E LS EV I ER
Journal of Chromatography A, 779 (1997) 195-203JOURNAL OF
CHROMATOGRAPHY A
Analysis of gangliosides by capillary zone electrophoresis and
capillary zone electrophoresis-electrospray mass spectrometry
Dar-Der Ju, Chien-Chen Lai, Guor-Rong
Her*
Department of Chemistry., National Taiwan University, Taipei, Taiwan
Received 12 December 1996; revised 7 April 1997; accepted 8 April 1997
Abstract
Gangliosides, sialic acid(s)-containing glycosphingolipids, were separated by capillary zone electrophoresis and detected with either UV or electrospray mass spectrometry. Several electrolyte systems were evaluated for the separation of underivatized gangliosides. The best result was obtained by using 50 mM borate and 50 mM phosphate buffer containing 20 mM cx-cyclodextrin at pH 9.9. The four major ganglioside forms (GM1, GDla, GDlb, GTlb) were successfully separated, and, moreover, each ganglioside yielded two peaks, splitting by the difference in chain length of the ceramide moiety. The resolution obtained in CE-UV could not be reproduced in CE-MS because of the incompatibility of the borate/phosphate buffer to ESI-MS. With the use of more volatile buffers, such as ammonium acetate or 2-[N-cyclohexylamino]-ethanesul- fonic acid, baseline resolution was obtained for gangliosides having different number of sugars, but the two dis- ialoganglioside isomers, GDla and GDlb, were coeluted. © 1997 Elsevier Science B.V.
Keywords: Buffer composition; Detection, electrophoresis; Gangliosides; Glycosphingolipids; Lipids
1. I n t r o d u c t i o n
Growing interest in the numerous and important biological functions of gangliosides [1,2] has en- gendered the need for capable separation techniques for their isolation and identification. Chromatography in its various forms has been widely used in the isolation of gangliosides [3-7]. Analysis of gan- gliosides in biological samples has been most often carried out by high-performance liquid chromatog- raphy and thin-layer chromatography. Recently, mainly because of its great potential for high sepa- ration efficiency, high-performance capillary electro-
*Corresponding author.
phoresis has been explored in the separation of underivatized gangliosides using low-wavelength UV detection [8,9] or derivatized ganglioside with chro- mophore tags [10].
Gangliosides are amphoteric conjugates of a cer- amide and a sialoglycan. The ceramide consists of a long chain base (sphingoids) and a fatty acid. It is known that many gangliosides have micro hetero- geneity in the ceramide part of the molecule. Yoo et al. [9] demonstrated the separation of gangliosides with different glycan structures but were unable to resolve gangliosides having the same glycans but different ceramides. Supercritical fluid chromatog- raphy (SFC) has shown its capability for the sepa- ration of gangliosides having the same glycans but
0021-9673/97/$17.00 © 1997 Elsevier Science B.V. All rights reserved
196 D.-D. Ju et al. / J. Chromatogr. A 779 (1997) 195-203
different ceramides [11,12]. However, unlike tile CE results [9], SFC lacks the capability to separate the disialoganglioside isomers, G D l a and GDlb. One objective of this study is the search for CE con- ditions capable of resolving not only gangliosides having different glycans but also gangliosides having the same glycans but different ceramides.
The primary detection method for CE is UV absorption [13-15]. As with most of the carbohy- drate species, one difficulty encountered in the analysis of gangliosides is the lack of chromophores in their molecules. This inherent property hinders high-sensitivity detection since UV absorption at wavelengths higher than 200 nm is relatively poor. There are two more problems associated with the analysis of gangliosides by CE-UV. Firstly, while the migration times are often used for compound identification in CE-UV, this technique is useful only if the analytes have been identified before and pure standards are available. Secondly, the migration times of CE are not as reproducible as in GC and HPLC and thus faces a greater risk in the identifica- tion of analytes based only on migration time.
