Estimation of Life Expectancy and the Expected Years of Life
Lost in Patients with Major Cancers: Extrapolation of Survival
Curves under High-Censored Rates
Po-Ching Chu, MD,1
Jung-Der Wang, MD, ScD,1,2
Jing-Shiang Hwang, PhD,3
Yu-Yin Chang, MS1
1Institute of Occupational Medicine and Industrial Hygiene, College of Public Health, National Taiwan University, Taiwan;2Departments of
Internal Medicine and Environmental and Occupational Medicine, National Taiwan University Hospital, Taipei, Taiwan;3Institute of Statistical
Science, Academia Sinica, Taipei, Taiwan
A B S T R AC T
Objectives: There exists a lack of extrapolation methods for
long-term survival analysis when censored rates are high (25–50%). This study aimed at estimating life expectancy (LE) after the diagnosis of cancer and the expected years of life lost (EYLL) using a newly developed semiparametric method.
Methods: Patients (n = 425,294) diagnosed with 17 different
types of major cancer were enrolled. All of the patients were registered with the Taiwan Cancer Registry between 1990 and 2001; their survivals were followed through the end of 2004. The survival function for an age- and sex-matched reference population was generated using the Monte Carlo method from the life table of the general population. Lifetime survival of the cancer patients (up to 50 years) were obtained using linear extrapolation of a logit-transformed curve of the survival ratio between the cancer and reference populations.
The estimates were compared with the results from the extrapolation of fitted Weibull models.
Results: The 15-year survival, LE, and EYLL for 17 different
types of cancer were determined, of which the LE of breast, cervical, ovarian, and skin cancers exceeded 15 years; nasopharyneal, leukemia, bladder, kidney, and colorectal cancers exceeded 10 years. Validity tests indicated that the relative biases of the extrapolated estimates were usually <5% under high censoring rates.
Conclusions: The newly developed method is feasible and
relatively accurate to project LE and EYLL, which could also be merged with data pertaining to quality of life, for a more detailed outcome assessment in the future.
Keywords: life expectancy, lifetime extrapolation, Monte
Carlo method, survival, years of life lost.
Introduction
Cancer is an issue of major public health concern, not only because it can cause substantial suffering and shorten the natural lifespan of cancer patients, but also because of the significant impact it can have upon society as a whole [1]. The estimation of life expectancy (LE) from the date the diagnosis of cancer is made until death has been performed in many medical fields to generate measures of cancer survival relevant to clini-cians, health economists, policymakers, and insurance companies [2]. In general, survival analysis provides an estimation of the survival rate during the observed study period, but there has been a lack of reliable method for lifetime extrapolation [3]. Parametric sur-vival modeling, such as the Weibull distribution [4], Gompertz extrapolation technique [5,6], exponential distribution [7], and the log-normal distribution [8], are
commonly used for lifetime extrapolation; however, the models may not be suitable for data with a high rate of right censoring, such as patients infected with human immunodeficiency virus [9].
We have developed a semiparametric method to incorporate LE information of the general population into the estimation process [9–14]. If the cancer-related excess hazard assumes constant after a period of time, cancer patients’ LE can be projected from the available follow-up data with this semiparametric method [10]. In addition to estimating the LE after diagnosis, the method can also be used to compute the expected years of life lost (EYLL), which is a measure of the overall burden on individuals and on society as a whole [1], and a more accurate reflection of the social and eco-nomic impact of cancer than that provided by crude incidence rates or mortality data [15]. Moreover, a valid estimation of LE and EYLL in cancer patients is crucial for the outcome assessment of effectiveness for cancer management and resource allocation of health services [3,16,17].
Using the data from the National Cancer Registry and vital statistics, we sought to estimate the mean
Address correspondence to: Jung-Der Wang, Institute of Occu-pational Medicine and Industrial Hygiene, College of Public Health, National Taiwan University, No.17 Xu-Zhou Road, Taipei, 100 Taiwan. E-mail: jdwang@ntu.edu.twf
10.1111/j.1524-4733.2008.00350.x
lifetime survival duration and EYLL for the major types of cancer in Taiwan. The estimates were com-pared with the results obtained from parametric sur-vival modeling.
