Substitution Reactions of Aluminum Organoamide Complexes [Al(
µ-NR
2)
nCl
3-n]
2(R
)
Et,
iPr; n e 3) with LiNR′R′′
(R′
)
R′′
)
iPr, Et; R′
)
H, R′′
)
iPr): Crystal Structure of
[Al(
µ-NEt
2)(N
iPr
2)X]
2(X
)
Cl, H) and AlCl
3(N
i
PrH
2
)
2{Al(NH
3)(NH
2)[Al(N
iPrH)(N
iPr)Cl]
2}
2Chung-Cheng Chang,*
,†Mung-Dar Li,
†Michael Y. Chiang,
†Shie-Ming Peng,
‡Yu Wang,
‡and
Gene-Hsiang Lee
‡Department of Chemistry, National Sun Yat-Sen University, Kaohsiung, Taiwan, ROC, and
Department of Chemistry, National Taiwan University, Taipei, Taiwan, ROC
Recei
V
ed May 4, 1995
XAs part of our ongoing study of different substituents of Al
2N
2ring systems, the synthesis, structural characterization,
and spectroscopic studies on several amido derivatives of four-coordinated aluminum are described. The compounds
Al
2(NEt
2)
2(N
iPr
2)
2Cl
2, 1, Al
2((N
iPr
2)
4Cl
2, 2, Al((N
iPr
2)
3, 3, Al(NEt
2)
3, 4,
{
Al(N
iPrH)
3}
3, 5, Al
2(NEt
2)
2(N
iPr
2)
2H
2,
6, and AlCl
3(N
iPrH
2)
2{
Al(NH
3)(NH
2)[Al(N
iPrH)(N
iPr)Cl]
2}
2, 7, were synthesized by substitution reactions and
characterized by mass spectra, IR spectra, elemental analysis, and
1H,
13C, and
27Al NMR data. The structures
of three compounds,
V
iz., 1, 6, and 7, have been determined by single-crystal X-ray diffraction analysis. Crystal
data with Mo K
R
(1 and 7,
λ
)
0.710 73 Å) or Cu K
R
(6,
λ
)
1.541 78 Å) radiation: (1) Al
2N
4C
20H
48Cl
2, a
)
7.747(2) Å, b
)
9.648(2) Å, c
)
10.110(3) Å,
R
)
102.91(2)
°
,
β
)
83.54(2)
°
,
γ
)
110.19(2)
°
, triclinic, space
group P1
h
, Z
)
1, R
)
0.053 for 1789 (I
>
3
σ(I)) reflections; (6) Al
2N
4C
20H
50, a
)
15.088(2) Å, b
)
14.506(3)
Å, c
)
12.439(3) Å, orthorhombic, space group Cmca (No. 64), Z
)
4, R
)
0.045 for 701 (I
>
3
σ(I)) reflections;
(7) Al
7N
14C
30H
86Cl
7, a
)
29.914(9) Å, b
)
10.197(3) Å, c
)
20.525(5) Å,
β
)
90.99(4)
°
, monoclinic, space
group C2/c, Z
)
4, R
)
0.042 for 2472 (I
>
2
σ(I)) reflections.
Introduction
Recently compounds with group 13 and 15 bonds were shown
to be potential precursors for semiconductor systems.
1-12Noteworthy are the organoaluminum compounds, which are
precursors for aluminum nitride. Most amido or imido
deriva-tives of aluminum tends to form oligomers with strong metal
-nitrogen
σ bonds. In complexes involving imido ligands,
especially (imino)aluminum monomers, the lone pairs on
nitrogen may not be involved in
σ-bonding and may in principle
contribute to the partial
π-bonds between aluminum and nitrogen
centers. Power and co-workers have reported the Al
-
N bond
in compounds possessing a possible weak
π-interaction.
13Low
valency, unsaturated coordination, and fascinating bonding are
noticeably emphasized in Al
-
N chemistry recently.
14Our
previous studies focused mainly on the reactivity of Al
-
N and
Mg
-
C bonds.
15-18Herein we report the synthesis,
character-ization, and crystal structures of Al
2(NR
2)
2(NR
′
2)
2X
2(R
)
Et,
iPr; R
′
)
iPr, X
)
Cl, H) and a novel adduct AlCl
3(N
i-PrH
2)
2{
Al(NH
3)(NH
2)[Al(N
iPrH)(N
iPr)Cl]
2}
2. The adduct
con-tains unusually short Al
-
N bonds indicating a possible
π-in-teraction or strong ionic attraction in the Al
-
N moieties.
Experimental Section
Apparatus and Materials. All manipulations were carried out in
a N2-flushed glovebag, drybox, or vacuum system. Solvents were
distilled and degassed prior to use. Lithium amides were synthesized by following a reported procedure.14 Single-crystal X-ray diffraction
data were obtained using an Enraf Nonius CAD-4 diffractometer. All
1H,13C, and27Al NMR spectra were measured on a Varian VXR-300
spectrometer. Chemical shifts are referenced relative to either TMS (1H) or benzene-d
6(1H,δ 7.15;13C{1H},δ 128.00), while27Al NMR
spectra were referenced relative to Al(H2O)63+
. Mass spectral data were obtained on a VG-7025 GC/MS/MS spectrometer. IR spectra were recorded as Nujol mulls between KBr disks on a BIO-RAD FT-IR spectrometer. Elemental analyses (C, H, N) were performed at the Analytische Laboratorien, Lindlar, Germany. Deviations in the results of the elemental analyses from calculated values are attributed to the extremely air sensitive and hygroscopic nature of these compounds.
Synthesis of Al2(NR2)2(NR′2)2Cl2(R)Et, R′)
iPr, 1; R
)R′)
iPr, 2). Bis((µ-dialkylamido)dichloroaluminum), Al
2(NR2)2Cl415(R)
†National Sun Yat-Sen University. ‡National Taiwan University.
XAbstract published in AdVance ACS Abstracts, April 1, 1997. (1) Liao, B.; Li, Y.; Lu, Y. J. Mater. Chem. 1993, 3 (2), 117. (2) Lindsell, W. E. In ComprehensiVe Organometallic Chemistry;
Wilkin-son, G., Stone, F. G. A., Abel, E. W., Eds.; Pergamon, Oxford, U.K., 1982; Vol. 1, Chapter 6, and references cited therein.
(3) Laubengayer, A. W.; Smith, J. D.; Ehrlich, G. G. J. Am. Chem. Soc. 1961, 83, 542.
(4) Gosling, K.; Smith, J. D.; Wharmby, D. H. W. J. Chem. Soc. A 1969, 1738.
(5) Alford, K. J.; Gosling, K.; Smith, J. D. J. Chem. Soc., Dalton Trans. 1972, 2203.
(6) (a) Hitchcock, P. B.; Smith, J. D.; Thomas, K. M. J. Chem. Soc., Dalton
Trans. 1976, 1433. (b) Amirkhalili, S.; Hitchcock, P. B.; Smith, J.
