Synthesis of the First Cluster Complexes Bearing Three Quadruply Bridging CO Ligands: X-ray Crystal Structure of [C5H3(SiMe3)2]WRu6(µ3
-H)-(CO)18
Yun Chi,*,†Chi-Jung Su,†Shie-Ming Peng,*,‡and Gene-Hsiang Lee‡
Department of Chemistry, National Tsing Hua UniVersity Hsinchu 30043, Taiwan, Republic of China Department of Chemistry and Instrumentation Center National Taiwan UniVersity, Taipei 10764 Taiwan, Republic of China ReceiVed June 24, 1997 The transformation from a regular C-bonded, terminal, or bridging CO ligand to a quadruply bridging CO ligand is recognized as an essential requirement for the cleavage of the C-O bond in metal cluster compounds and on metal surfaces.1 One of the interesting developments of recent years has been the realization that the tetrametallic butterfly clusters, such as [Fe4(µ-H)(CO)13]-or LMRu3(µ-H)(CO)
12(M)W and Mo; L )Cp and C5Me5), bearing one quadruply bridging CO ligand, are able to undergo equilibration in solution with the tetrahedral isomers possessing only the regular terminal or bridging CO ligands.2,3 Thus, these isomerization studies provide explicit evidence for the rapid exchange between these two different types of CO ligands. Recently, this chemistry has been extended into the preparation of complexes possessing twoµ4-CO ligands, as reported in the case of Ru6 cluster chemistry4 and its heterometallic domain.5 In the meantime, reactivity studies have demonstrated that these multisite-bound CO ligands can convert into carbide ligands through cleavage of C-O bond.6 In this communication, we extend the scope of these investigations, and report the first synthesis of WRu6compounds bearing three such uncommonµ4-CO ligands and a related cluster compound with twoµ4-CO ligands, generated from direct hydrogenation. The hydride complex [C5H3(SiMe3)2]W(CO)3H, synthesized from C5H4(SiMe3)2 and W(CO)3(NCEt)3, was added into a toluene solution of Ru3(CO)12at reflux over a period of 3 h. Workup of the reaction mixture by routine TLC gave a WRu6 complex [C5H3(SiMe3)2]WRu6(µ3-H)(CO)18(1a) in 28% yield.7 The analogous C5H4(SiMe3) and C5H4(CHMe2) substituted
complexes 1b and 1c were obtained using the hydride complexes [C5H4(SiMe3)]W(CO)3H and [C5H4(CHMe2)]W(CO)3H, respec-tively.7 All of these products were characterized by IR and1H and13C NMR spectroscopies and microanalysis. The X-ray structure of 1a was determined to reveal its identity.7
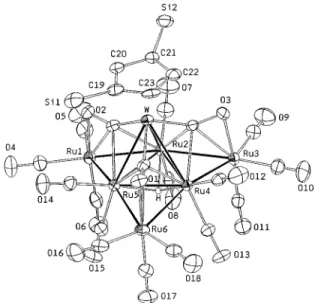
As depicted in Figure 1, the molecule consists of a tetrahedral WRu3core with the tungsten atom located at the apical position and capped by a C5H3(SiMe3)2 ligand, while the Ru atoms occupy the basal positions and each possess two terminal CO ligands. The W-Ru distances are nearly equal with distances 2.911(1)-2.917(1) Å, which are longer than the other Ru-Ru distances. The C5H3(SiMe3)2ligand seems to experience some steric congestion, as the W-C(SiMe3) distances (2.358(8) -2.368(8) Å) are relatively longer than the unsubstituted W-CH distances (2.324(7)-2.326(8) Å) of the C5H3(SiMe3)2ring. The hydride, which was located crystallographically, was found to span the Ru3basal triangle. Moreover, the Ru-Ru edges are each coordinated by a Ru(CO)3pendant, forming three WRu3 butterfly arrangements, supporting the observed µ4-η2-CO ligands. The associated metric parameters are similar to those of the tetrametallic butterfly clusters mentioned earlier2,3 and other polymetallic clusters bearing the same type of encapsulated µ4-CO ligand.8