Methods
Cancer Cohorts
The Taiwan National Cancer Registry, with a total of 425,294 patients diagnosed with 17 major cancers between 1990 and 2001, was the primary data source for this study. The anatomic sites of the 17 major cancers included the oral cavity, nasopharynx, esopha-gus, stomach, colon and rectum, liver, gallbladder and extrahepatic bile ducts, pancreas, lung, leukemia, skin, breast, cervix, ovary, prostate, bladder, and kidney and other urinary organs.
Survival in the Cancer Populations
Each cancer patient was followed through the end of 2004, and the survival status of each patient was further verified by cross-checking with the national mortality certification database maintained by Tai-wan’s Ministry of the Interior [18]. We used the Kaplan–Meier method to estimate survival function based on the follow-up data from 1990 to 2004. Extrapolation of Long-Term Survival for the Cancer Population after Follow-Up Limit
The detailed method, including the technical details and its proof, can be found in our previous articles [9,10]. The main idea of this approach is to borrow information from a reference population, of which the survival time is obtained from the available data of the national life table. Briefly, the extrapolation process comprised of three phases. First, we created a reference population of subjects whose age and sex matched with the cancer patients. The survival times of the reference population were generated from a general population with known survival times, using the Monte Carlo method. Second, we fitted a simple linear regression to the logit transform of the survival ratio between the cancer population and the reference popu-lation up to the end of the follow-up period. Finally, the estimated regression line and survival curve of the reference population was used to project a long-term survival curve beyond the follow-up limit. We pre-sented the major procedures of the method below. Survival in the Reference Population
The life tables for the general population were obtained from the national vital statistics, as published by the Department of Statistics, Ministry of the Inte-rior, Executive Yuan, Taiwan. Because the individual survival time of the subjects in a hypothetical cohort cannot be directly derived from the life table of the
general population, we used the Monte Carlo method to generate the simulated survival time of age- and sex-matched hypothetical subjects for each patient in the cancer cohorts. And the total collection of hypo-thetical subjects was used as the reference population. Then, the survival curve of the reference population is obtained by applying the Kaplan–Meier method to the simulated survival times [10].
Logit Survival Ratio Extrapolation
The semiparametric method was used to extrapolate the survival time beyond the follow-up limit of 15 years. The survival ratio between the survival func-tions of two populafunc-tions is defined by the formula:
W t S t S t ( ) = ( ) ( ) patient population reference population
Because the cancer population has a worse survival than the reference population, the value of survival ratio, W(t), initially equals 1, then gradually decreases due to disease-associated excess mortality. Because the value of W(t) is limited to the range from 0 to 1, linear regression for the temporal trend is not applicable. We therefore used the logit transformation of W(t). Furthermore, if the cancer-associated excess hazard remains constant over time, the curve of the logit of W(t) will converge to a straight line.
We then fitted a simple linear regression for the logit of W(t) from the time point, which was usually after the unstable period (e.g., initial active diagnostic or therapeutic management) to the end of the follow-up. Finally, given the least squares estimates of the inter-cept and slope parameters, αˆ and ˆβ, we projected the long-term survival curve of the patient population beyond the follow-up limits as:
ˆ
ˆ ( ˆ ˆ
S t
S t t
patient population
reference population exp )
( ) =
( ) α β+
1
1 exp(+ α βtˆ +ˆ ). The standard error of survival estimates was obtained through a bootstrap method by implementing the extrapolation process with data simulated by repeat-edly sampling techniques with replacement from a real data set 300 times [9,10,14]. To facilitate the compu-tation, we developed a software program, MC-QAS, which was built in the R statistical package, which can be freely downloaded from http://www.stat.sinica.edu. tw/jshwang.
Estimation of EYLL
The average EYLL of a cancer cohort was defined in this study as the mean survival difference between the specific cancer cohort and an age- and sex-matched reference population. In other words, the average EYLL was the difference in the area between the mean survival curves of the cancer and reference
popula-tions. This parameter provides us with a measure of the burden of cancer on individual patients and yields an estimation of how much a patient’s life is likely to be shortened by cancer [16]. The average EYLL was then multiplied by the total annual incidences of cancer for each cancer site in 1 year to obtain the subtotal of EYLL for the year; clearly, the subtotal of EYLL can be regarded as an indicator of the total burden of cancer on society as a whole [1].