D. J. Chem. Soc., Dalton Trans. 1979, 1206.
(7) Amirkhalili, S.; Hitchcock, P. B.; Jenkins, A. D.; Nyathi, J. Z.; Smith, J. D. J. Chem. Soc., Dalton Trans. 1981, 377.
(8) Atwood, J. L.; Stucky, G. D. J. Am. Chem. Soc. 1970, 92, 285. (9) McLaughlin, G. M.; Sim, G. A.; Smith, J. D. J. Chem. Soc., Dalton
Trans. 1972, 2197.
(10) Dozzi, G.; Cucinella, S.; Mazzei, A. J. Organomet. Chem. 1979, 164, 1.
(11) Piero, G. D.; Cesari, M.; Dozzi, G.; Mazzei, A. J. Organomet. Chem. 1977, 129, 281.
(12) (a) Sze, S. M. Physics of Semiconductor DeVices, 2nd ed.; Wiley: New York, 1981. (b) Rockeususs, W.; Roesky, H. W. AdV. Mater.
1993, 5, 443.
(13) Brothers, P. J.; Wehmschulte, R. J.; Olmstead, M. M.; Ruhlandt-senge, K.; Parkin, S. R.; Power, P. P. Organometallics 1994, 13, 2792. (14) (a) Collum, D. B. Acc. Chem. Res. 1993, 26, 227 and references cited
therein. (b) Lappert, W. F.; Power, P. P.; Sanger, A. R.; Srivastava, R. C. Metal and Metalloid Amides; Ellis-Horwood: Chichester, U.K., 1980; and references cited therein.
(15) Her, T. Y.; Chang, C. C.; Tsai, J. O.; Lai, Y. Y.; Liu, L. K.; Chang, H. C.; Chen, J. H. Polyhedron 1993, 12, 731.
(16) Her, T. Y.; Chang, C. C.; Liu, L. K. Inorg. Chem. 1992, 31, 2291. (17) Her, T. Y.; Chang, C. C.; Lee, G. H.; Peng, S. M.; Wang, Yu Inorg.
Chem. 1994, 33, 99.
(18) Chang, C. C.; Lee, W. H.; Her, T. Y.; Lee, G. H.; Peng, S. M.; Wang, Y. J. Chem. Soc., Dalton Trans. 1994, 315.
S0020-1669(95)00539-8 CCC: $14.00
© 1997 American Chemical Society
Downloaded by NATIONAL TAIWAN UNIV on August 14, 2009
Et, a; R)
iPr, b; 3.0 mmol; 1.02 g, a; 1.20 g, b), was added slowly to
an ether solution (100 cm3) of LiNR′′
2(R′′)Et, 0.48 g, 6.0 mmol; R′′ )
iPr, 0.65 g, 6.0 mmol) under nitrogen at
-30°C. The reaction
mixture was brought back to room temperature slowly, and after 1 h the solution was centrifuged to remove LiCl powder. Crude products of compound 1 and 2 were obtained after the removal of ether. Transparent colorless crystals of Al2(NR2)2(NR′2)2Cl2were obtained
by vacuum sublimation (compound 1) and recrystallization of the crude product from toluene (compound 2). Analytical data for 1 and 2 follows. Compound Al2(NEt2)2(NiPr2)2Cl2(1): Mp 96-97°C; yield
75%; IR (Nujol mull) 2980 (s), 2960 (s), 2910 (s), 2860 (s), 2810 (m), 1460 (m), 1370 (m), 1185 (br), 1055 (br), 690 (br) cm-1. Anal. Found: C, 51.3; H, 10.3; N, 11.8. Calcd: C, 51.2; H, 10.3; N, 11.9. Analysis by mass spectroscopy gave the fragments expressed as m/z (EI: 70 eV, relative intensity (%) and assignment in parentheses): 468 (9.3, [M]+), 453 (17.5, [M -CH3] +), 433 (9.1, [M -Cl] +), 390 (13.3, [M-(C3H7+Cl)] +), 368 (74.4, [M -N(C3H7)2] +), 334 (19.5, [MH -(Cl+N(C3H7)2)] +), 269 (19.5, [MH -2(N(C3H7)2)] +), 233 (26.4 [M-(Cl+2N(C3H7)2)] +), 219 (100, [M/2 -CH3] +), 303 (9.3, [M + Cl-N(C3H7)2] +). 1H NMR (C 6D6): δ 1.09 (t, 12H, CH3of NEt2, 3J H-H )6.6 Hz), 1.27 (d, 24H, CH3of N iPr 2,3JH-H )6.6 Hz), 2.94, 2.97, 3.37, 3.41 (quart, 8H, CH2of NEt2,3JH-H )6.6 Hz), 3.26 (sep, 4H, CH of NiPr 2,3JH-H )6.6 Hz). 13C NMR (C 6D6): δ 12.40 (CH3 of NEt2), 25.50 (CH3of NiPr2), 40.23 (CH2of NEt2), 46.69 (CH of NiPr 2). 27Al NMR (C6D6): δ 89 ppm (br). Compound Al2(NiPr2)4Cl2
(2): Mp (dec) 77°C; yield 40%; IR (in KBr pellets) 2965 (s), 2945 (s), 2890 (s), 1335 (m), 1230 (m), 1132 (w), 1063 (m), 817 (br), 641 (br) cm-1
. Anal. Found: C, 57.2; H, 11.0; N, 9.73; Cl, 11.9. Calcd: C, 54.8; H, 10.7; N, 10.6; Cl, 13.5. Analysis by mass spectroscopy gave the fragments expressed as m/z (EI: 70 eV, relative intensity (%) and assignment in parentheses): 524 (5.2, [M]+
), 509 (18.2, [M -CH3]+ ), 489 (13.3, [M-Cl] + ), 446 (12.1, [M-(C3H7+Cl)] + ), 424 (8.7, [M-N(C3H7)2] + ), 390 (12.7, [MH-(Cl+N(C3H7)2)] + ), 226 (32.6, [Al(N(C3H7)2-H] + ), 183 (32.6, [AlN(C3H7)2)-(C3H7+H)] + ), 126 (72.2, [AlNC3H7 - H] + ), 100 (100, [N(C3H7)2]+ ). 1H NMR (C6D6): δ 1.25 (d, 36H, CH3of NiPr2,3JH-H )6.6 Hz), 3.40 (sep, 4H, CH of NiPr 2,3JH-H )6.6 Hz). 13C NMR (C 6D6): δ 23.71 (CH3of NiPr 2), 45.31 (CH of NiPr2). 27Al NMR (C6D6): δ 112 ppm (br). Synthesis of Al(NR′R′′)3(R′)R′′) iPr, 3; R′ )R′′)Et, 4; R′ ) iPr, R′′ )H, 5). Bis((µ-dialkylamido)dichloroaluminum), Al2(NR2)2 -Cl415(R)Et, a;
iPr, b) (4.0 mmol; 1.36 g, a; 1.60 g, b), was added
dropwise to an ether solution (100 cm3) of lithium diisopropylamide
(1.71 g, 16 mmol) or lithium diethylamide (1.26 g; 16 mmol) under nitrogen at-30°C. The reactions took place when the temperature
was raised to room temperature. After 2.5 h the solution was centrifuged to remove the white precipitate. The crude product was recrystallized and characterized to be Al(NR′R′′)3(R′)R′′)
iPr, 3;
R′)R′′)Et, 4; R′)
iPr, R′′
)H, 5).
