SHORT COMMUNICATION
446
†To whom correspondence should be addressed.
E-mail: yuchih.lin0214@msa.hinet.net
Application of physical vapor deposition process to modify activated
carbon fibers for ozone reduction
Yu-Chih Lin†, Chung-Liang Chang, Tser-Sheng Lin*, Hsunling Bai**, Ming-Gu Yan**,
Fu-Hsiang Ko***, Chia-Tien Wu*** and Cheng-Hsiung Huang
Department of Environmental Engineering and Health, Yuanpei University, Hsinchu City, 300, Taiwan *Department of Safety, Health and Environmental Engineering, National United University, MiaoLi, Taiwan
**Institute of Environmental Engineering, National Chiao Tung University, Hsinchu City, Taiwan ***Institute of nanotechnology, National Chiao Tung University Hsinchu City, Taiwan
(Received 13 June 2007 • accepted 17 September 2007)
Abstract−This study utilized the activated carbon fiber (ACF) modified with metal catalyst via physical vapor deposi-tion (PVD) process (ACF/PVD) to diminish ozone. Furthermore, the ozone removal efficiency of ACF/PVD was com-pared with that of original ACF and ACF modified with metal catalyst via impregnation process (ACF/impregna-tion). In addition to the kinds of coated metal and the inlet ozone concentrations, the effects of the coating thickness and the reaction temperature on ACF/PVD for ozone removal were also examined. The results indicate that the ozone removal efficiency of ACF/PVD is better than that of original ACF and ACF/impregnation. The ozone removal effi-ciency of different metal-coated ACF/PVD in the superior order is gold (Au), and manganese (Mn). The increase of Au-coated thickness (3 nm to 80 nm) on ACF/PVD will enhance the ozone removal. However, when the Mn-coated thickness on ACF/PVD is larger than 15 nm, the ozone removal efficiency displays a declining trend. Furthermore, a higher reaction temperature will result in a better ozone removal of ACF/PVD and the original ACF.
Key words: Ozone, Activated Carbon, Physical Vapor Deposition, Adsorption
INTRODUCTION
The ozone in the ozonosphere benefits the environment, because it can adsorb UV light and screen out other harmful rays. Besides, ozone has also been verified as a superior oxidant that could assist in the control process of pollutants [1-5]. However, a high concen-tration of ozone at ground level impairs the health of individuals, and may cause monetary losses such as damage to products of rub-ber and plastics. In addition, ozone might cause headaches, laryn-gitis, asthma aggravation and damage to the respiratory system in the human body [6]. Thus, the USA OSHA has established a per-missible limit of 0.1 ppm for ozone in order to prevent workers from potential hazard. Forecasting the ozone concentration of atmo-sphere could remind asthma patients to take precautions and avoid the potential damage. Therefore, Oh et al. [7] developed two tech-niques to predict the formation of ozone, including schemes-time series analysis and parameter estimation method. Their results in-dicated that a prediction scheme based on the parameter estimation method gives a reasonable accuracy compared to the real data. With modern office equipment, such as photostats and the laser printers etc., can release ozone and may result in a maximum ozone con-centration of 2,000 ppb in an enclosed office [8]. Thus, it is of cru-cial concern to protect the welfare of the public from the increasing ozone concentration in our environment. The catalysts consisting of noble and transition metals have been verified for removing ozone efficiently [9]. Recently, activated carbon fiber (ACF) has been widely used as the catalyst carrier or filter in pollution control. In addition, ACF [8] and activated carbon (AC) [10] can remove high
concen-trations of ozone (200-1,600 ppm) efficiently. Both metal and ACF have the catalytic and adsorptive characteristics for pollution remov-al. The composite of those elements, synthesized by impregnation process, could improve the pollution removal efficiency or extend their lifetime, etc. [11,12]. Synthesis of metal/ACF could be sim-ply achieved through the impregnation process. Takeuchi and Itoh [13] examined ozone removal by ACF impregnated with Ag, Cu, and Na reagents. However, the drawbacks of this process include the difficulty in controlling the amount and the distribution of the metal coating on ACF etc. These drawbacks will produce unstable quality of the composites, or consume the precursor resource [14,15]. Thus, physical vapor deposition (PVD) was developed to overcome those problems occurring during the impregnation process, and uti-lized in catalyst synthesizing. Sasaki et al. [16] employed the PVD to coat palladium onto SiO2, Al2O3, and ZrO2, for decomposing meth-anol. Gaffney et al. [17] also anchored Mo, V, Nb, Te, and the metal alloy of Mo-V-Nb-Te on wafer and honeycomb cordierites via the PVD process. Such synthesized composite was utilized for inverting propylene to acrylic acid. Apart from the aforementioned literature, no information is available on synthesizing composite catalyst via the PVD process, and coating active metal on ACF for decomposing pollutant.