Validation of the Monte Carlo Extrapolation and Comparison with the Parametric Method
Empirical cancer data from the National Cancer Reg-istry provided us with an opportunity to validate actual performance. Thus, a selected subcohort of patients diagnosed between 1990 and 1996 with those cancers of interest to this study was created for each cancer site. It was assumed that the cohorts were only followed up until the end of 1996, or for a period of 7 years. We extrapolated the data through the end of 2004 using both the Monte Carlo method and the Weibull model for comparison. For every subcohort that was followed up until the end of 2004, the Kaplan–Meier method was calculated as the “gold standard” to determine the accuracy. The relative biases for each cancer site were also computed to compare the differences in values between the Kaplan– Meier estimates and the two extrapolation methods.
Results
LE and EYLL
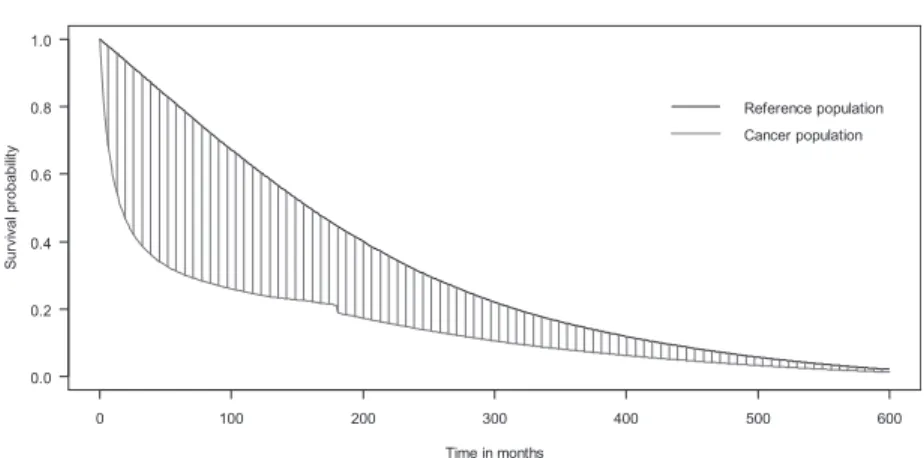
The 15-year follow-up data were used to extrapolate the lifetime survival time to the 50th year after diag-nosis for the estimation of the LE. The Kaplan–Meier estimate was applied to estimate the mean survival time in the 15-year follow-up. The LE and EYLL for the 17 major cancer sites are summarized in Table 1. The censoring rates for the survival analysis were between 8% and 67% by the end of the 15-year follow-up period. In terms of population sizes, liver cancer had the largest cohort, while gallbladder and extrahepatic bile duct cancers had the smallest cohort. We found that the cohorts with the longest LE were breast (20.01 years), cervical (19.77 years), and ovarian cancers (17.71 years), and the shortest LE were pancreatic (2.81 years), lung (3.09 years), and liver cancers (3.45 years). The estimated average EYLL of a cancer cohort is the difference between the areas of estimated survival curves for the reference population and the cancer cohort. As an example shown in Fig. 1, estimated EYLL for a gastric cancer patient was 8.8 years. The cohorts with the largest average estimated EYLL were leukemia (19.34 years) and cancers of the liver (15.61 years) and nasopharynx (14.79 years).
Following multiplication by the total annual inci-dences for the different types of cancer in 2001, the
greatest health impacts on society, in terms of the subtotal of EYLL for the year, were cancers of the liver, lung, and oral cavity. Furthermore, the average expected life span could be obtained simultaneously after the consideration of the mean age at diagnosis and the mean LE after diagnosis. The patterns of the average expected life spans for the different types of cancer were different from those of LE using our method and are listed in Fig. 2. Leukemia (53.41 years), and cancers of the nasopharynx (62.19 years) and oral cavity (63.38 years), had the shortest average expected life span.