Because of its low detection limit, high specificity, and, more importantly, abundant structural infor- mation, mass spectrometry (MS) has been consid- ered as one of the ideal devices for chromatographic detection. The merits of using MS as the chromato- graphic detector are best demonstrated with the highly successful G C - M S . The coupling of HPLC and CE with MS used to be a much more difficult task than the interfacing of GC with MS: however, recent developments in MS such as electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI) make the coupling of HPLC and CE with MS much easier [16-20]. In this paper, we described the merits and problems of on-line cou- pling capillary zone electrophoresis (CZE) with ESI- MS in the analysis of gangliosides.
2. Experimental
2. I. Chemicals
All chemicals were of analytical-reagent grade unless stated otherwise. Ganglioside standards, G M I , GDla, G D I b , G T l b and type III gangliosides
(purified from bovine brain containing approximately 20% N-acetylneuraminic acid) were all purchased from Sigma (St. Louis, MO, USA). The structures of these gangliosides are listed in Scheme 1.
Approximately 1 Ixg/ml ganglioside solutions were used in CE and C E - M S analysis. These solutions were prepared by dissolving gangliosides in running buffers, c¢-CD, [3-CD and ~/-CD were ob- tained from Nacalai Tesque (Japan). Sulfated [3-CD and hydroxyl-[3-CD were obtained from Aldrich (USA). 2-[N-cyclohexylamino]ethanesulfonic acid (CHES) and 3-[N-cyclohexylamino]-l-propanesul- fonic acid (CAPS) were purchased from Sigma. Disodium hydrogenphosphate and sodium tetraborate anhydrous were obtained from Janssen (Belgium). Ammonium acetate and 2-propanol of HPLC grade were purchased from J.T. Baker (Phillipsburg, N J, USA). Deionized (18 Mf~) water (Milli-Q water system, Millipore, Bedford, MA, USA) was used in the preparation of the samples and buffer solution.
2.2. Capillary. electrophoresis
Two CZE systems were used. C Z E - U V was
G M I
G D l a
Gall31 ~ 3 G a l N A c 131 ~ 4Gal]~ I ~ 4 G l c 1 ~ 1Cer 3
0c2 N e u N A c
G a l ~ 1 ~ 3GalNAc[51 ~ 4Gal[5 ! ~ 4 G l c 1 ~ 1Cer
3 3
t
o~2 o~2 N e u N A c N e u N A c G D I b G T I bGall31 ~ 3 G a l N A c [51 ~ 4G al[51 ~ 4 G l c 1 ~ 1Cer 3
c~2 NeuNAco~2 ~ 8 N e u N A c
Gal[51 ~ 3GalNAc131 ~ 4Gal[51 ~ 4 G l c 1 ~ 1Cer
3 3
t
c~2 0c2
N e u N A c N e u N Ac 0t2 - ' " " 8 N e u N A c S c h e m e 1. S t r u c t u r e s o f t h e g a n g l i o s i d e s .
D.-D. Ju et al. / J. Chromatogr. A 779 (1997) 195-203 197
conducted on a BioFocus 2000 capillary electro- phoresis system (Bio-Rad, Hercules, CA, USA). A custom-made CE system, using a CZE 1000R high- voltage power supply (Spellman, Plainview, NY. USA) was used to couple with MS. CE columns were fused-silica capillaries (Polymicro Tech- nologies, Phoenix, AZ, USA) 100 cm (94 cm to detector)×50 txm I.D.×375 txm O.D. A small area of the polyimide coating was burned off to form a window for UV detection. The detector (UV-C, Rainin, Emeryville, CA, USA) wavelength was set at 200 nm. Before use, the capillary column was washed with 1 M NaOH (4 min) followed by 0.1 M NaOH (4 min), water (4 min) and running buffer (5 rain). The washing buffers were introduced by pressure injection (100 psi helium; 1 psi=6894.76 Pa). The capillary was then equilibrated with the running buffer under + 2 0 kV for 20 min.