The compounds Al(NR′R′′)3(3-5) were also obtained from the
reaction of Al2Cl6with LiNR′R′′(R′)R′′)
iPr for 3; R′
)R′′)Et
for 4, R′)
iPr, R′′
)H for 5) in a molar ratio of 1:6. Analytical data
for 3-5 follows. Compound Al(N
iPr
2)3 (3): Solid; mp 58-59°C;
yield, 80%. 3 was purified by sublimation at 50°C, 10-3Torr. IR (KBr pellets): 2998 (s), 2976 (s), 2895 (s), 1441 (m), 1410 (m), 1111 (m), 1102 (w), 995 (m), 911 (br), 643 (br) cm-1. Anal. Found: C, 58.5; H, 11.7; N, 11.1. Calcd: C, 66.0; H, 12.9; N, 12.8. Analysis by mass spectroscopy gave the fragments expressed as m/z (EI: 70 eV, relative intensity (%) and assignment in parentheses): 328 (2.2, [M+
H]+ ), 313 (4.5, [M+H-CH3] + ), 298 (5.2, [M+H-2Me] + ), 283 (5.1, [M+H-3Me] + ), 226 (12.8, [M-H-N(C3H7)2] + ), 211 (32.6, [AlN(C3H7)2-H-Me] + ), 183 (10.6, [AlN(C3H7)2)-(C3H7+H)] + ), 126 (32.6, [AlNC3H7 - H] + ), 100 (72.2, [N(C3H7)2]+ ), 58 (100, [HNC3H7]+ ). 1H NMR (C 6D6): δ 1.27 (d, 36H, CH3of NiPr2,3JH-H )6.6 Hz), 3.41 (sep, 4H, CH of N iPr 2,3JH-H )6.6 Hz). 13C NMR (C6D6): δ 25.66 (CH3of NiPr2), 46.24 (CH of NiPr2) and27Al NMR
(C6D6): δ 162 ppm (br). Compound Al(NEt2)3(4): Solid; mp 68-70
°C; yield, 60%; IR (Nujol mull) 2994 (s), 2980 (s), 2891 (m), 1456 (w), 1412 (m), 1370 (m), 1080 (m), 1035 (m), 990 (br), 960 (m) cm-1. Anal. Found: C, 58.9; H, 12.2; N, 17.1. Calcd: C, 59.2; H, 12.4; N, 17.3. Analysis by mass spectroscopy gave the fragments expressed as
m/z (EI: 70 eV, relative intensity (%) and assignment in parentheses):
487 (18.7, [2M+H] +), 413 (5.5, [Al 2(NEt2)5-H] +), 398 (12.3, [Al 2 -(NEt2)5-Me-H] +, 384 (15.6, [Al 2(NEt2)5-2Me-H] +), 340 (17.2, [Al2(NEt2)4-2H] +), 313 (13.8, [Al(NEt 2)4-2H] +), 272 (72.3, [EtAl-(NEt2)3]+), 242 (92.3, [M -H] +) 171 (33.0, [Al(NEt 2)2]+), 100 (100, [HAl(NEt2)]+). 1H NMR (C 6D6): δ 1.24 (t, 15H, CH3of NEt2,3JH-H )6.6 Hz), 3.14 (quart, 8H, CH2of NEt2, 3J H-H )6.6 Hz). 13C NMR (C6D6): δ 12.38 (CH3of NEt2), 39.75 (CH2of NEt2). 27Al NMR
(C6D6): δ 157 ppm (br). Compound{Al(NiPrH)3}3(5): Mp (dec)
189°C; yield, 45%; IR (Nujol mull) 3340 (w), 3290 (w), 2950 (m), 2920 (s), 2850 (w), 1460 (m), 1370 (w), 1295 (w), 1190 (s), 1105 (w), 1065 (m), 1050 (m), 1010 (w), 925 (m), 840 (m), 760 (br), 690 (br), 650 (w) cm-1. Analysis by mass spectroscopy gave the fragments expressed as m/z (EI: 30 eV, relative intensity (%) and assignment in parentheses): 603 (55.3, [3Al(NiPrH) 3]+), 601 (100, [3Al(NiPrH) 3 -2H]+), 559 (5.5, [3Al(NiPrH) 3 -iPr -H] +), 549 (52.2, [2Al(NiPrH) 3 +H2Al(N iPrH 2)]+ ), 384 (5.2, [2Al(NiPrH) 3-Me-H] + ), 143 (7.7, [Al(NiPrH) 2]+ ), 71 (4.3, [HAliPr]+ ), 57 (6.4, [iPrN]+ ), 44 (23.2, [HC3H7]+ ), 43 (24.7, [C3H7]+ ). 1H NMR (C 7D8): δ 0.19 (br, NH of NiPrH), 1.23 (d, CH 3oftNiPrH,3JH-H )6.3 Hz), 1.38 (d, CH3of bNi -PrH,3J H-H )6.3 Hz), 3.40, 3.41 (sep, CH of N iPrH,3J H-H )6.3 Hz). 13C NMR (C 7D8): δ 29.59 (CH3oftNiPrH), 29.74 (CH3ofbNiPrH), 44.84 (CH oftNiPrH), 46.83 (CH ofbNiPrH). 27Al NMR (C 6D6): δ 111 ppm (br).