Validity of Extrapolation
The cancer cohorts established during the 7 years between 1990 and 1996 were extrapolated to an addi-tional 8 years and were then compared with actual survival estimated with the Kaplan–Meier method using the complete 15 years of follow-up, from 1990 to 2004. The calculations of the relative biases for the two methods are summarized in Table 2. The censor-ing rates at the end of the first 7-year follow-up period ranged between 21% and 81%. The absolute values of the relative biases for the Monte Carlo method ranged between 0.76% and 14.41% after 8 years of extrapo-lation; these values were generally much smaller than those obtained under the Weibull model, with the notable exceptions of skin and prostate cancers. Nev-ertheless, the standard errors for the Weibull model were generally smaller than those for the Monte Carlo method. There are some variations or differences between the Kaplan–Meier estimates in Tables 1 and 2 because they were calculated from two different periods of cohorts of cancer, namely, those diagnosed during 1990 to 2001 and 1990 to 1996, respectively.
Discussion
The method adopted for this study incorporated data simultaneously from the mortality patterns of the general population based on vital statistics and actual experience of the cancer patients, which would be better than potential years of life lost, assuming an arbitrarily chosen potential limit of life such as 65 years [19]. Moreover, the method can estimate the lifetime survival for different types of cancer with a reasonable accuracy after about 7 years of follow-up, as shown in Table 2.
Some researchers assume that the survival of cancer patients at the end of follow-up is similar to that in the general population [2], such as children with acute lymphoblastic leukemia, who may enjoy an event-free survival for longer than 10 years [20]. In fact, the above assumption can be regarded as a special case in this method with the excess hazard presumed to be 0 after an initial period of treatment, and it was also applicable in other diseases, such as long-term survival
Ta b le 1 Fr equency distributions and sur vival estimates fo r differ ent types of major cancers after 15 years of follo w-up––estimates of mean sur vival time in 15-y ear follo w-up sur vival using Ka plan–Meier (K–M) estimate Cancer site Cohor t size Mean age at diagnosis (SD) Censoring rate (%) 15-y ear sur vival based on K–M estimate Lif etime sur vival based on MC method (SE) A verage EYLL based on MC method (SE) Subtotal of EYLL based on MC method Pancr eas 7,931 65.6 (12.7) 8.28 1.75 (0.05) 2.81 (0.17) 12.87 12,769 Lung 58,773 66.6 (11.7) 9.97 2.20 (0.02) 3.09 (0.07) 11.79 79,584 Liv er 68,585 60.4 (13.5) 13.28 2.63 (0.02) 3.45 (0.08) 15.61 133,282 Esophagus 9,710 63.0 (12.1) 11.28 2.42 (0.04) 3.54 (0.20) 13.25 16,279 Gallblad der and extrahepatic bile duct 5,097 66.5 (12.0) 17.72 3.37 (0.07) 4.98 (0.20) 10.36 6,312 Stomach 35,477 64.9 (13.6) 26.80 4.78 (0.03) 7.51 (0.14) 8.80 30,794 Pr ostate 14,288 73.1 (8.0) 44.65 7.05 (0.06) 8.17 (0.13) 1.72 3,433 Oral ca vity 26,681 53.8 (12.9) 35.94 5.96 (0.04) 9.58 (0.61) 14.00 49,671 Colon and rectum 60,789 63.8 (13.7) 41.94 7.00 (0.03) 10.86 (0.11) 6.36 45,905 Kidne y and other urinar y organs 11,671 62.7 (15.1) 43.68 7.07 (0.07) 10.97 (0.85) 6.74 10,120 Blad der 15,092 66.7 (12.6) 46.93 7.71 (0.05) 10.99 (0.20) 3.83 6,727 Leuk emia 9,224 41.8 (25.5) 28.49 4.97 (0.08) 11.61 (0.94) 19.34 18,602 Nasophar ynx 15,231 49.6 (13.4) 43.06 7.42 (0.05) 12.59 (0.74) 14.79 20,271 Skin 14,005 63.3 (16.9) 62.18 9.71 (0.05) 16.16 (0.22) 1.59 2,873 Ovar y 6,436 49.3 (17.0) 52.59 8.46 (0.11) 17.71 (0.80) 11.91 8,775 Cer vix uteri 29,636 54.7 (13.8) 63.92 10.21 (0.03) 19.77 (0.30) 6.18 14,978 Br east 36,668 50.5 (12.5) 66.94 10.41 (0.03) 20.01 (0.80) 9.35 43,633 Extra polation to lif etime sur vival and EYLL was based on the Monte Carlo (MC) method pr ojected fr om 15 years to 50 years. SD ,standar d d e viation; SE, standar d e rr or ;EYLL, expected years of lif e lost.
