.4 Chemotherapy
3.4.1 Protocol
A 15-week or 25-week chemotherapy protocol was administered as presented in Table 2. or Table 3., respectively. Dogs presented after 2016 were generally given a 15-week protocol after the design proposed by Thamm et al. was more well-studied [1, 24]. For 25-week protocol, there were two types of the setting of chemotherapy agents.
One was the modified University of Wisconsin-Madison CHOP protocol without maintenance, and the other was similar to the general construction of the former but named as CMOP where substitution of mitoxantrone 6 mg/m2 for doxorubicin was
applied. The study reported by Wang et al. [49] showed CMOP provided similar efficacy to CHOP in both progression-free survival and median survival time. Therefore, dogs administered with either set of the 25-week protocol, or experienced drug switching from doxorubicin to mitoxantrone under the decision of the attending doctor during treatment were all included in this study.
Alteration of the protocol may be necessary during treatment under the following situations in NTUVH. Substitution of cyclophosphamide with chlorambucil at 20 mg/m2 was performed when the patient was presented with sterile hemorrhagic cystitis suspected to the side effect of cyclophosphamide. In dogs with a high risk of developing acute tumor lysis syndrome (ATLS), dose reduction for the 1st dose of vincristine in the first cycle of chemotherapy may be made from 0.7 mg/m2 to 0.5 mg/m2 according to the preference of the attending clinician. Due to the retrospective nature of this study, this dosage modification was acceptable and documented accordingly.
3.4.2 Monitor
At each visit for chemotherapy, a CBC was performed. Other parameters in biochemistry panel were performed based on the judgment from the attending clinician.
A dose was delayed for seven days if the absolute neutrophil count was less than 3000/
µL, platelet count was less than 100,000/ µL, or any clinical condition where chemotherapy was contraindicated (i.e. grade 3-5 toxicity). Dose reduction ranging from 10 to 20% were performed at the discretion of the attending clinician according to the severity of previous adverse events.
3.5 Response
Response to treatment was evaluated according to Response evaluation criteria for peripheral nodal lymphoma in dogs (v1.0) proposed by veterinary cooperative oncology group (VCOG) [50]. The lymph node size was measured by caliper and documented in diameters from three dimensions. Complete remission (CR) was defined as disappearance of all measurable disease; partial remission (PR) was characterized by at least a 30% but < 100% decrease in the mean sum longest diameter of target lesions.
The progressive disease (PD) was characterized by at least a 20% increase in the mean sum longest diameter of target lesions or development of a new lesion; The stable disease (SD) was determined when neither sufficient decrease to qualify for PR nor sufficient increase to qualify for PD.
3.6 Toxicity
Toxicity was evaluated from the outcomes of hematology, biochemistry profiles and clinical performance at each visit and subsequently graded from 1 to 5 based on Veterinary Cooperative Oncology Group Common Terminology Criteria for Adverse Events (VCOG-CTCAE) [51] (Table 4).
3.7 Statistical analysis
For all patients, objective response rate (ORR) was calculated by dividing the number of cases experiencing CR or PR with the total number of cases treated. Time to progression (TTP) was calculated from the date of treatment initiation to the date of PD.
Overall survival time (OST) was calculated from the date of treatment initiation to the date of tumor-related death or euthanasia
Demographic distribution between the group of 15-week protocol and the group of 25-week protocol was analyzed to see if a significant difference existed. Continuous data including age, body weight and days between diagnosis and treatment initiation was analyzed by Mann-Whitney U test. Categorical data, including breed, sex, stage, substage, immunophenotype and the status of steroid pretreatment, were compared by Pearson’s chi-square test. However, if the expected value of a given cell in the comparison was less than five, Fisher’s exact test was used instead.
Among parameters for evaluating clinical outcomes, ORR between two groups were compared by Pearson’s chi square test; median TTP and OST were analyzed by Kaplan-Meier estimation and the distribution was displayed by Kaplan-Meier curve.
Difference of TTP and OST between two groups was analyzed by log-rank test. For TTP, cases were censored at the time when they were lost to follow-up or still remained progression-free until the study closure. For OST, cases which were dead from unrelated causes, lost to follow-up or still alive at the time of study closure were censored.
However, cases that were lost follow up were considered to have died from their disease if they were known to be out of remission at their last follow-up.
The influence of potential prognostic factors on achieving CR or not, TTP and OST of all patients were analyzed by Pearson’s chi-square test and log-rank test, respectively.
Factors analyzed included age, body weight, breed, sex, stage, substage, presence of thoracic involvement in imaging, pattern of thoracic involvement, immunophenotype, hematologic and biochemistry parameters (i.e. presence of anemia, thrombocytopenia, lymphocytosis, leukocytosis and hypercalcemia, baseline globulin and baseline neutrophils), status of steroid pretreatment, presence of adverse events (i.e. neutropenia,
avoiding ATLS and the use of mitoxantrone in protocol. Univariate factors with P ≤ 0.05 were further analyzed by multivariate analysis using the Cox proportional hazards model.