2.3. Mass spectromet~
A VG Platform single quadrupole mass spectrome- ter (Fisons Instruments/VG Bio Tech, Altrincham, UK) equipped with a CE interface was used for this study. The interface utilizes a triaxial flow arrange- ment whereby the CZE eluent is mixed with a suitable make-up solution (sheath liquid) at the probe tip and then nebulized using N 2 gas. In the negative ion mode, a potential about - 3 . 5 kV was applied to the probe tip. When the high voltage used for the CZE separation was maintained at + 2 0 kV, the overall potential across the separation capillary was about 23.5 kV. A syringe pump (Model H-74900-00, Cole-Parmer, Niles, IL, USA) was used to deliver the sheath flow at a flow-rate of 10 jxl/min. Nitrogen gas at a flow-rate of 0.5 1/min was used as the nebuliz- ing gas. The warm (80°C) bath gas (nitrogen) at a flow-rate of 1.6-2.5 l/rain aided desolvation of the electrospray droplets. Mass spectral data were col- lected at selected ion monitoring mode (0.2 s dwell time, 0.2 mass unit span).
3. Results and discussion
3.1. CE-UV analysis of gangliosides
A ganglioside molecule has an anion hydrophilic
sialooligosaccharide chain and a hydrophobic cer- amide moiety. In aqueous media, gangliosides have been shown to form stable micelles. This phenom- enon hinders the efficient separation of gangliosides as monomeric species in neat aqueous media. There- fore, an organic solvent or an additive capable of breaking the micelles is needed.
Cyclodextrins (CDs), neutral oligomers of D-(+)- glucopyranose, have been used as buffer additives to obtain better resolution [9]. These compounds are capable of forming inclusion complexes with many molecules. The formation of an inclusion complex is determined by the hydrophobicity and size of the solute, The inner diameters of the cavities of ~-, [3- and y-CDs are different. Perhaps the size of the cavity of c~-CD provides the best fit for the lipid moiety of gangliosides; addition of cx-CD to the running electrolyte has been proved to be capable of breaking-up the aggregation of amphiphilic gan- gliosides and allowed for their efficient separation as monomers in aqueous media.
Gangliosides are known to possess heterogeneity in their glycan as well as in their ceramide portion. As mentioned earlier, the reported results [9] showed the capability of resolving gangliosides with different glycan but not with different ceramide moieties. One goal of this study is to search for CE conditions which enable the differentiation of gangliosides with the same glycan but with different ceramide moi- eties. Several electrophoretic conditions including CHES, CAPS, borate, borate-phosphate, phosphate and ammonium acetate have been studied with and without the addition of different CDs. The results suggested that CDs were critical for the break-up of the micelles. Among the five CDs studied, only ~-CD provided the separation of di- and trisialogan- gliosides. As mentioned earlier, the size of the cavity of ~x-CD probably provides the best fit for the lipid moiety of gangliosides. Borate was found to be important for the separation of the disialoganglioside isomers, G D l a and GDlb. The separation could be due to the complexation between borate and sugars. The best electropherogram, as shown in Fig. 1, was obtained by using 50 mM borate and 50 mM phosphate buffer containing 20 mM e~-CD at pH 9.9. In comparison with the published results [9], two peaks rather than one were detected for each gan- glioside. According to our previous mass spectromet-
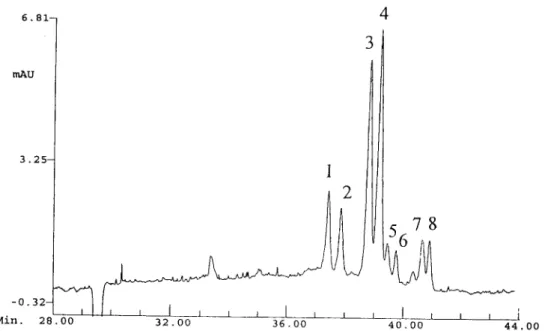
198 D.-D. Ju et al. / J. Chromatogr. A 779 (1997) 1 9 5 - 2 0 3 6 . 8 1 - m A U 3 . 2 5 -
!