after a head injury [21]. There are other methods dealing with censoring, which can be applied under different circumstances [22,23]. Nevertheless, the method proposed by us directly extrapolates the unfin-ished survival curves to lifetime and seems the most straightforward both in concept and in actual clinical practice for following up cancer cohorts, as our method only relies on the availability of vital statistics of the general population and an assumption of con-sistent premature mortality throughout lifetime.
In cost-effectiveness analyses, the Markov model is often applied to estimate LE, which uses a finite number
of the hypothetical cohort to simulate effectiveness (e.g., survival) and cost, but requires external data and/or methods to facilitate the sensitivity analysis on some assumptions. Our method, which is based on the actual cohort data of follow-up, could be used to vali-date the results from the Markov model [9]. In fact, the result of the long-term survival (40 years) for breast cancer cohort in this study is similar with those based on a simulation from the Markov model, with and without treatment of trastuzumab [24], as shown in Fig. 3.
The method may also be applied in clinical trials, in which at least two arms or cohorts of patients, say,
0 100 200 300 400 500 600 0.0 0.2 0.4 0.6 0.8 1.0 Time in months S u rv iv al pr obabi lit y
Mean Survival Difference
Reference population Cancer population
Figure 1 Mean survival difference between
gastric cancer population and reference popu-lation after 50 years of extrapopopu-lation.
0 10 20 30 40 50 60 70 80 90 Leu kemi a Nas opha rynx Oral c avity Liver Esoph agus Ova ry Pan crea s Lung Brea st Gall blad der & ex trahe pat ic bil e duc t Stom ach Kidney & other uri nar y or gans Cer vix u teri Colo n & rec tum Blad der Skin Prost ate Cancer type Av erage ex pec ted li fe span in y ears
Mean age at diagnosis Life expectancy after diagnosis
treatment versus placebo, are followed for a period of time to observe their actual survival and qua-lity of life. Thus, we would obtain two survival ratios, S(t|treatment)/S(t|reference) and S(t|placebo)/ S(t|reference), at a different time t and they both could be extrapolated to lifetime with confidence intervals obtained through the bootstrap method for compari-son [10]. Our method does not need to classify the study disease into a limited number of health states to obtain the transition probabilities. Instead, the quality of life data collected during clinical trials at each time t can provide the mean value that can be directly multiplied with the survival probability and summed up to obtain the quality-adjusted LE [12].
Based on our results, the methodology presented here could be more useful for cancer or some diseases in which survival is longer or prognoses are better, or there is a high censoring rate at the end of the follow-up, such as cancers of the breast, cervix, ovary, and nasopharynx. Furthermore, the incidence rates of the cancers of the oral cavity and breast have increased recently in Taiwan [25] and the ages at diagnosis are about 50 years of age, which is earlier than other common cancers, as shown in Fig. 2. From the results of this study, oral cancer had a large average EYLL (14.00 years) and subtotal of EYLL (49,671 years), and breast cancer also had a large subtotal of EYLL (43,633 years). Thus, we recommend that policymak-Table 2 Estimates of mean survival years in 15 years of follow-up using the Monte Carlo method and the Weibull model approaches on the first 7 years of follow-up data with high censored rates were compared with the Kaplan–Meier estimates based on 15 years of follow-up
Cancer site
15-year follow-up Extrapolation based on the first 7-year follow-up
Kaplan–Meier estimate Monte Carlo method Weibull model
Censoring rate (%)
Estimates Estimates SE Relative bias (%) Estimates SE Relative bias (%)
Colon and rectum 7.12 7.06 0.14 -0.76 6.40 0.06 -10.07 62.73
Leukemia 4.99 5.05 0.17 1.19 4.10 0.11 -17.83 43.41 Skin 9.74 9.85 0.28 1.21 9.71 0.14 -0.26 80.53 Bladder 7.76 7.66 0.27 -1.25 7.38 0.12 -4.81 68.21 Stomach 4.82 4.93 0.08 2.19 3.78 0.05 -21.61 42.85 Cervix uteri 10.10 9.82 0.16 -2.82 9.01 0.09 -10.84 77.69 Breast 10.03 9.73 0.18 -2.99 8.93 0.10 -10.98 80.52 Esophagus 2.59 2.68 0.1 3.58 1.73 0.05 -33.02 27.91 Ovary 8.38 8.70 0.25 3.89 7.47 0.18 -10.87 67.34