Therefore, this study utilizes the ACF modified with metal cata-lyst, via the PVD process (ACF/PVD) to diminish ozone. The coated metals are chosen as gold (Au), and manganese (Mn), which are the common noble and transition metals for catalysis, respectively. Furthermore, the ozone removal efficiency of ACF/PVD is com-pared with that of the original ACF, and the ACF modified with metal catalyst via the impregnation process (ACF/impregnation). In addition to the kinds of coated metal, the inlet ozone concentra-tions, the reaction temperature, and the effects of the coating
thick-ness on ACF/PVD for ozone removal were also examined.
EXPERIMENTAL METHODS
1. Preparation for Activated Carbon Fiber Modified with Me-tal CaMe-talyst
In this study, both the PVD and the impregnation process were used to modify ACF (AW1101 KoTHmex, Taiwan Carbon Com-pany, Taiwan) with metal catalyst. The PVD system (ULVAC-R/H, Tokyo Japan) consisted of an evaporator chamber, a current trans-former, and a vacuum pump. The PVD process processes were as follows: 1) adjusting the current to the required high temperature, greater than the melting point of the chosen metal, and 2) promot-ing Au and Mn precursors (99.99% purity, Summit-Tech Resource Corp, Taiwan) to evaporate the metal, after the evaporator chamber was vacuumed (pressure less than 10−6torr). The evaporative metal would then diffuse on the ACF surface to form thin-film. The com-posite materials (metal-ACF/PVD) were obtained after the temper-ature, and pressure of the PVD system achieved normal condition. The thickness of metal-coated layer was defined by the quartz crys-tal microbalance (QCM) method [18].
The impregnation process was also utilized to prepare the Mn-ACF composite (Mn-Mn-ACF/impregnation) by impregnating Mn-ACF with an aqueous solution of 0.027 M Mn(NO3)2·4H2O (analytical grade, Merck, Germany). The ACF and the impregnating aqueous solu-tion were placed in a custom-made furnace, equipped with a rotary device for 12 hours, at a rotation speed of 5 rpm and a temperature of 120oC. The impregnated ACF was dried in air at 110oC for 12 hours, and later calcined at 400oC for another hour [11].
2. Measurement of Ozone Removal Efficiency
Ozone decomposition reactions were carried out in a flow-type reactor. The inner diameter and length of reactor were 16 mm and 150 mm, respectively. The original ACF, the metal-ACF/PVD, or the metal-ACF/impregnation (0.03 g) was packed in the reactor for testing, respectively. The temperature and pressure of all experiments were controlled at 25oC and 1 atm. The dry-grade air (80% N
2 and 20% O2) cylinder was used to supply air streams, and their flow rates were regulated and monitored by mass flow controllers (Model 5850, Brooks, USA). This study used a dielectric barrier discharge (DBD) system [19], and a UV-typed ozone generator to generate high con-centration (>100 ppm), and low concon-centration (<10 ppm) ozone, respectively. The concentration of ozone in the inlet and outlet gas flows was analyzed by a Fourier transformation infrared spectrom-eter (FTIR, BRUKER, VECTOR 22, USA) (ozone range: 100-2,000 ppm), equipped with a gas cell device (Infrared Analysis Inc., model 2.4-P. A., USA), or by an ozone analyzer (Model 49C, Thermo Environmental Instruments, USA) (ozone range: 1-100 ppm) de-pendent on its level. The ozone removal efficiency was defined as
η=(Cinlet−Coutlet/Cinlet)×100%, where Cinlet and Coutlet were the
concen-trations of inlet and outlet ozone, respectively. The standard devia-tion of the repeated experiment was around 3% in terms of the ozone removal efficiency and packed weight of ACFs.