- 0 . 3 2 - I M i n . 28 00 3 2 . 0 0 3 6 . 0 04
3
J t I ~ i 4 0 . O0 4 4 . O0Fig. 1. Electropherogram of type III ganglioside mixture. Conditions: 20 kV, 5 psi pressure injection, UV detection at 200 nm. Running buffer: 50 mM borate, 50 mM phosphate, 20 mM ct-CD, pH 9.9. Peaks: 1,2=GM1; 3,4=GDIa; 5,6=GDlb: 7,8=GTIb. The minor peak in front of the peak 7 was not identified.
ric study [21], there are two different long chain bases sphingosine (C, 8 ) and eicosasphingosine (C 20 ) in the ganglioside molecule. The observation of two peaks for each ganglioside suggested that gan- gliosides having the same glycan but different cer- amide moieties might have been separated. The separation could be due to the small difference in mobility resulting from the difference in molecular mass (different chain length). Because of the lack of ganglioside standards with single ceramide, co-in- jection, the technique used for the assignment of ganglioside peaks in the C E - U V study, was not applicable for the identification of the doublets. On line C E - M S would be an ideal technique for the identification of the doublets. Unfortunately, phos- phate and borate electrolytes were found to be not suitable for coupling CZE with MS. The ion current was not stable and white solid deposit was observed in the skimmer resulting in the gradual loss of sensitivity during a C E - M S run. These doublets were identified indirectly by comparison of the ESI mass spectra with C E - U V electropherograms. Gan- glioside standards were injected directly into the electrospray mass spectrometry and analyzed in the
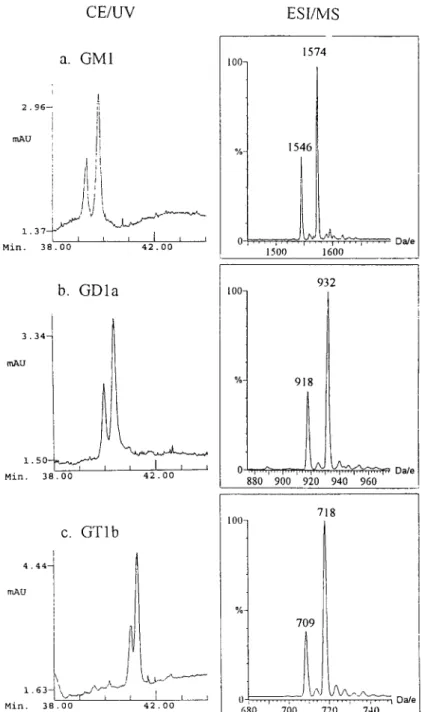
negative ion mode. The similarity between relative peak intensities in C E - U V and ion currents in MS, as shown in Fig. 2, suggested that the doublets were most likely the same ganglioside species with a long chain base of Ct8 and C20, respectively. A similar phenomenon was found for type III gangliosides. As shown in the electropherogram (Fig. 1) and ESI mass spectrum (Fig. 3), there was a good agreement for GMI and GDla. (since G D l b is a minor com- ponent, the ion current of the double charged ions at
m/z
918, 932 originated mainly from GDIa.) There was some discrepancy in GTlb. The reason for the discrepancy is not known.3.2. CE-MS analysis of gangliosides
CE is a modem technique known for its superior separation efficiency per unit time. The much smaller loading capacity and higher separation efficiency makes fraction collection followed by off-line analy- sis a much more difficult task than in HPLC meth- ods. This engendered the need of on-line coupling CE with MS.