In order to compare the incidence and severity of adverse events (AEs) between two protocols, Pearson’s chi-square test was used to analyze the presence and distribution of adverse events including grading of neutropenia, thrombocytopenia, anorexia, vomiting and diarrhea occurred during treatment.
Mainly, for the group of 25-week protocol, we still wanted to see the influence of mitoxantrone administration on achieving CR, TTP and OST. Therefore, data from the subgroup of CMOP and CHOP was analyzed by Pearson’s chi-square test and Kaplan-Meier estimation for achieving CR, and TTP and OST, respectively.
All analyses were performed by SPSS v. 20 software and were considered significant at P ≤ 0.05.
Chapter 4 Results
4.1 Demography
4.1.1 Patient characteristics
Sixty-four dogs met the inclusion criteria between January 2010 and February 2018 and was enrolled in the present study. 42 dogs received the 25-week protocol as their initial treatment; 22 dogs were treated with the 15-week protocol. There were 18 (28%) mixed breed dogs, 16 (25%) Golden retrievers, 6 (9%) Welsh corgis, 4 (6%) Schnauzers, 3 (5%) Beagles, 3 (5%) Malteses, 3 (5%) Shi-tzu, 2 (3%) West highland white terriers, 2 (3%) Poodles, and the rest included 1 Dachshund, 1 Labrador, 1 Husky, 1 Cavalier King Charles Spaniel, 1 Sheltie, 1 Shiba inu and 1 Yorkshire. The most common breeds in two groups both were mixed breed dogs and Golden Retrievers (Table 5). Median age was 7.8 (range 2-13 years) in the 25-week group and 9.5 (range 4-12 years) in 15-week group. Median body weight was 16.15 kg (range 5.06-54.4 kg) in 25-week group and 15.95 (range 1.46-40 kg) in the 15-week group. There were 17 spayed females, 5 intact females, 14 castrated males and 6 intact males in the 25-week group; 8 spayed females, 4 intact females, 9 castrated males and 1 intact male in the 15-week group. There was no significant difference in the distribution of patient characteristics between two groups. Detailed information was summarized in Table 5.
4.1.2 Clinical stage
Fifty-six dogs had sufficient information for staging, with 8 dogs in the 25-week
both abdominal radiography and ultrasonography were available for evaluation in 9 dogs; only abdominal radiography or ultrasonography were available for 7 dogs and 12 dogs, respectively. In the 15-week group, only 1 dog had information of both abdominal radiography and ultrasonography. The rest were staged via abdominal ultrasonography for determination of stage IV. No dogs in either group performed a splenic or hepatic cytology. The thoracic image for pulmonary infiltration and intrathoracic lymph node evaluation was available in 27 (64.3%) dogs of the 25-week group and 20 (90%) dogs of 15 weeks. In 25-week group, 10 dogs had abnormal change in thoracic radiography, including pulmonary infiltration in 7 dogs; enlargement of sternal and tracheobronchial lymph node both in 6 dogs and widen cranial mediastinum in 4 dogs. In the 15-week group, 9 dogs were identified with abnormal findings, including 2 dogs with pulmonary infiltration, 3 dogs with enlarged sternal lymph node, 4 dogs with enlarged tracheobronchial lymph node and 3 dogs with widening cranial mediastinum.
Overall, among the 34 dogs available for complete staging in 25-week group, there were 1 dog (2.9%) in stage I, 8 (23.5%) dogs in stage III, 16 (47.1%) dogs in stage IV, and 9 (26.5%) dogs in stage V. The remaining 8 dogs without sufficient information were in either stage III or IV. In 15-week group, 2 (9.1%) dogs were in stage III, 14 (63.6%) dogs were in stage IV and 6 (37.3%) dogs were in stage V. No significant difference in stage allocation between two groups (P=0.408) (Table 5).