Reaction of Al2(NEt2)2(NiPr2)2Cl2 (1) with LiNiPrH. Bis((
µ-dialkylamido)chloro(diisopropylamido)aluminum), Al2(NEt2)2(NiPr2)2
-Cl2(1.87 g, 4.0 mmol), was added slowly to an ether solution (100
cm3) of lithium isopropylamide (2.60g; 24.0 mmol) under nitrogen at
-30 °C. The reaction took place when the reaction mixture was
warmed to room temperature. The solution was centrifuged to remove the white precipitate. The products Al2(NEt2)2(NiPr2)2H2(6) and{Al(Ni
-PrH)3}3(5) were obtained by gradient vacuum sublimation (10-3Torr) at 115 and 185°C, respectively. Analytical data for Al2(NEt2)2(Ni
-Pr2)2H2(6): Mp (dec) 145°C; yield 10%; IR (Nujol mull) 2980 (s),
2960 (s), 2910 (s), 2860 (s), 1725 (m), 1460 (m), 1370 (m), 1185 (br), 1055 (br), 690 (br) cm-1. Anal. Found: C, 59.0; H, 12.3, N, 12.7. Calcd: C, 59.2; H, 12.6; N, 14.0. Analysis by mass spectroscopy gave the fragments expressed as m/z (EI: 70 eV, relative intensity (%) and assignment in parentheses): 401 (8.7, [M+H] +), 385 (17.2, [M -CH3]+), 384 (8.3, [M -H -Me] +), 357 (5.2, [M -C3H7] +), 300 (5.2, [M-N(C3H7)2] +, 258 (12.7, [MH -(C3H7+N(C3H7)2)] +), 201 (12.7, [M/2+H] +), 100 (100, [N(C 3H7)2)]+), 58 (57.2, [HNC 3H7]+), 43 (72.3, [C3H7]+). 1H NMR (C 7D8): δ-0.20 (br, 2H, Al-H), 1.08 (t, 12H, CH3of NEt2,3JH-H )6.6 Hz), 1.24 (d, 24H, CH3of N iPr 2, 3J H-H )6.6 Hz), 2.97, 3.00, 3.34, 3.37 (q, 8H, CH2of NEt2, 3J H-H ) 6.6 Hz), 3.24 (sep, 4H, CH of NiPr 2, 3JH-H ) 6.6 Hz). 13C NMR (C7D8): δ 12.40 (CH3of NEt2), 25.53 (CH3of NiPr2), 40.30 (CH2of NEt2), 46.77 (CH of NiPr2). 27Al NMR (C7D8): δ 108 ppm (br).
Synthesis of AlCl3(NiPrH2)2{Al(NH3)(NH2)[Al(NiPrH)(NiPr)Cl]2}2
(7). Al2Cl6 (2.67 g; 10.0 mmol) was added slowly to a solution
containing an ether solution (100 cm3) of lithium isopropylamide (2.14
g; 60.0 mmol) with excess isopropylamine (20.0 mmol) under nitrogen at-30°C. The reaction took place when the temperature returned to
room temperature. The crude product was obtained after removal of solvent and LiCl precipitate. The product AlCl3(NiPrH2)2{[Al(NH3
)-(NH2)[Al(NiPrH)(NiPr)Cl]2}2(7) was obtained from recrystallization
from a hexane/toluene mixture (1:1).
The compound AlCl3(NiPrH2)2{Al(NH3)(NH2)[Al(NiPrH)(NiPr)Cl]2}2
(7) (solid) was purified by sublimation at 55°C, 10-3Torr: Mp 73
-75°C; yield, 38%; IR (Nujol mull) 3361 (w), 3295 (w), 3205 (w), 2980 (s), 2958 (s), 2950 (s), 2870 (s), 1460 (m), 1370 (m), 1172 (br), 1151 (br), 893 (m), 651 (br) cm-1
. Anal. Found: C, 38.3; H, 6.7; N, 15.0. Calcd: C, 33.3; H, 8.2; N, 18.1. Analysis by mass spectroscopy gave the fragments expressed as m/z (EI: 70 eV, relative intensity (%) and assignment in parentheses): 568 (17.2, [AlCl2(NiPrH2) +
[Al(NH3)(NH2)[Al(NiPrH)(NiPr)Cl]2 - 2H] + ), 509 (47.2, [AlCl2 + [Al(NH3)(NH2)[Al(NiPrH)(NiPr)Cl]2-2H] + ), 435 (5.2, [AlCl3(NiPrH2)2 +Al(N iPr 2)(NiPrH)]+ ), 413 (2.1, [Al(NH3)(NH2)[Al(NiPrH)(NiPr)Cl2 -2H] +), 378 (7.2, [AlCl 3(NiPrH2)2+Al(N iPr 2)]+), 224 (8.6, [Al(Ni -PrH)(NiPr)Cl +AlCl-Me] +), 69 (5.4, [AliPr -H] +), 58 (21.8, [Ni -PrH]+), 44 (61.8, [HiPr]+), 43 (100, [iPr]+). 1H NMR (C 6D6): δ 0.87 (br, 10H, Al(NH2)(NH3)), 0.95 (d, 12H, CH3of NiPrH2,3JH-H )6.6 Hz), 1.23 (d, 24H, CH3of NiPr,3JH-H )6.6 Hz), 1.26 (br, 4H, NH of NiPrH 2), 1.31 (br, 24H, CH3of NiPrH), 2.80 (br, 2H, CH of NiPrH2,
Downloaded by NATIONAL TAIWAN UNIV on August 14, 2009
3J H-H )6.6 Hz), 3.23 (sep, 4H, CH of N iPf,3J H-H )6.6 Hz), 3.38 (sep, 4H, CH of NiPrH,3J H-H )6.6 Hz), 3.89 (br, 4H, NH of N iPrH, 3J H-H )6.6 Hz). 13C NMR (C 6D6): δ 23.70 (CH3of NiPrH2), 25.66 (CH3of NiPrH), 25.90 (CH3of NiPr), 46.01 (CH of NiPrH2), 46.29 (CH of NiPrH), 65 45 (CH of NiPr). 27Al NMR (C 6D6): δ 112 ppm (br).
X-ray Structure Analysis. Single crystals for X-ray measurements
were sealed in glass capillaries. Intensity data were collected using theθ-2θ scan mode and corrected for absorption and decay. All
structures were solved by direct methods and refined with full-matrix least squares on F. In the final cycle all non-hydrogen atoms were fixed at idealized positions. Scattering factors for neutral atoms and anomalous scattering coefficients for non-hydrogen atoms were taken from the literature.19 All calculations were carried out with either a
Micro VAX 3600 computer using the NRCVAX program package20a
(for 6) or a SGI R4000 computer using the teXsan program package20b
(for 1 and 7). Data collection and crystal parameters are summarized in Table 1, and selected bond distances and angles of 1, 6, and 7 are listed in Table 2. The final positional parameters of the refined atoms are presented in Table 3.
Results and Discussion
The compound
{
Al(NR
′
R
′′
)
3}
ncan be synthesized either by
the reaction of aluminum hydride with amine or by treatment
of aluminum chloride with amine and lithium amide.
2,29The
latter reaction can be controlled by stoichiometry. For the main
group metals, substitution reaction via lithium amide is a
common method to generate Al/NR
2/halide ternary compounds.
In order to study the selectivity in substitution and stabilization
of the Al
2(NR
2)
nCl
6-ncomplexes, we have used different
stoichiometric ratios of aluminum complexes and lithium amide
to form the new Al
2(NR
2)
nCl
6-ncomplexes.
The X-ray
structural determinations of the complexes are described in the
following section.