D.-D. Ju et al. I J. Chromatogr. A 779 (1997) 195-203 199 CE/UV ESI/MS a. G M 1 i 2.96-- m A U 1. 37- ~ f j I Min, 3 8 . 0 0 i I I i I 4 2 , 0 0 100 % 1574 1546 1500 1600 b. G D l a 3.34- mAU Min, 38.00 I { I I 42.00 10 % 932 918 0 ~ 7 1 I . . . I I 1 I ' 1 ' ' ' ' 1 ' ' ' ' 1 ' ' ' ~ ' ' Dale 880 900 920 940 960 c. GTlb 4.44- rnAU 1.63- Min. 38.00 L.Lt / v ~ - - - ~ - I I 42 . 00 718 1001 709 06~0 ... ' 7 ~ ' 7~0 ''' ; 7~0 ~' Dale
Fig. 2. C E - U V and negative ESI-MS mass spectrum of (a) GM1, (b) G D l a , (c) GTlb. Two ions with 28 amu interval, indicating the difference in chain length (sphingosine and eicosasphingosine), were observed for each ganglioside. The ions at m/z 1546/1574, 918/932, 709/718 were the singly, doubly and triply charged negative molecular ions of GM1, GDIb and GTlb, respectively.
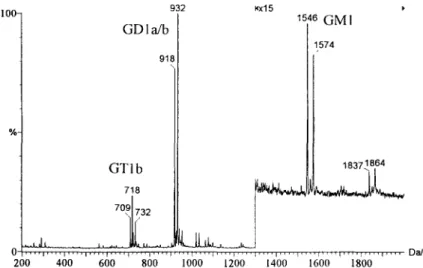
200 D.-D. Ju et al, / J. Chromatogr. A 779 (1997) 195-203 10ft %. 932 G D 1 a/b 918 \ G T I b 718 ~x15 1546 G [ V [ I 1574 18371864 0 ' ' ' 1 . . . . I . . . . I . . . . r . . . . i . . . . i . . . . p . . . . [ . . . . I . . . . ~ . . . . t . . . . I . . . . I . . . . l . . . . I . . . . r . . . . r . . . . D a / e 200 400 600 800 1000 1200 1400 1600 1800
Fig. 3. Negative ESI-MS mass spectrum of type III gangliosides mixture. In addition to the ions explained in Fig. 2, the ions at m/z 1837. 1864 were the singly charged molecular ions of GDla/b.
( f l o w F A B ) and E S I h a v e b e e n s u c c e s s f u l l y c o u p l e d with C E [ 2 2 - 2 4 ] . In c o m p a r i s o n w i t h F A B , E S I has the a d v a n t a g e o f o p e r a t i n g at a t m o s p h e r i c pressure so that h y d r o d y n a m i c flow due to the v a c u u m o f M S d o e s n o t occur. T h e r e f o r e , the c h r o m a t o g r a p h i c r e s o l u t i o n can be p r e s e r v e d .
As m e n t i o n e d earlier, a l t h o u g h b o r a t e - p h o s p h a t e b u f f e r p r o v i d e d e x c e l l e n t separation p o w e r , this
b u f f e r s y s t e m was not suitable for C E c o u p l e d with E S I - M S . In order to r e d u c e the influence o f the buffer, less c o n c e n t r a t e d buffers w e r e tested. Un- fortunately, it was f o u n d that the separation ef- ficiency d r o p p e d significantly w h e n the b u f f e r con- centration d e c r e a s e d to less than 15 m M .
M o r e v o l a t i l e buffers such as C H E S , C A P S and a m m o n i u m acetate h a v e b e e n s h o w n to be c o m p a t - 1 2 . 0 0 - m A U 4 . 6 7 - - 2 . 6 6 -
i
M i n . 1 4 0 0 I J _ _ [ _ _ _ . 1 8 . 0 0GD 1 a/b
GM1
I I I I , 2 . 0 0G T l b
I q I I I 2 6 . 0 0 3 0 . 0 0Fig. 4. Electropherogram of type III gangliosides mixture. Conditions: 20 kV, 5 psi pressure injection, UV detection at 200 nm. Running buffer: 50 mM ammonium acetate, 20 mM a-CD, pH 7.5.
D.-D. Ju et al. I J. Chromatogr. A 779 (1997) 195-203 201
ible with ESI-MS [25]. However, the separation
powers of these systems were not as good as that of
the phosphate-borate system as shown in Fig. 4. The
two disialoganglioside isomers (GDla and GDlb)
were coeluted and only partial separation was found
for gangliosides with different ceramide structure.