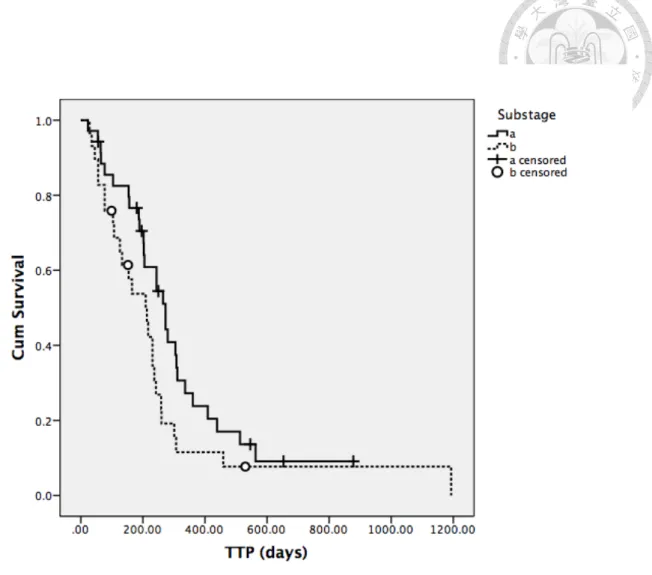
For substage, in the 25-week group 23 (54.8%) dogs and 19 (45.2%) dogs were classified as substage a and b, respectively. In the 15-week group, 12 (54.5%) dogs were substage a and 10 (45.5%) dogs were substage b. Most common clinical signs presented in dogs with substage b were lethargy, anorexia and panting. Other less frequent signs included ecchymosis, vomiting, diarrhea and vision impairment. No significant difference in substage distribution between two groups (P=0.987) (Table 5).
4.1.3 Immunophenotype
Examination for immunophenotype was performed in 51 dogs. All dogs but 2 dogs from the 25-week group who were defined by immunohistochemistry were analyzed by flow cytometry. In 32 dogs available for immunophenotype from 25-week group, 28 (87.5%) dogs were B-cell; 1 (3.1%) dog was T-cell; 3 (9.4%) dogs were null type. In 19 dogs with available information from the 15-week group, 14 dogs (73.7%) were classified as B-cell; 2 (10.5%) dogs were T-cell; 3 (15.8%) dogs were null type. No significant difference in the distribution of immunophenotype between two groups (P=0.406) (Table 5).
4.1.4 Laboratory findings
More than half dogs from either group had no specific findings in laboratory examinations. Hypercalcemia as a possible paraneoplastic syndrome was only noted in 1 dog from the 25-week group among the whole population. Abnormal hematologic laboratory findings possibly associated with poor prognosis (e.g. thrombocytopenia and anemia) were not overrepresented in either group, for there was no significance recognized in the distribution of these factors between two groups (Table 5). Detailed features for two groups were listed in Table 6.
4.2 Chemotherapy protocol
Twenty-seven (64.3%) dogs from the 25-week group and 18 (81.8%) dogs from 15-week group were treated with the protocol on the same day of diagnosis. 5 (11.9%)
between diagnosis and treatment and numbers of population pretreated with steroid before chemotherapy between two groups (Table 5).
Particularly, the 1st dose adjustment for prevention of ATLS was more significantly presented in the 15-week group (4.8% in 25-week group versus 40.9% in the 15-week group, P=0.000) (Table 9).
4.2.1 Treatment of the 25-week group
Forty-two dogs were treated by the 25-week protocol. In respect of the construct of 25-week protocol, CHOP was used in 32 (76.2%) dogs and CMOP protocol was used in 7 (16.6%) dogs. 2 (4.8%) dogs were given 3 cycles of CMOP followed by 1 cycle of CHOP; 1 (2.4%) dog was given 3 cycles of CHOP and then 1 cycle of CMOP.
Thirty (71.4%) dogs completed the 25-week protocol. Among the 12 dogs who didn’t finish the treatment, 4 dogs failed to retain complete remission through the current protocol and 8 dogs relapsed during treatment. Available information regarding rescue protocol for the 39 dogs who eventually relapsed included continuous incorporation of L-asparaginase into CHOP-based protocol (n=23), CCNU (n=2) and Dactinomycin (n=1).
Seven (16.7%) dogs were given L-asparaginase in the protocol. All dogs, except for 1 dog who was given 3 doses, received only 1 dose of L-asparaginase (10,000 IU/m2, intramuscularly or subcutaneously). All doses were administered during treatment based on the judgement of the attending clinician, with none at the initiation of treatment as part of chemotherapy induction. The number of patients administered with L-asparaginase was not significantly different between two groups (Table 5).
Apart from the dose reduction for prevention of ATLS at initiation of chemotherapy, 13 (31%) dogs experienced a total of 20 dose reductions during treatment. Of these 13
dogs, 9 dogs required one dose reduction, 2 dogs experienced dose reduction for twice;
1 dog for 3 times and 1 dog for 4 times. 16 (80%) events were due to neutropenia. As for preventive dose reduction for ATLS, 2 (4.8%) dogs whose large tumor burden recognized as a risk factor of developing ATLS received dose reduction of vincristine (0.5 mg/m2) at treatment initiation.
Twenty-nine (69%) dogs experienced a total of 62 dose delays during treatment. 8 dogs required one dose delay, 12 dogs required two dose delays, 7 dogs required 3 dose delays, 1 dog required 4 dose delays and 1 dog required 5 dose delay. 43 (69.4%) episodes were due to neutropenia. 7 (11.3%) times were due to gastrointestinal toxicity.