Kidney and other urinary organs
7.10 6.75 0.24 -4.92 6.51 0.14 -8.33 62.34
Oral cavity 5.68 5.38 0.17 -5.38 4.43 0.07 -21.94 54.28
Prostate 6.73 6.30 0.32 -6.44 6.37 0.14 -5.28 68.85
Gallbladder and extrahepatic bile duct
3.59 3.82 0.19 6.57 2.63 0.11 -26.77 33.62
Pancreas 2.09 2.24 0.13 6.73 1.23 0.04 -41.21 20.89
Nasopharynx 7.10 6.55 0.24 -7.71 5.86 0.10 -17.41 62.52
Lung 2.38 2.57 0.05 8.00 1.62 0.02 -31.77 25.18
Liver 2.48 2.83 0.08 14.41 1.81 0.02 -26.70 27.58
The censoring rates were computed at the end of the first 7-year follow-up period. SE, standard error.
0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 0 5 10 15 20 25 30 35 40 45 50 Years Surv ival pr obabi lity
Monte Carlo method Markov model: with trastuzumab Markov model: without trastuzumab
Figure 3 Long-term survival curve for patients with breast cancer (including all stages) in Taiwan using the Monte Carlo method, which was compared with published [24] survival curves of patients with early breast cancer simulated under the Markov model with and without treatment of trastuzumab.
ers place the prevention of major causes of oral cancer, such as betel quid chewing, tobacco usage, and excess consumption of alcohol [26], at a higher priority. The screening program for breast cancer should be targeted to an earlier age group, such as the 40 to 49 years of age group, to possibly save more life years.
Although we have used the best national data cur-rently available in Taiwan, the study still had some limitations that need to be addressed before wide adoption for outcome evaluation. The first limitation of the method was the uncertainty regarding the sta-bility of excess hazard in the extrapolation period. Because the cancer-related excess hazard is unlikely to be exactly constant throughout the extrapolation period, a certain degree of prediction error is unavoid-able [10]. In spite of the uncertainty, our semiparamet-ric method avoids the large deviations in long-term projections seen with the parametric model, such as under the Weibull distribution, with the advantage of an input of information from the life table of the background general population. Even if the assump-tion of a constant excess hazard between the cancer and reference populations may not hold, the method used the median of slopes near the end of the follow-up, which was generally the least biased in the extrapo-lation [9,10,14]. Excluding those cancers with a low censoring rate, i.e., <25%, for which there is usually less need for a long period of extrapolation, the rela-tive biases were usually<5.0%. The second limitation was that the extrapolation method required an assumption of premature mortality, which does not hold for skin cancer, and is therefore, slightly less accurate. Third, the lifetime extrapolation is based on current and prior experiences, such as life tables; however, it is clear that such a method could easily underestimate the actual survival of future cancer populations because it does not consider the active development and adoption of newer technologies for cancer diagnosis and management. Thus, our estima-tion of lifetime survival of cancer patients may be a conservative one, while the EYLL could easily be over-estimated. Finally, because LE is also a function of comorbidity, disability, and cancer stage, in addition to age and cancer type [27], the current estimate provides only a rough estimation of the average loss of LE. It may be possible, in future studies, to stratify the cancer cohorts into subcohorts based on more available data on the stage of cancer, and/or other comorbidities, to improve the accuracy of the survival estimates. For example, because more than one-half of the patients with prostate cancer in Taiwan were detected at advanced ages and earlier stages based on the Cancer Registry Annual Report (1999–2004) [28], the fre-quent comorbidity and mixing of different stages in the same cohort may make the extrapolation of survival rates less accurate. Furthermore, if the data regarding quality of life at each duration to date for cancer could
be collected, they could also be integrated with the survival curves to obtain the quality-adjusted LE [10,12,21,29], which could serve as the basis for outcome evaluation in cost–utility analysis.