For optimal sensitivity and also for avoiding the
interference of background ions, the mass spectrome-
ter was operated in the selected ion monitoring
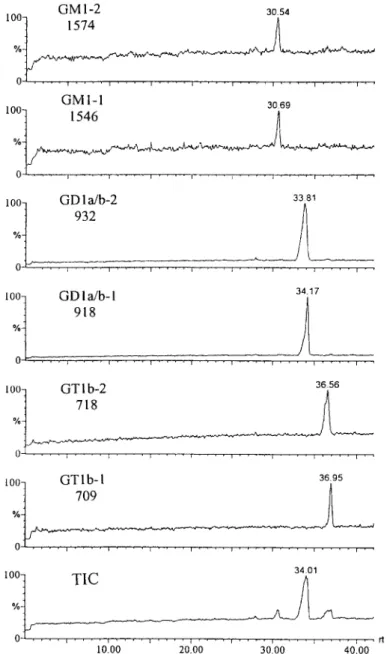
(SIM) mode. The mass electropherogram of type III
gangliosides using ammonium acetate as the running
buffer is shown in Fig. 5. The two molecular ions in
each ganglioside were selected for real-time moni-
toring. A small difference in migration time was
GM 1-2
30.54
GM
1 - 1306g
'°° 1
1546
A
%100
GDla/b-2
3381,/o]~
932
_
~
L 0 0 q G D l a / b - I 34.17°i_~
- - - - ; , ,2
36.561001
aTlb- l
36;95 0 I . . . . ~ . . . . ~ . . . t , , , ~ . . . . J . . . . i . . . . f1oo]
TIC
0 - 1 . . . . t . . . . i . . . . I . . . . i . . . . 10.00 20.00 3 4 0 1 i . . . . i . . . . ~ . . . . i r t 30.00 40.00Fig. 5. CE-MS mass electropherogram of type III gangliosides. Running buffer: 50 mM ammonium acetate, 20 mM (x-CD, pH 7.5. The migration times were different from those in Fig. 4 because the mass electropherogram was acquired 5 min after starting electrophoresis.
202 D.-D. Ju et al. / J. Chromatogr. A 779 (1997) 1 9 5 - 2 0 3 observed for gangliosides having different ceramide
moieties (Fig. 5). It was surprising to note that, unlike with the phosphate-borate buffer, the heavier one eluted a little bit earlier than the lighter one. It has been shown that CHES and CAPS are compat- ible with negative ESI-MS [25]; the mass elec- tropherograms obtained with CHES and CAPS were very similar to those obtained with a m m o n i u m acetate (data not shown).
Like borate-phosphate, o~-CD is also a nonvolatile compound. The intensity of the ion signals decreased when (x-CD was added to the buffer. The effect of et-CD on the C E - M S signal, however, was not as serious as that of the phosphate-borate buffer.
Make-up solution (sheath liquid) was reported critical to the performance of the C Z E - E S I interface [22]. Sheath liquids of different composition have been tested for sensitivity and stability under nega- tive ESI conditions. The results showed that 2-pro- panol produced a much more stable signal than methanol and ethanol. Furthermore, the addition of a m m o n i a also improved the sensitivity. The best result was obtained with a solution of w a t e r - 2 - propanol (20:80) containing 0.5% ammonia.
One major function of the sheath liquid is to supplement the CZE flow to a level suitable for electrospray operation. With the use of a sheath liquid of w a t e r - 2 - p r o p a n o l (20:80) containing 0.5% ammonia, a flow of 8 - 1 2 m l / m i n was found to be best suited for ESI operation. The flow-rate of the sheath liquid was very high compared to the flow from the capillary. The high dilution degree might partially account for the compatibility of c~-CD with ESI-MS. One drawback of using a high flow sheath liquid is the deterioration of the signal intensity due to the decrease in analyte concentration.