3 (4.8%) episodes were due to elevated liver indexes. Other miscellaneous causes were such as leukocytosis, foreign body digestion before visit or owner’s request. Median and mean dose delay were both 1.5 times (range: 0-5).
4.2.2 Treatment of the 15-week group
Twenty-two dogs were treated with 15-week protocol. Most dogs received the construct of CHOP, except for 1 dogs who was initially given mitoxantrone for the first 2 cycles in consideration of cardiac toxicity by the attending clinician according to the finding of heart murmur but then switched to Adriamycin after evaluation by a cardiologist and it turned out no previous concern. 16 (72.7%) dogs completed the protocol. Of the 6 dogs failing to complete, 1 was unable to reach complete remission, 2 were due to development of progressive disease during treatment and 3 were due to owner’s financial concern. Available information regarding rescue protocol for the 13 dogs who eventually developed progressive disease included continuous incorporation
Two dogs were given L-asparaginase during treatment. One dog was given 1 dose and the other dog was given 2 doses due to multiple wounds on skin over the body and was therefore given L-asparaginase due to contradiction to chemotherapy. All doses were administered during treatment, rather than the treatment initiation.
Nine (40.9%) dogs experienced dose reduction during treatment. 5 dogs required 1 dose reduction, 3 dogs required 2 dose reductions and 1 dog required 4 dose reductions.
As in 25-week group, most dogs were due to neutropenia. Other causes included gastrointestinal side effect (n=1), elevated liver indexes (n=1), or both (n=1). For prevention of ATLS, 9 (40.9%) dogs received dose reduction for 1st vincristine (0.5-0.6 mg/m2) at treatment initiation.
A total of 30 episodes of dose delay were presented in 15 (68.2%) dogs. 8 dogs experienced 1 dose delay; 3 dogs required 2 dose delays; 2 dogs required 3 dose delays;
1 dog needed 4 dose delays and 1 dog needed 6 dose delays. 19 (63.3%) episodes were due to neutropenia; 5 (16.7%) were due to elevated liver indexes; 4 (13.3%) were due to gastrointestinal toxicity. The median and mean time of dose delay were 1 and 1.36, respectively (range: 0-6)
4.3 Outcome
4.3.1 Response
For the whole population in this study, ORR was 98.4%, with CR in 56 (87.5%) dogs, PR in 7 (10.9%) dogs and SD in 1 (1.6%) dog. In the 25-week group where ORR was 97.6%, 35 (83.3%) dogs reached CR; 6 (14.3%) dogs experienced PR and 1 (2.4%) dog experienced SD as their best response. In the 15-week group with ORR of 100%, 21 (95.5%) dogs reached CR and only 1 (4.5%) dog attained PR. There was no
significant difference in the ORR between two groups (P=0.466) (Table 7).
4.3.2 Time to progression and overall survival
For all dogs, the median TTP was 231 days. In the 25-week group, the median TTP was 242 days (range: 23-1193 days). 2 dogs were lost to follow-up on Day 546 and Day 653 and censored on the day of last follow-up. 2 dogs still remained progression-free and were censored on the day of study closure (Day 878 and Day 530). In the 15-week group, the median TTP was 217 days (range: 28-563 days). 1 dog was lost to follow up on Day 56 and 5 dogs were still in remission by the day of study closure. The median follow-up duration for these 5 dogs was 180 days (range: 99-250 days). No significance was noted in median TTP between two groups (P=0.503) (Figure 1).
The median OST for the whole population was 354 days. The median OST in 25-week group was 354 days. 6 dogs were lost to follow-up and censored on the last day of presentation, with median follow-up duration of 498 days (range: 266-653 days).
1 dog died of unrelated cause (cardiac disease) on Day 878, and 3 dogs were still alive and censored by the closure of study on Day 530, 594 and 1193. The median OST in 15-week group was 326 days. 1 dog was lost to follow-up and censored on the day of last presentation (Day 56), 1 dog died of unrelated cause (cardiac disease) on Day 563, and 9 dogs were still alive and censored at the completion of the study with median follow-up duration of 250 days (range: 99-775 days). There was no significance in the median OST between two groups (P=0.999) (Figure 2).
The median follow-up duration for all dogs in the 25-week group and the 15-week group was 313 days (range: 126-1190 days) and 275 days (range: 56-775 days),
The median TTP and median OST for two groups were summarized in Table 8.
4.4 Adverse events
A total of 283 episodes of adverse events were noted for all 64 dogs in this study, with 192 ones from the 25-week group and 91 ones from the 15-week group.