Description of Structures. Compound Al(NEt
2)(N
iPr
2)Cl
uses diethylamide as a bridging group to form dimer 1, and the
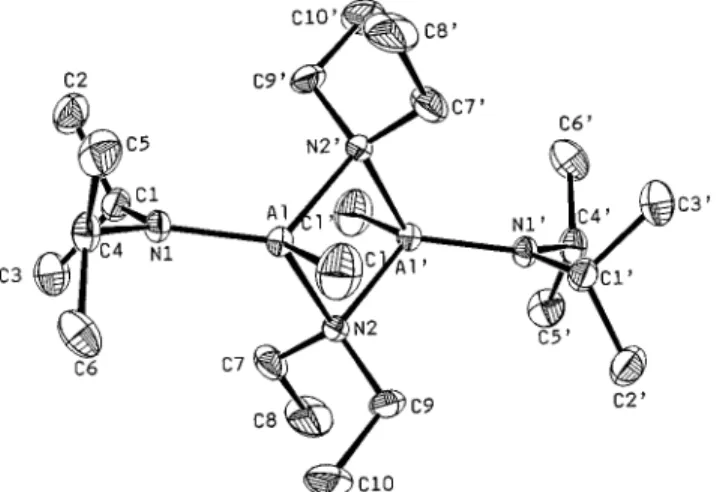
dimer sits on an inversion center as shown in Figure 1. The
four atoms Al, N(2), Al
′
, and N(2
′
) form a coplanar
four-membered ring with equal Al
-
N distances of 1.967(4) Å, and
the internal angles of the Al
2N
2ring are all near 90
°
. In the
terminal amide the Al, N(1), C(1), and C(4) atoms lie on a plane
as a result from the sp
2hybridization of the N(1) atom.
However, the Al
-
N(1) bond distance is quite short (1.791(4)
Å) reaching the lowest values found in the literature (1.79
-(19) Ibers, J. A., Hamilton, W. C., Eds. International Tables for X-rayCrystallography; Kynoch Press: Birmingham, U.K., 1974; Vol. IV,
Tables 2.2.B and 2.3.1.
(20) (a) Gabe, E. J.; Le Page, Y.; White, P. S.; Lee, F. L. Acta Crystallogr. 1987, A43, S294. (b) teXsan: Crystal Structure Analysis Package; Molecular Structure Corp.: College Station, TX, 1985, 1992. Table 1. Crystallographic Data Refinement Details for Compounds 1, 6, and 7 compound 1 6 7 formula C20H48Al2N4Cl2 C24H50Al2N4 C30H88Al7N14Cl7 fw 469.49 400.51 1082.15 a, Å 7.747(2) 15.088(2) 29.914(9) b, Å 9.648(2) 14.506(3) 10.197(3) c, Å 10.110(3) 12.439(3) 20.525(5) R, deg 102.91(2) β, deg 83.54(2) 90.99(4) γ, deg 110.19(2)
cryst system triclinic orthorhombic monoclinic space group P1 Cmca (No. 64) C2/c
2θ range, deg 16.66-28.06 60.9-78.3 15.50-20.00 cryst size, mm 0.25× 0.30 × 0.50 0.16× 0.45 × 0.45 0.25× 0.50 × 0.55 V, Å3 691(1) 2722(1) 6260(3) Z 1 4 4 Dcalc, Mg M-3 1.129 0.977 1.148 µ, mm-1 0.31 1.02 (Cu K R) 0.45 λ, Å 0.710 70 1.541 78 0.710 70 no. of rflns measd 2449 1162 4068 no. of unique rflns 2423 4068 no. of rflns I0>2.0σ(I0) 1789 697 (I0>3.0σ) 2472 transm factors (min; max) 0.942; 0.997 0.883; 1.000 RF 0.062 0.051 0.047 Rw 0.053 0.042 0.043 GoF 4.47 3.61 1.76 max∆/σ 0.015 0.01 0.023
Figure 1. ORTEP view of the compound Al2(µ-NEt2)2(NiPr2)2Cl2(1).
Table 2. Selected Bond Distances (Å) and Angles (deg) for
Compounds 1, 6, and 7 Compound 1 Al-N(1) 1.791(4) N(2)-C(7) 1.50(1) Al-N(2) 1.967(4) C(1)-C(2) 1.53(1) Al-Cl 2.127(2) C(7)-C(8) 1.52(1) N(1)-C(4) 1.46(1) Al-N(2)-Al′ 91.4(2) Al-N(1)-C(4) 124.9(3) N(2)-Al-N(2′) 88.6(2) C(1)-N(1)-C(4) 113.1(3) Cl-Al-N(2) 105.8(1) Al-N(2)-C(7) 112.6(3) Cl-Al-N(1) 115.2(1) C(2)-C(1)-C(3) 109.1(4) Al-N(1)-C(1) 122.0(3) N(2)-C(7)-C(8) 115.0(4) Compound 6 Al(1)-N(1) 1.792(5) N(2)-C(5) 1.49(1) Al(1)-N(2) 1.962(3) C(1)-C(2) 1.51(1) Al(1)-H(14) 1.67 C(5)-C(6) 1.51(1) N(1)-C(1) 1.47(1)
Al(1)-N(2)-Al(1′) 92.0(2) Al(1)-N(1)-C(3) 122.4(4)
N(2)-Al(1)-N(2′) 88.0(2) C(1)-N(1)-C(3) 112.3(5) N(2)-Al(1)-H(14) 107.5 Al(1)-N(2)-C(5) 115.6(2) N(1)-Al(1)-H(14) 114.4 C(2)-C(1)-C(2′) 109.6(6) Al(1)-N(1)-C(1) 125.3(4) N(2)-C(5)-C(6) 115.6(2) Compound 7 Al(1)-N(1) 1.926(4) Al(3)-N(7) 1.874(6) Al(1)-N(2) 1.926(4) Al(1)-Cl(1) 2.124(2) Al(1)-N(3) 1.784(4) N(1)-C(1) 1.50(1) Al(2)-N(4) 1.788(5) N(3)-C(7) 1.51(1) Al(3)-N(3) 1.718(4) Al(4)-Cl(4) 2.169(2) Al(3)-N(4) 1.735(4) Al(4)-N(5) 2.037(4) Al(3)-N(6) 1.880(6) N(5)-C(13) 1.51(1)
Al(1)-N(1)-Al(2) 86.9(2) Al(2)-N(4)-Al(3) 122.9(2)
N(1)-Al(1)-N(2) 84.3(2) Al(1)-N(3)-C(7) 123.8(3) Cl(1)-Al(1)-N(1) 113.1(1) Al(3)-N(3)-C(7) 113.5(3) Cl(1)-Al(1)-N(3) 123.1(2) N(6)-Al(3)-N(7) 106.8(3) Al(1)-N(1)-C(1) 123.3(3) N(7)-Al(3)-N(3) 110.4(3) Al(2)-N(1)-C(1) 122.1(3) N(7)-Al(3)-N(4) 110.1(3) Al(1)-N(3)-Al(3) 122.7(2) C(2)-C(1)-C(3) 111.0(5) N(3)-Al(3)-N(4) 109.0(2)
Downloaded by NATIONAL TAIWAN UNIV on August 14, 2009
1.85 Å).
21This possibly indicates more s-character in the Al
-N(1) bonding. The Al
-
Cl(1) bond distance of 2.127(2) Å is
in the same range as found in other organoaluminum chloride
compounds.