Interestingly, the cluster framework of 1 possesses a virtual C3 axis which coincides with the vector linking the W atom and the hydride ligand. This symmetrical feature is best revealed by the13C NMR data of 1a, which exhibits five CO signals atδ 288.1 (JWC) 168 Hz), 207.6, 200.5 (JCH)2.4 Hz), 189.6 (JCH)12 Hz), and 184.5 with an intensity ratio of 3:3:3:3:6 at room temperature. Thus, the signal atδ 288.1 with the characteristic1J
WCcoupling is assigned to theµ4-CO ligands. The signals atδ 200.5 and 189.6, which show the2J
CHcoupling, are due to the equatorial and the axial CO ligands associated with the Ru atoms on the inner metal triangle, while the signals at δ 207.6 and 184.5 with the ratio 3:6 are derived from the axial and the equatorial CO ligands of the Ru(CO)3pendants. Some attempts were made to investigate theµ4-CO reactivity. Thus, treatment of 1a-c with CO in toluene (1 atm, 5 h, 110
°C) resulted a slow decomposition. However, as a general example, hydrogenation of 1a gave a trihydride complex [C5H3-(SiMe3)2]WRu6(µ-H)3(CO)17 (2) in 71% yield.7 The X-ray diffraction study disclosed that it now adopts a double edge-bridged trigonal bipyramidal core structure (Figure 2), which is formally derived from the previously mentioned, triple edge-bridged tetrahedra by generation of a W-Ru bond. Again, the hydride ligands are located, and they span the newly formed W-Ru(1) edge, the Ru(2)-Ru(3) edge, and the Ru(2)- Ru(4)-Ru(5) face, respectively. These assignments are fully consistent with the1H NMR data which exhibit three signals atδ-14.14 (JWH)84.8 Hz),-14.70, and-20.71; the last one is obviously due to the face-bridging hydride because of the large distinction in chemical shift.9
More striking behavior of 2 was found by VT NMR. For example, theµ4-CO ligands occur atδ 292.1 and 289.2 in the 13C NMR spectrum at 294 K. This observation agrees with †National Tsing Hua University.
‡National Taiwan University.
(1) (a) Horwitz, C. P.; Shriver, D. F. AdV. Organomet. Chem. 1984, 23,
219. (b) Ichikawa, M.; Lang, A. J.; Shriver, D. F.; Sachtler, W. M. H. J. Am. Chem. Soc. 1985, 107, 7216. (c) Blyholder, G.; Lawless, M. J. Chem. Soc., Chem. Commun. 1990, 632. (d) Chisholm, M. L.; Hammond, C. E.; Johnston, V. J.; Streib, W. E.; Huffman, J. C. J. Am. Chem. Soc. 1992, 114, 7056.
(2) (a) Manassero, M.; Sansoni, M.; Longoni, G. J. Chem. Soc., Chem. Commun. 1976, 919. (b) Horwitz, C. P.; Shriver, D. F. Organometallics
1984, 3, 756. (c) Horwitz, C. P.; Holt, E. M.; Brock, C. P.; Shriver, D. F.
J. Am. Chem. Soc. 1985, 107, 8136. (d) Wang, J.; Sabat, M.; Horwitz, C. P.; Shriver, D. F. Inorg. Chem. 1988, 27, 552.
(3) (a) Chi, Y.; Wu, F.-J.; Liu, B.-J.; Wang, C.-C.; Wang, S.-L. J. Chem. Soc., Chem. Commun. 1989, 873. (b) Chi, Y.; Su, C.-J.; Farrugia, L. J.; Peng, S.-H.; Lee, G.-H. Organometallics 1994, 13, 4167.
(4) (a) Ingham, S. L.; Johnson, B. F. G.; Martin, C. M.; Parker, D. J. Chem. Soc., Chem. Commun. 1995, 159. (b) Blake, A. J.; Dyson, P. J.; Lngham, S. L.; Johnson, B. F. G.; Martin, C. M. Inorg. Chem. Acta. 1995, 240, 29. (d) Kolehmainen, E.; Rissanen, K.; Laihia, K.; Kerzina, Z. A.; Rybinskaya, M. I.; Nieger, M. J. Organomet. Chem. 1996, 524, 219.
(5) (a) Adams, R. D.; Babin, J. E.; Tasi, M. Angew. Chem., Int. Ed. Engl. 1987, 26, 685. (b) Adams, R. D.; Alexander, M. S.; Arafa, I.; Wu, W. Inorg. Chem. 1991, 30, 4717. (c) Davies, J. E.; Nahar, S.; Raithby, P. R.; Shields, G. P. J. Chem. Soc., Dalton Trans. 1997, 13.