Hematologic AEs comprised 32.2% of total episodes, while gastrointestinal AEs made up 67.8% of all. 5 dogs didn’t experience any toxicity, with 3 from the 25-week group and 2 from the 15-week group. Instead, 9 dogs experienced at least one grade 4 toxicity, with 5 dogs from the 25-week group and 4 dogs from the 15-week group. The detailed distribution of frequency and severity of adverse events was summarized in Table 9.
Among the AEs of hematologic in nature, 4 (57.1%) dogs in the 25-week group and 10 (45.5%) dogs in the 15-week group experienced neutropenia during treatment.
Grade 1 neutropenia was the most frequently presented in both groups, accounting for 51% of neutropenia episodes in the 25-week group and 40% of that in the 15-week group. Grade 4 neutropenia was noted in 4 dogs from both groups, being 9.5% and 18.2% in each population, and among them only 1 dog from the 15-week group experienced grade 4 neutropenia for twice. Febrile neutropenia with grade 1 fever (39.9
) was noted in 1 dog from the 15-week group on the third day after administration of 1st vincristine. The blood examination on the same day revealed grade 2 anemia (PCV=24.9%), grade 1 neutropenia (neutrophil=1581/µL) with decreased segmentation and mild toxic reaction, and grade 2 thrombocytopenia (platelet=84000/µL, by manual calculation). This dog recovered after administration of oral antibiotics (i.e. Augmentin).
There was neither significance in the occurrence of neutropenia, nor occurrence of grade 4 neutropenia between two groups. (P=0.869 and 0.320, respectively). For
thrombocytopenia, only 5 (21.4%) dogs in the 25-week group and 5 (22.7%) dogs in the 15-week group experienced the toxicity. Grade 1 thrombocytopenia predominated for both groups. Toxicity other than grade 1 was only noted in a minority of 23.5% of episodes from the 25-week group being grade 2. No significance was noted in the occurrence of thrombocytopenia between two groups (P=0.905). Generally, the frequency and severity of neutropenia were more profound than those of thrombocytopenia in both groups.
For AEs of gastrointestinal in nature, more than half of dogs in both groups experienced anorexia and vomiting. Grade 1 and grade 2 toxicity were in majority, with grade 2 anorexia and grade 1 vomiting being the most frequently presented situations in both two groups. Grade 4 toxicity was only noted once in 1 dog of the 25-week group with anorexia. No significance was noted in the occurrence of anorexia, vomiting and diarrhea between two groups (P=0.299, 0.749 and 0.487, respectively).
Two dogs from the 25-week group and 1 dog from 15-week group developed sterile hemorrhagic cystitis after first, second and third administration of cyclophosphamide, respectively. Cyclophosphamide was subsequently substituted by chlorambucil in the following cycles for these dogs.
One dog from the 15-week group developed dilated cardiomyopathy speculated to be resulted from cumulative cardiotoxicity of doxorubicin. This was a 5-year old Corgi.
Panting and exercise intolerance had been noted for 1 month after last dose of doxorubicin injection, with the cumulative dose of doxorubicin being 180 mg/m2. Deterioration of respiratory signs and presence of pleural effusion and ascites were noted at second month after last dose. Echocardiography revealed dilated
Three days after discharge, this dog eventually died at home (one week after diagnosis of cardiomyopathy). Re-evaluation of echocardiography was thus unavailable.
Overall 20 dose reductions were observed in 13 (31%) dogs of the 25-week group and 15 dose reductions in 9 (40.9%) dogs from the 15-week group. The most common cause for dose reduction was neutropenia, accounting for 16 (80%) of episodes from the 25-week group and 8 (53.3%) episodes from the 15-week group. Other miscellaneous causes included gastrointestinal toxicity (n=6), elevated liver enzyme (n=3). There was no significant difference in the rate of dogs requiring dose reduction between two groups (P=0.326).
A total 62 episodes of dose delay from 29 (69%) dogs in the 25-week group and 30 episodes in 15 (68.2%) dogs from the 15-week group were noted. Neutropenia still remained the major cause for dose delay, accounting for 43 (69.4%) times of delay in the 25-week group and 19 (63.3%) times of delay in the 15-week group.
Gastrointestinal toxicity resulted in 7 (11.3%) times of delay from the 25-week group and 4 (13.3%) times of delay from the 15-week group. Elevated liver indexes were associated with 3 (4.8%) episodes and 5 (16.7%) episodes from the 25 and 15-week group respectively. Other miscellaneous causes were such as leukocytosis, foreign body digestion before visit or owner’s request. There was no significant difference in the rate of dogs requiring dose delay between two groups (P=0.385).
Other than the dose reductions meant for ATLS prevention at treatment initiation, 11 (26.2%) dogs from the 25-week group and 6 (27.3%) dogs from the 15-week group required neither dose reduction nor delay during treatment.