22-25A molecular plot of compound Al(NEt
2)(N
iPr
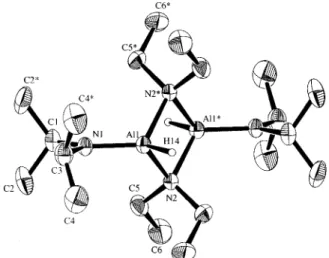
2)H (6), a dimer
with a center of inversion, is shown in Figure 2. The selected
bond lengths and bond angles are shown in Table 2. Both
compound 6 and compound 1 possess an identical framework.
The Al
2N
2four-membered ring skeleton is nearly square planar.
The distance of 1.792(4) Å between Al(1) and terminal N(1)
atom is the same as that in 1.
13Although the Al
-
H distance
may not be reliable in the X-ray analysis, the observed Al(1)
-H(14) distance of 1.67 Å falls between that in a
trialkylalumi-num (1.75(3) Å)
26and that in a lithium organotrihydroaluminate
(1.61(2) Å).
27The H
-
Al
-
N angles involving H(14) (108,
114
°
) are consistent with distorted tetrahedral geometry around
the aluminum atom.
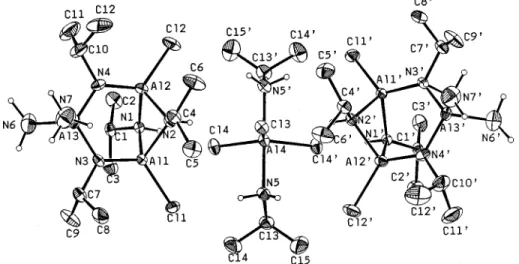
Compound 7 is composed of two parts of
{
Al(NH
3)(NH
2)-[Al(N
iPrH)(N
iPr)Cl]
2
}
2(X) with one part of AlCl
3(N
iPrH
2)
2(Y).
The exact molecular symmetry of 7 is C
2with the 2-fold axis
passing through Al(4) and Cl(3). Coordinated to the seven
aluminum atoms are ammonia, amide, imide, and free amine
as shown in Figure 3. The selected bond lengths and bond
angles are given in Table 2. The X part contains a plane formed
from atoms Al(1), N(3), N(4), and Al(2) and a
cyclobutane-like Al
2N
2ring made of Al(1), Al(2), N(1), and N(2) atoms. In
the three-coordinate N(3) moiety, the Al(1), N(3), C(7), and
Al(3) atoms lie on a plane caused by the sp
2hybridization of
N(3) atom, and so is the N(4) atom. The short distances of
Al
-
N involving N(3) (1.784(4), 1.718(4) Å) and N(4)
(1.735-(4) and 1.788(5) Å) could be caused by ionic resonance effects.
14The Y molecule is an amine adduct of electronically neutral
AlCl
3. Hence, the X molecule is also neutral. The nitrogen
ligands coordinated to Al(3) have to be neutral amine and amido
anion in order to balance the charge on X. After careful
examination of the final difference electron density map, the
N(7) is assigned as belonging to the amine ligand and N(6) is
assigned as belonging to the amido ligand.
Stoichiometric Reaction of Compound Al
2(NR
2)
2Cl
4(R
)
Et, a; R
)
i
Pr, b) with LiN
iPr
2
. The reactions of Al
2(NR
2)
2-Cl
4(R
)
Et, a; R
)
i
Pr, b) and lithium diisopropylamide, LiN
i-Pr
2, in 1:2 stoichiometric ratio produced 1 and 2 as shown in
eq 1. The
1H NMR spectrum of 1 displayed a chemical shift
at
δ
)
1.09 (t, 6H), 2.98 (q, 4H), and 3.28 (q, 4H) due to the
methyl and methylene protons in the ethyl groups and at
δ
)
1.27 (d, 12H) and 3.38 (sep, 4H) due to the methyl and methine
protons in the isopropyl groups. The
13C NMR spectrum
showed a chemical shift at
δ
)
12.40 and 40.23 for the methyl
and methylene groups of the ethyl and at
δ
)
25.50 and 46.69
(21) Vieth, M. Chem. ReV. 1990, 90, 1.(22) Bott, S. G.; Elgamal, H.; Atwood, J. L. J. Am. Chem. Soc. 1985, 107, 1796.
(23) Robinson, G. H.; Self, M. F.; Sangokoya, S. A.; Pennington, W. T. J.
Am. Chem. Soc. 1989, 111, 1520.
(24) Piero, G. D.; Perego, G.; Cucinella, S.; Cesari, M.; Mazzei, A. J.
Organomet. Chem. 1977, 136, 13.
(25) Cucinella, S.; Salvatori, T.; Busetto, C.; Perego, G.; Mazzei, A. J.
Organomet. Chem. 1974, 78, 185.
(26) Uhl, W. Z. Anorg. Allg. Chem. 1989, 570, 37.
(27) Eaborn, C.; Gorrell, I. B.; Hitchcock, P. B.; Smith, J. D.; Tavakkoli, K. Organometallics 1994, 13, 4143.