(6) (a) Whitmire, K. H.; Shriver, D. F. J. Am. Chem. Soc. 1981, 103, 6754. (b) Kolis, J. W.; Holt, E. M.; Drezdzon, M.; Whitmire, K. H.; Shriver, D. F. J. Am. Chem. Soc. 1982, 104, 6134. (c) Martin, C. M.; Dyson, P. J.; Ingham, S. L.; Johnson, B. F. G.; Blake, A. J. J. Chem. Soc., Dalton Trans.
1995, 2741. (d) Su, C.-J.; Su, P.-C.; Chi, Y.; Peng, S.-M.; Lee, G.-H. J.
Am. Chem. Soc. 1996, 118, 3289.
(7) Complete experimental details and characterization data for all new complexes isolated during this work are provided as Supporting Information. (8) (a) Brun, L. P.; Dawkins, G. M.; Green, M.; Miles, A. D.; Orpen, A. G.; Stone, F. G. A. J. Chem. Soc., Chem. Commun. 1982, 926. (b) Johnson, B. F. G.; Lewis, J.; McPartlin, M.; Pearsall, M.-A.; Sironi, A. J. Chem. Soc., Chem. Commun. 1984, 1089. (c) Gibson, C. P.; Dahl, L. F. Organometallics 1988, 7, 535. (d) Chi, Y.; Chuang, S.-H.; Liu, L.-K.; Wen, Y.-S. Organometallics 1991, 10, 2485. (e) Adams, R. D. Li, Z.; Lii, J.-C.; Wu, W. Organometallics 1992, 11, 4001. (f) Corrigan, J. F.; Doherty, S.; Taylor, N. J.; Carty, A. J. Organometallics 1993, 12, 993. (g) Wang, J.-C.; Lin, R.-C.; Chi, Y.; Peng, S.-M.; Lee, G.-H. Organometallics 1993, 12, 4061. (h) Su, P.-C.; Chi, Y.; Su, C.-J.; Peng, S.-M.; Lee, G.-H. Organo-metallics 1997, 16, 1870.
(9) Chao, W.-J.; Chi, Y.; Way, C.-J.; Mavunkal, I. J.; Wang, S.-L.; Liao, F.-L.; Farrugia, L. J. Organometallics 1997, 16, 3523.
11114 J. Am. Chem. Soc. 1997, 119, 11114-11115
S0002-7863(97)02092-1 CCC: $14.00 © 1997 American Chemical Society
Downloaded by NATIONAL TAIWAN UNIV on August 14, 2009
the asymmetric nature of the cluster skeleton indicated by the X-ray analysis. Upon raising of the temperature to 325 K, these CO signals coalesce to form a broad signal centered atδ 291.1. In addition, two of the three distinct olefinic hydrogen of the C5H3(SiMe3)2 substituent atδ 5.23 and 4.85 in its 1H NMR spectrum at 294 K merge to form a broad signal atδ 5.06 at 325 K. These temperature-dependent behaviors are best interpreted in terms of the hydride hopping between the Ru-(2)-Ru(3) edge and the Ru(5)-Ru(6) edge of the Ru6 basal plane, forming a dynamic plane of mirror symmetry. Interest-ingly, the scrambling of the Ru-H-Ru hydride and the face-bridging hydride was also noted under the same conditions, suggesting that the proposed hydride hopping is relayed by the face-bridging hydride ligand (Scheme 1). Consistent with this
observation, the signals atδ-14.70 and-20.71 are broaden extensively on warming to 303 K, suggesting the presence of pairwise exchange between these sites. The nonselective scrambling of all hydride ligands becomes possible only at the higher temperature, as the signal atδ-14.14, due to the third W-H-Ru hydride ligand, has turned much broader upon warming to 333 K, confirming the existence of such a higher energy process.
In summary, this paper reports the first example of transition metal clusters bearing threeµ4-CO ligands. Two observations deserve additional comments. First, the formation of WRu6 clusters 1a-c is in sharp contrast to that of the condensation reactions using CpW(CO)3H or (C5Me5)W(CO)3H, which gave only the tetrametallic counterparts LWRu3(µ-H)(CO)12(L)Cp and C5Me5).3The key factors leading to the formation of the WRu6compounds can be deduced by comparing the spectro-scopic data of the substituted group 6 hydride complexes7with those of their parent CpW(CO)3H. It suggests that the electronic effect imposed by the trimethylsilyl or isopropyl groups is minimal because the positions of their IR ν(CO) absorptions and the1H NMR chemical shifts for hydrides are very similar. As a result, these cyclopentadienyl groups seem to exert their influence through steric interaction exclusively.10 However, this interligand repulsion is very subtle and can be depressed by removing of a CO ligand through ligand displacement.11 Thus, complex 2 is available through hydrogenation of 1a, demon-strating the elevation of such a steric interaction.