4.5 Prognostic factors
4.5.1 Prognostic factors analysis for CR
The following factors were analyzed to see if they played a role in attaining CR or not: age (< or ≥ median age), body weight (< or ≥ median body weight), breed (Golden retriever or other breeds), sex (spayed female, intact female, castrated male or intact male), stage (< 5 or 5), substage (a or b), immunophenotype (B-cell, T-cell or null), administration of steroid before chemotherapy, presence of thoracic involvement in imaging, characteristics of thoracic imaging (infiltration or swollen lymph node), presence of laboratory abnormalities present at diagnosis (including anemia, thrombocytopenia, lymphocytosis and leukocytosis), baseline globulin (< or ≥ median value of globulin), baseline neutrophil (< or ≥ median value of neutrophil), presence and severity of AEs during treatment (including neutropenia, thrombocytopenia, anorexia, vomiting and diarrhea), need for dose delay, number of dose delay (< or ≥ 2 delays), need for dose reduction, number of dose reductions (< or ≥ 2 reductions), the use of L-asparaginase in protocol, dose reduction for ATLS prevention at treatment initiation, and the use of mitoxantrone in protocol. Hypercalcemia is not evaluated because only one dog has hypercalcemia among the whole population.
In univariate analysis of the above factors, body weight, breed and thoracic involvement in imaging were showed result in statistically significance in attaining CR or not during treatment. All dogs with body weight lower than median value (i.e. 16.15 kg) achieved CR; however, only 75% of dogs with body weight higher than median value attained CR (P=0.002). For dogs who were not Golden retrievers, the rate of CR
dogs with an abnormality in thoracic imaging (P=0.018). Interestingly, no significance was noted between different characteristics of thoracic imaging (85.7% rate of CR for infiltration versus 73.7% for swelling of lymph nodes, P=0.417).
Relatively higher rate of CR was shown in dogs presented with neutropenia during the treatment (94.1% versus 80.0%) and dogs encountering more than 2 times of dose delay (96.4% versus 80.6%), but both were not significant enough (P=0.088 and 0.057, relatively). The result of analysis was summarized in Table 10-12.
No statistical significance was found between dogs ever receiving mitoxantrone during the treatment and those who did not (P=0.517). Particularly in 25-week group, either the use of CHOP or CMOP resulted no significant difference in achieving CR (P=0.780).
4.5.2 Prognostic factors analysis for TTP
Factors mentioned previously in univariate analysis for attaining CR or not, along with response to treatment, were analyzed for TTP in all patients as well. Body weight, breed and response to treatment were factors shown to have significant influence on TTP (Table 13-15). Dogs with body weight higher than median value had significantly shorter median TTP than those whose body weight lower than median value (231 days versus 209 days, P=0.010). Golden retrievers had median TTP of 126 days which was significantly shorter than that of 244 days in other breeds (P=0.024). In dogs able to attain CR during the treatment, median TTP was 244 days and significantly longer than 56 days from those failed to achieve CR (P=0.000). Dogs with substage a also had relatively longer median TTP than those with substage b, but significance was not reached (273 days versus 209 days, P=0.069, Figure 6). The Kaplan-Meier curves for the above factors with significance were shown in Figure 3 to 5.
In multivariate analysis for factors with significance (P<0.05) in TTP, only response to treatment remained statistically significant. Hazards ratio for dogs without CR and those who achieving CR was 3.560 (95% confidence interval, 1.433-8.842, P=0.006). Both body weight and breed were not significant (P=0.176 and 0.999,
respectively) in multivariate analysis. Results of multivariate analysis with hazards ratio (HR) and 95% confidence interval (95% CI) of factors for TTP were summarized in Table 16.
No significance was found between dogs ever receiving mitoxantrone during the treatment and those who didn’t (242 days versus 231 days, P=0.209). In the 25-week group, the median TTP between subgroup of CHOP and CMOP was revealed no statistically significant (242 days versus 273 days, P=0.274).