4. Conclusion
Several buffer systems were evaluated for the separation of underivatized gangliosides. The best result was obtained by using 50 m M borate and 50 m M phosphate buffer containing 20 m M c~-CD at pH 9.9. Under this condition, the four major forms of ganglioside mixture were successfully separated (GM1, G D l a , G D I b , G T l b ) and each sample pro- duced two peaks, with splitting occuring according
to the difference in the long chain base of the ceramide moiety. This study showed that the best separation obtained in the C E - U V study could not be reproduced in C E - M S because of the incompatibility of the phosphate-borate buffer with ESI-MS. Further improvements in ESI-MS to accommodate the use of inorganic buffers are needed to obtain the full potential of CE.
Acknowledgments
This work was supported by the National Research Council of Taiwan.
References
[1] A. Handa, H. Hoshino, K. Nakajima, M. Adachi, K. Ikeda, K. Achiwa, T. Itoh, Y. Suzuki, Biochem. Biophys. Res. Commun. 175 (1991) 1.
[2] D.F. Emerich, T.J. Walsh, Brain Res. 527 (1990) 299. [3] D.F. Smith, B.V. Torres, Methods Enzymol. 179 (1989) 30. [4] S. Ladisch, B. Gillard, Methods Enzymol. 138 (1987) 300. [5] R. Kannagi, K. Watanabe, S. Hakomori, Methods Enzymol.
138 (1987) 3.
[6] H. Nakabayashi, M. Iwamori, Y. Nagai, J. Biochem. 96 (1984) 977.
[7] R.F. Menzeleev, Yu.M. Krasnopolsky, E.N. Zvonkova, V.I. Shvets, J. Chromatogr A 678 (1994) 183.
[8] Y. Liu, K.-F.J. Chan, Electrophoresis 12 (1991)402. [9] Y.S. Yoo, Y.S. Kim, J. Chromatogr A 652 (1993) 431. [10] Y. Mechref, G.K. Ostrander, Z.E. Rassi, J. Chromatogr A
695 (1995) 83.
[ 11 [ J. Kuei, G.R. Her, V.N. Reinhold, Anal. Biochem. 172 (1988) 228.
[ 12] V.N. Reinhold, D.M. Sheely, J. Kuei, G.R. Her, Anal. Chem. 60 (1988) 2719.
[13] F.E.P. Mikkers, F.M. Everaerts, Th.P.E.M. Verheggen, J. Chromatogr. 169 (1979) 11.
[14] S. Terabe, K. Otsuka, K. Ichikawa, A. Tsuchiya, T. Ando, Anal. Chem. 56 (1984) 111.
[15] K. Ganzler, K.S. Greve, A.S. Cohen, B.L. Karger, A. Guttman. N.C. Cooke, Anal. Chem. 64 (1992) 2665. [16] G. Hopfgartner, T. Wachs, K. Bean, J. Henion, Anal. Chem.
65 (1993) 439.
[17] S. Jun, U. Yoshihisa, K. Akihiro, K. Ikunoshin, Anal. Biochem. 207 (1992) 1.
[18] R.D. Smith, J.A. Olivares, N.T. Nguyen, H.R. Udseth, Anal. Chem. 60 (1988) 426.
[19] M.A. Moseley, J.W. Jorgenson, J. Shabanowitz, D.F. Hunt, K.B. Tomer, J. Am. Soc. Mass Spectrom. 3 (1992) 289.
D.-D. Ju et al. / J. Chromatogr. A 779 (1997) 195-203 203 [20] S. Pleasance, P. Thibault, J. Kelly, J. Chromatogr. 591
(1992) 325.
[21] D.D. Ju, G.J. Wei, G.R. Her, J. Am. Soc. Mass Spectrom. 5 (1994) 558.
[22] F. Foret, T.J. Thompson, P. Vouros, B.L. Karger, Anal. Chem. 66 (1994) 4450.
[23] M.A. Moseley, L.J. Deterding, K.B. Tomer, J.W. Jorgenson, Anal. Chem. 63 (1991) 109.
[24] M.A. Moseley, L.J. Deterding, K.B. Tomer, J.W. Jorgenson, J. Chromatogr. 516 (1990) 167.