Table 3. Atomic Parameters x, y, z and BeqValues, Where Esd’s
Refer to the Last Digit Printed
x y z Beq Compound 1 Al 0.0926(2) 0.1008(2) 0.1135(1) 2.43(7) C(1) 0.3787(2) 0.1307(2) 0.1017(2) 6.2(1) N(1) 0.01448(2) 0.2055(4) 0.2632(3) 2.5(2) N(2) -0.0335(5) -0.1179(4) 0.0652(3) 2.8(2) C(1) -0.1763(7) 0.2018(5) 0.2843(5) 3.1(2) C(2) -0.1981(7) 0.3543(6) 0.2903(5) 4.9(3) C(3) -0.2744(7) 0.1395(6) 0.4084(5) 4.9(3) C(4) 0.1328(6) 0.3057(5) 0.3748(5) 3.3(3) C(5) 0.2829(7) 0.4372(6) 0.3304(5) 4.8(3) C(6) 0.2138(8) 0.2222(7) 0.4474(5) 5.2(3) C(7) -0.2012(8) -0.1712(6) 0.1547(5) 4.4(3) C(8) -0.3141(9) -0.3370(6) 0.1141(6) 7.2(4) C(9) 0.0898(8) -0.2092(6) 0.0586(5) 4.6(3) C(10) 0.151(1) -0.2140(7) 0.1951(6) 7.0(4) Compound 6 Al(1) 1.0000 0.0902(1) 0.4575(1) 4.87(5) N(1) 1.0000 0.1302(3) 0.3212(4) 4.9(1) N(2) 0.9693(3) 0.0000 0.5000 5.1(1) C(1) 1.0000 0.0709(5) 0.2254(5) 6.4(2) C(2) 0.9182(4) 0.0802(4) 0.1563(4) 9.9(2) C(3) 1.0000 0.2281(5) 0.2936(6) 7.2(2) C(4) 0.9183(4) 0.2774(4) 0.3359(4) 11.2(2) C(5) 0.8530(3) -0.0362(3) 0.4109(4) 7.4(2) C(6) 0.7893(3) -0.1124(4) 0.4417(4) 10.9(2) Compound 7 Al(1) 0.40561(5) 0.1164(2) 0.07064(7) 3.25(7) Al(2) 0.35275(6) 0.0849(2) 0.17133(8) 3.95(8) Al(3) 0.31763(5) -0.0287(2) 0.04034(8) 3.94(8) Al(4) 1/ 2 0.3494(2) 1/4 3.5(1) Cl(1) 0.46783(5) 0.1950(2) 0.04125(8) 5.18(8) Cl(2) 0.34654(6) 0.1250(2) 0.27266(8) 7.1(1) Cl(3) 1/ 2 0.1331(2) 1/4 4.3(1) Cl(4) 0.44918(5) 0.4529(1) 0.19362(7) 5.1(1) N(1) 0.3718(1) 0.2357(4) 0.1234(2) 3.1(2) N(2) 0.4100(1) 0.0137(4) 0.1493(2) 3.7(2) N(3) 0.3674(1) 0.0371(4) 0.0157(2) 3.8(2) N(4) 0.3116(1) 0.0005(4) 0.1230(2) 4.4(2) N(5) 0.5405(1) 0.3419(4) 0.0128(2) 4.4(2) N(6) 0.2697(2) 0.0463(7) -0.0068(3) 10.1(4) N(7) 0.3160(2) -0.2093(6) 0.0237(3) 10.6(4) C(1) 0.3394(2) 0.3331(5) 0.0956(2) 3.9(3) C(2) 0.0721(2) 0.4078(6) 0.1485(3) 6.8(4) C(3) 0.3622(2) 0.4258(6) 0.0489(3) 6.1(3) C(4) 0.4187(2) -0.1301(5) 0.1492(3) 4.8(3) C(5) 0.4631(2) -0.1621(6) 0.1199(3) 7.6(4) C(6) 0.4157(3) -0.1852(5) 0.2176(3) 8.5(4) C(7) 0.3756(2) 0.0205(5) -0.0563(3) 5.1(3) C(8) 0.4166(2) -0.0595(7) -0.0694(3) 7.7(4) C(9) 0.3768(3) 0.1497(7) -0.0905(3) 8.3(5) C(10) 0.2687(2) -0.0515(7) 0.1486(3) 7.6(4) C(11) 0.2396(2) 0.0552(9) 0.1747(4) 11.6(6) C(12) 0.2768(3) -0.1582(8) 0.1993(4) 12.4(6) C(13) 0.5585(2) 0.4672(6) 0.1422(3) 5.5(3) C(14) 0.5406(3) 0.4845(7) 0.0739(3) 8.8(4) C(15) 0.6081(2) 0.4675(7) 0.1455(4) 9.6(5)
Figure 2. ORTEP drawing of the compound Al2(µ-NEt2)2(NiPr2)2H2
(6).
Downloaded by NATIONAL TAIWAN UNIV on August 14, 2009
assigned to methyl groups the and methine carbon of the
isopropyl group. The
27Al NMR spectrum showed a chemical
shift at
δ
)
89 ppm which was assigned to a four-coordinated
environment of the organoaluminum compound.
28Mass
spec-tral data showed the base-ion peak at m/z
)
468 which was
assigned to the molecular ion. The above spectral data are in
good agreement with the crystal structure obtained from X-ray
diffraction techniques.
After several days, the
1H NMR
spectrum of 1 gradually appeared to have the additional peaks
δ
)
0.95 (d) and 2.81 (sep), which were assigned to the methyl
protons and methine protons of the cis form of Al
2(
µ-NEt
2)
2(N
i-Pr
2)
2Cl
2. In the characterization of 2, the
1H NMR spectrum
displayed chemical shifts at
δ
)
1.25 (d) due to methyl groups
of the isopropyl and at
δ
)
3.40 (sep) which was assigned to
the methine groups. Mass spectral data contain the base-ion
peak at m/z
)
489 was assigned to the dimer ion less one
chloride group. Compound 2 was isolated by sublimation at
75
°
C after solvent removal. It is reasonable to suggest two
species, cis and trans, to exist in the solutions of compounds 1
and 2. On variation of the molar ratio of compound b to LiN
i-Pr
2from 1:2 to 1:4, product 3 was obtained as shown in eq 2,
while compound 1 did not react further with lithium
diisopro-pylamide.
The
1H NMR spectrum of 3 displayed a chemical shift at
δ
)
1.27 (d) due to methyl groups of isopropyl, and the septet at
δ
)
3.41 was assigned to the methine groups of isopropyl. Mass
spectral data contained the base-ion peak at m/z
)
328 assigned
to the monomeric species plus one hydrogen group. The X-ray
diffraction data
35for compound 3 showed identical parameters
with the published report.
13Substitution Reactions of Al
2(NR
2)
2nCl
6-2n(R
)
Et,
i
Pr;
n
)
1
-
3) with Excess LiNR
′
R
′′
. Some intermediates were
difficult to purify when different chemical stoichiometries
were used. For instance, we were able to isolate the compounds
2 and 3 using Al
2(N
iPr
2)
2Cl
4and LiN
iPr
2in 1:2 and 1:4
stoichiometric ratios while we could not isolate the rest of the
intermediates when the stoichiometry was 1:1, 1:3, 1:5, etc. They
may form aluminum complexes with more amido substituents.
So we used excess lithium amide to isolate the products.
Compounds 4 and 5 were synthesized by the reactions of a, b,
1, and 2 with excess lithium amide LiNR
′
R
′′
as shown in eqs
3 and 4. However, one additional compound 6, Al
2(NEt
2)
2(N
i-Pr
2)
2H
2, was isolated during the synthesis of 5. We presume
that compounds 5 and 6 were obtained through the substitution
(28) Benn, R.; Rufinsky, B.; Lehmkuhl, H.; Janssen, E.; Kruger, C. Angew.
Chem., Int. Ed. Engl. 1983, 10, 779.
Figure 3. ORTEP drawing of the compound AlCl3(iPrNH2)2{Al(NH3)(NH2)[Al(NiPrH)(NiPr)Cl2}2(7).
Figure 4. ORTEP drawing of the compound{Al(NH3)(NH2)[Al(Ni
-PrH)(NiPr)Cl] 2}2(X).