4.5.3 Prognostic factors analysis for OST
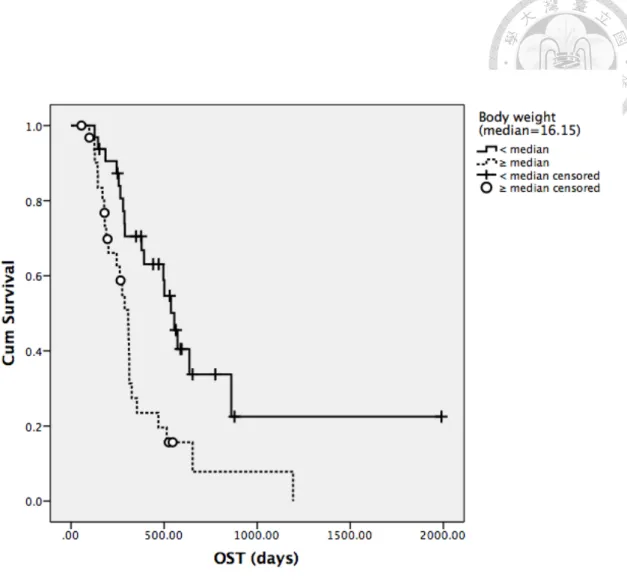
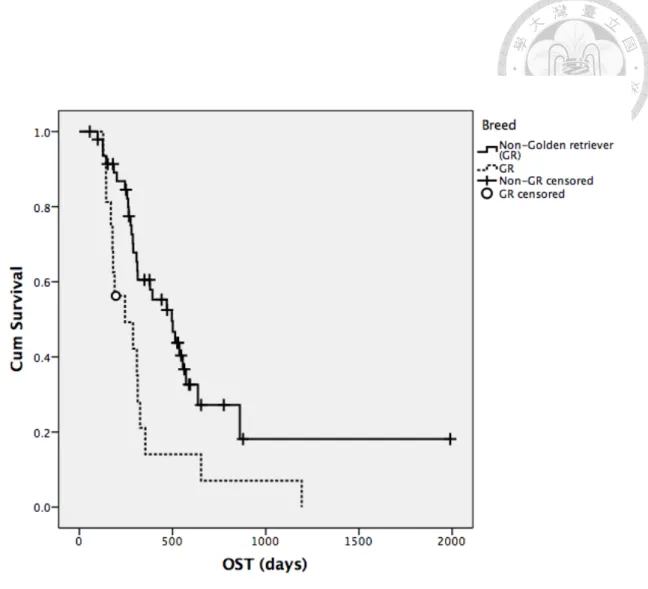
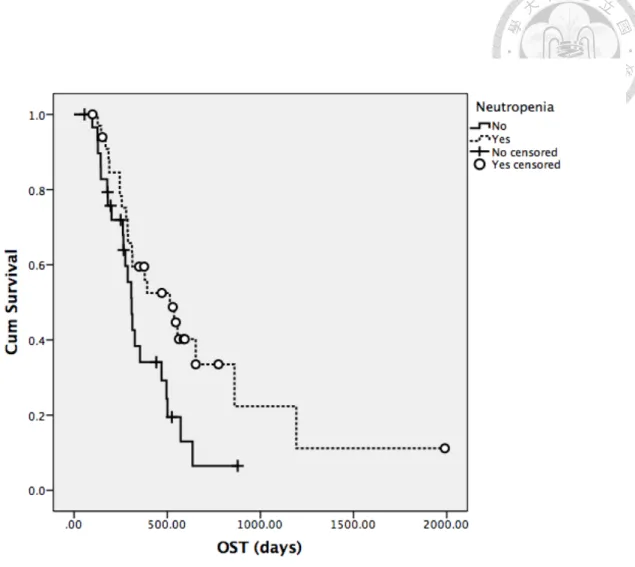
Factors mentioned previously in univariate analysis for TTP were analyzed for OST in all patients (Table 17-19). Body weight, breed, presence of neutropenia during treatment and response to treatment were significantly associated with median OST.
With similar findings as in TTP, dogs with body weight higher than median value and Golden retrievers both had significantly shorter median OST, with 307 days versus 555 days (P=0.002) for the former subgroup and 245 days versus 496 days for the latter subgroup (P=0.007). Response to treatment remained significant for median OST as well, with 392 days in dogs attaining CR and 189 days in dogs without CR. In the aspect of adverse events from treatment, dogs ever experiencing neutropenia had significantly higher median OST (514 days versus 309 days, P=0.029). The
However, no factors with significance (P ≤ 0.05) from univariate analysis remained significant in multivariate analysis for median OST. Results of multivariate analysis with hazards ratio (HR) and 95% confidence interval (95% CI) of factors for OST were summarized in Table 20.
For dogs ever receiving mitoxantrone during the treatment and those who didn’t, median OST was not statistically significant between two subgroups (313 days versus 379 days, P=0.136). In 25-week group, the median OST for subgroup of CHOP and CMOP was 313 days and 379 days, respectively (P=0.176), without significance found.
4.5.4 Prognostic factors analysis for large-sized population and non-GR population
According to the results above, we further recognized the dogs with body weight over 20 kg from the other relatively small-sized dogs. In this subgroup of large-sized population, the rate of complete remission, TTP and OST were compared between Golden retrievers and other large-sized non-GR dogs. Detailed distribution for body weight was listed in Table 21. Although the rate of CR, median TTP and median OST were all numerically lower in GR than in large-breed non-GR dogs, statistical significances were not observed for either endpoint between two groups (Table 22).