Regarding the choice between semiparametric and parametric methods, one disadvantage of semiparamet-ric methods is that they are less efficient than parametsemiparamet-ric methods. Therefore, the standard errors of the Weibull model were generally smaller than those of the Monte Carlo method, as shown in Table 2, indicating a trade-off between bias and efficiency for the two methods. In fact, the Monte Carlo method is generally less biased except for two cancers, skin and prostate, of which the assumption of premature mortality might be violated. Moreover, there is a general tendency of underestima-tion for the Weibull model, as the relative biases are all accompanied with a negative sign. In general, one would consider the efficiency of estimation only if the relative biases for different methods are very close or similar. Thus, we recommend the Monte Carlo method whenever the assumption of premature mortality seems to hold in extrapolation of survival curves for cancer.
In conclusion, by incorporating information from the life table of the general population, estimation using the logit survival ratio extrapolation method is a robust approach to calculating the lifetime survival of cancer patients, as based on national data. The estimation of average EYLL provides a quick overview of an indi-vidual’s LE after diagnosis and potential loss of life due to a specific type of cancer, as well as being helpful in the outcome evaluation for cancer treatment and preven-tion. To communicate the cancer risk with a lay person, this would seem to be much more understandable than simply giving the 5-year survival rate or cumulative survival and could also be used directly to empower people to engage in proactive prevention. The subtotal of EYLL represents the greatest quantity that society could possibly save by the prevention of the cancer. In the future, the method could be integrated with quality of life data for the comparative assessment of the out-comes of cancer patients under different treatment pro-tocols or different health-care plans for people to judge the competitiveness [11,12,30].
Supplementary material for this article can be found on http://www.ispor.org/publications/value/ViHsupplementary. asp.
Source of financial support: Two grants, one from the Bureau of Health Promotion at the Department of Health, Taiwan (DOH94-HP-1801) and another from the National Health Research Institute, Taiwan (NHRI-EX94–9204PP). References
1 Brown ML, Lipscomb J, Snyder C. The burden of illness of cancer: economic cost and quality of life. Annu Rev Public Health 2001;22:91–113.
2 Viscomi S, Pastore G, Dama E, et al. Life expectancy as an indicator of outcome in follow-up of population-based cancer registries: the example of childhood leukemia. Ann Oncol 2006;17:167–71. 3 Messori A, Trippoli S. A new method for expressing
survival and life expectancy in lifetime cost-effectiveness studies that evaluate cancer patients (review). Oncol Rep 1999;6:1135–41.
4 Gaitatzis A, Johnson AL, Chadwick DW, et al. Life expectancy in people with newly diagnosed epilepsy. Brain 2004;127:2427–32.
5 Mark DB, Hlatky MA, Califf RM, et al. Cost effec-tiveness of thrombolytic therapy with tissue plasmi-nogen activator as compared with streptokinase for acute myocardial infarction. N Engl J Med 1995; 332:1418–24.
6 Messori A, Messori A. Survival curve fitting using the Gompertz function: a methodology for conducting cost-effectiveness analyses on mortality data. Comput Methods Programs Biomed 1997;52:157–64. 7 Beck RJ, Kassirer JP, Pauker SG. A convenient
approximation of life expectancy (The ‘DEALE’). I. Validation of the method. Am J Med 1982;73:883– 8.
8 Haybittle JL. Life expectancy as a measurement of the benefit shown by clinical trials of treatment for early breast cancer. Clin Oncol (R Coll Radiol) 1998;10: 92–4.
9 Fang CT, Chang YY, Hsu HM, et al. Life expectancy of patients with newly-diagnosed HIV infection in the era of highly active antiretroviral therapy. QJM-An Int J Med 2007;100:97–105.
10 Hwang JS, Wang JD. Monte Carlo estimation of extrapolation of quality-adjusted survival for follow-up studies. Stat Med 1999;18:1627–40.