Second, the formation of the smaller, tetrametallic complex LWRu3(µ-H)(CO)12as the potential intermediate is also ruled out by the unsuccessful coupling experiment using [C5H4-(CHMe2)]WRu3(µ-H)(CO)12 and Ru3(CO)12 as reagents; the former was prepared from condensation of Ru3(CO)12 and [{C5H4(CHMe2)}W(CO)3]Na in THF, followed by acidifica-tion.12 Thus, the the formation of 1 occurs not via the expected step-by-step cluster building reaction
but via an unknown trimolecular coupling involving one tungsten hydride complex and two Ru3(CO)12 molecules simultaneously.
Acknowledgment. We are grateful to the National Science Council of the Republic of China for financial support (Grant No. NSC 86-2113-M007-035).
Supporting Information Available: Text describing experimental details and spectroscopic data for 1a-c and 2 and tables of bond
distances, atomic coordinates, and anisotropic thermal parameters for 1a and 2 (17 pages). See any current masthead page for ordering and Internet access instructions.
JA9720927
(10) (a) Lappert, M. F.; Pickett, C. J.; Riley, P. I.; Yarrow, P. I. W. J. Chem. Soc., Dalton Trans. 1981, 805. (b) Antinolo, A.; de Ilarduya, J. M.; Otero, A.; Royo, P.; Lanfredi, A. M. M.; Tiropicchio, A. J. Chem. Soc., Dalton Trans. 1988, 2685. (c) Okuda, J. Top. Curr. Chem. 1992, 160, 97. (11) (a) Churchill, M. R.; Hollander, F. J.; Shapley, J. R.; Foose, D. S. J. Chem. Soc., Chem. Commun. 1978, 534. (b) Chi, Y.; Cheng, C.-Y.; Wang, S.-L. J. Organomet. Chem. 1989, 378, 45.
(12) (a) Cazanoue, M.; Lugan, N.; Bonnet, J.-J.; Mathieu, R. Organo-metallics 1988, 7, 2480. (b) Su, C.-J.; Chi, Y.; Peng, S.-M.; Lee, G.-H. Organometallics 1995, 14, 4286.
Figure 1. Molecular structure of 1a and the atomic numbering scheme; the methyl groups of the trimethylsilyl substituents were omitted for clarity. Selected bond lengths (Å): W-Ru(2))2.917(1), W-Ru(4) )2.913(1), W-Ru(5))2.911(1), Ru(2)-Ru(4))2.801(1), Ru(2)
-Ru(5))2.799(1), Ru(4)-Ru(5))2.815(1), Ru(1)-Ru(2))2.811(1),
Ru(1)-Ru(5))2.828(1), Ru(2)-Ru(3))2.820(1), Ru(3)-Ru(4))
2.804(1), Ru(4)-Ru(6))2.805(1), Ru(5)-Ru(6))2.820(1).
Figure 2. Molecular structure of 2 and the atomic numbering scheme; the methyl groups of the trimethylsilyl substituents were omitted for clarity. Selected bond lengths (Å): W-Ru(1))2.996(1), W-Ru(2) )2.882(1), W-Ru(4))2.922(1), W-Ru(5))2.986(1), Ru(2)-Ru(4) ) 2.838(1), Ru(2)-Ru(5) ) 2.824(1), Ru(4)-Ru(5) ) 2.740(1),
Ru(1)-Ru(2))2.832(1), Ru(1)-Ru(5))2.741(1), Ru(2)-Ru(3))
2.888(1), Ru(3)-Ru(4))2.827(1), Ru(4)-Ru(6))2.851(1), Ru(5)
-Ru(6))2.727(1).
Scheme 1
[W complex]+Ru
3(CO)12f [WRu3intermediate] f f
[WRu6cluster]
Communications to the Editor J. Am. Chem. Soc., Vol. 119, No. 45, 1997 11115
Downloaded by NATIONAL TAIWAN UNIV on August 14, 2009