Again, we eliminated Golden retrievers and recognized the subgroup of non-GR population. In this subgroup, the comparisons were focused between dogs < 20 kg and dogs ≥ 20 kg. No significant difference was noted in median TTP and OST (P=0.171 and 0.100, respectively) between two groups. However, the rate of complete remission was significantly higher in dogs < 20 kg than dogs ≥ 20 kg (100% vs. 81.8%, P=0.049) (Table 22).
Chapter 5 Discussion
5.1 Outcomes of different protocols
For canine multicentric lymphoma, chemotherapy remains a primary treatment which has been by far the most effective way to achieve the best response and prolong the survival time to the most in these patients. Reported response rate generally ranges from 89% to 100% [1, 27, 30, 49, 52, 53]. Different protocols had been proposed, and the efficacy of a protocol sometimes could be only assessed by comparison with that from historical or published data due to lack of a control group in the same study. To the author’s knowledge, this should be the first study focusing on the comparison of the two widely used protocols for canine multicentric lymphoma, namely the modified 25-week UW-Madison protocol and the 15-week maintenance-free CHOP protocol, in the same study. In the present study, 42 dogs were given the 25-week protocol and 22 dogs were administered the 15-week protocol. The objective response rate of 15-week group (100%) was not significantly different from that of 25-week group (98.4%), and both were comparable to previous data of response rate in chemotherapy reported for canine multicentric lymphoma. This finding may more likely reflect and be related to the chemosensitive nature of lymphoma as long as chemotherapeutic agent, especially a multiagent protocol, is administered. In the research by Hosoya et al. [52], the substitution of doxorubicin with cytosine arabinoside in protocol did result in a significantly shorter median duration of first remission (94 days versus 174 days, P
<0.01), but such change actually didn’t influence the initial response (92% versus 100%). Modification of protocol in a multi-agent chemotherapy setting for canine
One of the primary intentions of this study is to see whether a more dose-intense and shorter protocol contributes to a comparable or even better outcome, through the setting of two-armed comparison. The 15-week protocol used in this study was first investigated in the study by Curran and Thamm [1]. In that study, the objective response rate was 98%, the median progression-free survival (PFS) was 176 days and the median OST was 311 days. A relatively short PFS was noted. Possible explanation provided by the authors was the fact that 50% of the patient population was substage b, hypercalcemia or stage V at the time of diagnosis, possibly indicating a population with poorer prognosis. Interestingly, there was also 50% of population in the present study met at least one of the criteria mentioned above possibly associated with a poor prognosis. However, the TTP and OST from the 15-week group (217 days and 326 days, respectively) here were longer than the data by Curran and Thamm above, and both were comparable to those from our 25-week group. Even so, it should be interpreted with caution when the comparison was made between studies which may be possibly highly biased due to different backgrounds and numbers of populations, difference in standard of diagnostic, staging and treatment procedures between institutions and the methods of response assessment. Overall, the outcome of a more dose-intense and shorter protocol was acceptable and comparable according to the data presented here.
The median TTP was 217 days for the 15-week group and 242 days for the 25-week group (P=0.503), without significance noted. However, among the censored individuals, dogs that hadn’t reached progressive disease by the closure of this study were more frequently presented in the 15-week group with significance (4.8% in the 25-week group versus 22.7% in the 15-week group, P=0.029), although the distribution of all the censored data for TTP among two groups was not significantly different (P=0.08). This finding indicates that there is still possibility that the median TTP from
the 15-week group potentially may be longer than the data currently available.
Nevertheless, if that’s the case, whether significance in TTP between two groups will exist is still not known. The same situation was noted in the analysis of median OST as well. For OST, all the censored data and those who were still alive and thus censored were both significantly more presented in the 15-week group (for the former 23.8% in the 25-week group versus 50% in the 15-week group, P=0.034; for the latter 7.9% in the 25-week group versus 40.9% in the 15-week group, P=0.002). Although the numbers of the median OST in both group (354 days for the 25-week group and 326 days for the 15-week group) were comparable to the previous published data, ranging from 10 to 12 months [3] and no significance was noted between two groups (P=0.999), the result may be different if longer follow-up duration for the same population in the 15-week group was allowed in the future.
Particularly in the 25-week group, the administration of CHOP or CMOP didn’t result in significant differences in the response rate, median TTP (242 days versus 273 days) and OST (313 days versus 379 days). Similar findings were noted in the investigation by Wang et al. [49] as well, where CHOP or CMOP were given in each 22 dogs, resulting in median TTP of 222 days and 162 days (P=0.75) and median OST of 318 days and 242 days (P=0.63) , respectively.
5.2 Adverse events
In the present study, most of the adverse events were low-grade toxicities and generally well-tolerated, similar to previous findings. Although the incidence of adverse events among different studies were variable and couldn’t be actually compared due to