11 Hsu C, Wang JD, Hwng JS, et al. Survival-weighted health profile for long-term survivors of acute myel-ogenous leukemia. Qual Life Res 2003;12:503–17. 12 Hwang JS, Wang JD. Integrating health profile with
survival for quality of life assessment. Qual Life Res 2004;13:1–10.
13 Ho WL, Lin KH, Wang JD, et al. Financial burden of national health insurance for treating patients with transfusion-dependent thalassemia in Taiwan. Bone Marrow Transplant 2006;37:569–74.
14 Ho JJ, Hwang JS, Wang JD. Life-expectancy estima-tions and the determinants of survival after 15 years of follow-up for 81,249 workers with permanent occupational disabilities. Scand J Work Environ Health 2006;32:91–8.
15 Mettlin C. Trends in years of life lost to cancer: 1970– 1985. CA Cancer J Clin 1989;39:33–9.
16 Burnet NG, Jefferies SJ, Benson RJ, et al. Years of life lost (YLL) from cancer is an important measure of population burden—and should be considered when
allocating research funds. Br J Cancer 2005;92: 241–5.
17 Wright JC, Weinstein MC. Gains in life expectancy from medical interventions—standardizing data on outcomes. N Engl J Med 1998;339:380–6.
18 Hsieh GY, Chen PC, Wang JD. Verification and cor-rection of error for death registration data of the Department of Health R.O.C. between 1980 and 1997. Taiwan J Public Health 2002;21:329–38 (in Chinese).
19 Murray CJL, Mathers CD, Salomon JA, et al. Health gaps: an overview and critical appraisal. In: Murray CJL, Salomon JA, Mathers CD, et al., eds. Summary Measures of Population Health: Concepts, Ethics, Measurement and Applications. Geneva: World Health Organization, 2002.
20 Pui CH, Cheng C, Leung W, et al. Extended follow-up of long term survivors of childhood acute lymphoblas-tic leukaemia. N Engl J Med 2003;349:640–9. 21 Tsauo JY, Hwang JS, Chiu WT, et al. Estimation of
expected utility gained from the helmet law in Taiwan by quality-adjusted survival time. Accid Anal Prev 1999;31:253–63.
22 Lin CC, Johnson NJ. Decomposition of life expect-ancy and expected life-years lost by disease. Stat Med 2006;25:1922–36.
23 Baser O, Gardiner JC, Bradley CJ, et al. Longitudinal analysis of censored medical cost data. Health Econ 2006;15:513–25.
24 Millar JA, Millward MJ. Cost effectiveness of trastu-zumab in the adjuvant treatment of early breast cancer: a lifetime model. Pharmacoeconomics 2007; 25:429–42.
25 Bureau of Health Promotion. Cancer Registry Annual Report, 1999–2002. Taipei: Bureau of Health Promo-tion, Department of Health, Taiwan, 2002–2005 (in Chinese).
26 Ko YC, Huang YL, Lee CH, et al. Betel quid chewing, cigarette smoking and alcohol consumption related to oral cancer in Taiwan. J Oral Pathol Med 1995; 24:450–3.
27 Repetto L, Comandini D, Mammoliti S. Life expect-ancy, comorbidity and quality of life: the treatment equation in the older cancer patients. Crit Rev Oncol Hematol 2001;37:147–52.
28 Bureau of Health Promotion. Cancer Registry Annual Report, 1999–2004. Taipei: Bureau of Health Promo-tion, Department of Health, Taiwan, 2002–2007 (in Chinese).
29 Wang JD. Study design. In: Wang JD, ed. Basic Prin-ciples and Practical Applications in Epidemiological Research. Singapore: World Scientific, 2002. 30 Porter ME, Teisberg EO. Redefining Health Care.
Creating Value-Based Competition on Results.
![Figure 3 Long-term survival curve for patients with breast cancer (including all stages) in Taiwan using the Monte Carlo method, which was compared with published [24] survival curves of patients with early breast cancer simulated under the Markov model wi](https://thumb-ap.123doks.com/thumbv2/9libinfo/8693294.198532/6.891.98.797.155.464/survival-patients-including-compared-published-survival-patients-simulated.webp)