Al
2(NR
2)
2Cl
4a, b
+
2LiN
iPr
2f
Al
2(NR
2)
2(N
iPr
2)
2Cl
2R
)
Et, 1
R
)
iPr, 2
+
2LiCl (1)
Al
2(N
iPr
2)
2Cl
4b
9
8
2 LiNiPr2 -2LiClAl
2(N
iPr
2)
4Cl
22
9
8
2LiNiPr2 -2LiCl2Al(N
iPr
2)
33
(2)
Al
2(NR
2)
2Cl
4R
)
Et, a
R
)
iPr, b
+
6 LiNR
′
R
′′
9
8
-4LiCl{
Al(NR
′
R
′′
)
3}
nR
′
)
R
′′
)
Et, 4
R
′
)
iPr, R
′′
)
H, 5
+
2LiNR
2(3)
Al
2(NR
2)
2(NR*
2)
2Cl
2R
)
Et, R*
)
iPr
R
)
R*
)
iPr
9
8
6LiNR′R′′ -2LiCl{
Al(NR
′
R
′′
)
3}
nR
′
)
R
′′
)
Et, 4
R
′
)
iPr, R
′′
)
H, 5
+
2LiNR
2+
2LiNR*
2(4)
Downloaded by NATIONAL TAIWAN UNIV on August 14, 2009
reaction of compound 1, Al
2(NEt
2)
2(N
iPr
2)
2Cl
2, by lithium
hydride and lithium isopropylamide, respectively. The lithium
hydride may be a side product in the synthesis of lithium
isopropylamide. Compounds 3
-
5 can be synthesized by other
routes. Reaction of Al
2Cl
6with LiNR
′
R
′′
in 1:6 stoichiometric
ratio yields all three compounds. This result is similar to that
found by Ruff et al.
29-32Also, compounds 4 and 5 could also
be synthesized by substitution reaction of 3 and 4 with lithium
amide LiNR
′
R
′′
, respectively (eq 5). The
1H NMR spectrum
of 4 displayed a chemical shift at
δ
)
1.24 (t) due to the methyl
groups of the ethyl, and the quartet at
δ
)
3.14 was assigned
to the methylenyl groups. Mass spectral data contained the
base-ion peak at m/z
)
487 assigned to the dimeric ion plus one
hydrogen group.
The FT-IR spectrum of the complex 5 showed a broad peak
in the 3340 cm
-1region which was assigned to the N
-
H
stretching vibrations. The
1H NMR spectrum of 5 displayed a
chemical shift at
δ
)
0.19 (br) due to NH of isopropylamide;
the doublet peaks at
δ
)
1.23 and 1.38 were assigned to the
terminal and bridged methyl groups of isopropylamide,
respec-tively. Mass spectral data contained the base-ion peak at m/z
)
603 assigned to be a trimeric ion. Hence it is suggested that
5 exists as a trimer.
Formation of AlCl
3(N
iPrH
2)
2{[Al(NH
3)(NH
2)[Al(N
iPrH)-(N
iPrH)(N
iPr)Cl]
2}2(7). Reaction of aluminum trichloride with
lithium isopropylamide yielded a single product, Al(N
iPrH)
3
,
5. However, similar reactions of aluminum trichloride with
lithium isopropylamide in the presence of isopropylamine have
generated compound 7.
In an attempt to rationalize the
formation of 7, we propose the possible involvement of an
unidentified species LiNH
2. A logical retrosynthesis scheme
is shown in eqs 6
-
9.
AlCl
3(N
iPrH
2)
2{
Al(NH
3)(NH
2)[AlCl(N
iPrH)(N
iPr)]
2}
27
r
2
{
Al(NH
3)(NH
2)[AlCl(N
iPrH)(N
iPr)]
2}
X
+
AlCl
3(N
iPrH
2)
2Y
(6)
Al
2(N
iPrH)
4Cl
28
7
9
4 LiNiPrH -4LiClAl
2C
6(7)
Al
2(N
iPrH)
2(NH
2)
49
7
9
2 LiNiPrH -2LiClAl
2(NH
2)
4Cl
27
9
4LiNH2 -4LiClAl
2Cl
6(8)
{
Al(NH
3)(NH
2)[AlCl(N
iPrH)(N
iPr)]
2}
X
7
9
-NiPrH 2Al
2(N
iPrH)
4Cl
28
+
1/
2Al
2(N
iPrH)
2(NH
2)
49
(9)
The compound 7 contains two different moieties with a
general composition of X
2Y.
The X part possess the
π
interaction delocalized in the Al
3N
2planar framework, which
may be hypothetically built from Al
2(N
iPrH)
4Cl
2(8) and Al(N
i-PrH)(NH
2)
2(9). Moreover, the synthesis of compounds 8 and
9 could be rationalized by the above substitution reactions. The
clarification of the mechanistic details would require extensive
work. However, it seems worthy of further investigation.
The FT-IR spectrum of the compound 7 showed three broaden
peaks in the region 3205
-
3361 cm
-1
which were attributed to
the stretching vibrations of the N
-
H groups. The
1
H NMR
spectrum of 7 displayed chemical shift at
δ
)
0.89 (br) and
3.87 (br) due to NH of Al(NH
3)(NH
2) and N
iPrH, respectively.
The chemical shifts of various protons of Al(NH
2)(NH
3) are
comparable to those of the corresponding values in the
{
tBu
2-AlNH
2}
3derivative.
33The chemical shifts at
δ
)
0.95 (d), 1.23
(d), and 1.31 (br) with integral ratio 1:2:2 are due to methyl
groups of isopropyl of AlCl
3(N
iPrH
2), isopropylimido, and
isopropylamido, respectively. The
13C NMR spectrum showed
chemical shifts at
δ
)
23.70, 25.66, and 25.90 assigned to the
methine carbon of isopropyl of imido, amido, and amine,
respectively. The peaks at
δ
)
65.45, 46.29, 46.01 were
assigned to methyl carbon of isopropyl of imido, amido, and
amine. The
27Al NMR spectrum showed a chemical shift at
δ
)
112 ppm assigned to a four-coordinated environment of
organoaluminum complex.
28These spectral data support the
structure as determined by X-ray diffraction technique.
Acknowledgment. We thank the National Science Council
of the Republic of China for financial support.
Supporting Information Available: Text describing X-ray
pro-cedures and tables of crystal data, complete bond distances and bond angles, final fractional coordinates, and thermal parameters (21 pages). Ordering information is given on any current masthead page. IC950539B
(29) Ruff, J. K. J. Am. Chem. Soc. 1961, 83, 2835.
(30) Ruff, J. K.; Hawthorne, M. F. J. Am. Chem. Soc. 1960, 82, 2141. (31) Ruff, J. K.; Hawthorne, M. F. J. Am. Chem. Soc. 1961, 83, 535. (32) Ruff, J. K. Inorg. Chem. 1962, 1, 612.
(33) Interrante, L. V.; Sigel, G. A.; Garbauskas, M.; Hejna, C.; Slack, G. A. Inorg. Chem. 1989, 28, 252.
(34) Janik, J. F.; Duesler, E. N.; Paine, R. T. Inorg. Chem. 1988, 27, 4335. (35) AlN3C18H42(3): a)15.747(2) Å, b)12.648(2) Å, c)10.110(3) Å,R)102.91(2)°,β)83.54(2)°,γ)110.19(2)°, V)2251(4) cm
3, triclinic, space group P1.
(36) Loopy, A.; Tchoubar, B. Salt Effects in Organic and Organometallic
Chemistry; VCH: Weinheim, Germany, 1992; Chapter 7, p 241.
(5)
Downloaded by NATIONAL TAIWAN UNIV on August 14, 2009