RESULTS AND DISCUSSION
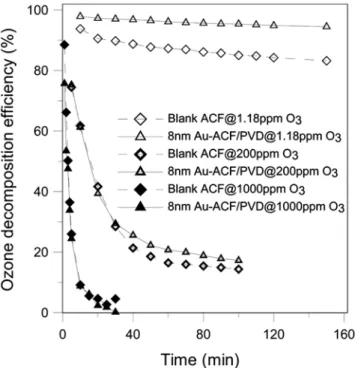
Fig. 1 illustrates the comparisons of processing efficiency under different concentrations, between the original, and the modified ACF
coated with metal Au 8 nm via the PVD process (Au-ACF/PVD) at a total flow rate of 3 lpm.
When the ozone concentration was 1.18 ppm, the efficiency of the 8 nm Au-ACF/PVD was significantly greater than that of the original fiber. After 150 minutes, the Au-ACF/PVD still maintained high decomposition efficiency beyond 95%. By contrast, the origi-nal ACF maintained merely around 85%. However, when the ozone concentration was elevated to 200 ppm, the efficiencies of the orig-inal and the Au-ACF/PVD were not significantly different in the first 30 minutes. After that, the efficiency of the 8 nm Au-ACF/ PVD was 3-5% better than that of the original ACF. If the ozone concentration was elevated to 1,000 ppm, the loading of ozone on the catalyst materials in the reactor would exceed the capacity of the catalyst tested. As a result, both the processing efficiencies of the original and the Au-ACF/PVD dropped below 10% in the first 10 minutes with negligible differences in their performance. Ac-cording to the literature [8], with the use of AC and metal catalyst, the reactions between the ozone, AC, and the catalyst would pro-duce oxidized substances on the surface. Furthermore, the efficiency of the composite catalyst would progressively decrease due to the poisoning of the surface layer. At high concentration level, the ozone removal rate was close to 0% after a short period of the beginning of experiments. These results agree with the observation of Sub-rahmanyamet al. [9] that showed the ACF would collapse in four minutes when the ACF was treated with 1,600 ppm ozone.
From the appearance of both blank ACF and Mn-ACF/impreg-nation before ozone exposure illustrated in Fig. 2, the structure of the Mn-ACF/impregnation was obviously deteriorated (Fig. 2(b)). However, the Mn-ACF/PVD (Fig. 2(c)) showed no signs of deteri-oration, and the thin film of metal coating on the surface was visible to the human eye.
Fig. 3 shows the differences in ozone removal efficiency, under
Fig. 1. Effect of inlet concentration on decomposing ozone efficiency (ozone flowrate: 3 lpm, ACFs packed weight: 0.03 g).
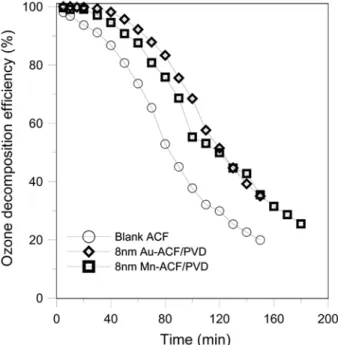
the same conditions, between the Mn-ACF/PVD, and Mn-ACF/ impregnation. The ozone inlet concentration was 200 ppm, and the total flow rate was 3 lpm. The Mn-ACF/PVD posed high efficiency,
and dropped down to 22% only after 60 minutes. Then, the perfor-mance maintained for another 40 minutes. On the contrary, the Mn-ACF/impregnation underperformed in comparison to the Mn-ACF/ PVD, and maintained efficiency of 10-20% only. The poor perfor-mance of the Mn-ACF/impregnation on ozone decomposition was ascribed to the deteriorated structure of Mn-ACF/impregnation.
Fig. 2. Appearance of blank ACF, ACF/impregnation and ACF/ PVD before O3 exposure.
Fig. 3. Effects of impregnation and PVD process on decomposing ozone efficiency (ozone flowrate: 1 lpm, ozone concentra-tion: 200 ppm, ACFs packed weight: 0.05 g).
Fig. 4. Effects of metal-coated varieties via PVD on ozone removal efficiency (ozone flowrate: 1 lpm, ozone concentration: 200 ppm, ACFs packed weight: 0.05 g).
The results of the experiments with a flow rate of 3 lpm cannot distinguish the different characteristics of the original ACF and ACF/ PVD on ozone decomposition, probably because the amount of ozone introduced into the testing system much exceeded the capacity of the packed ACF and ACF/PVD, or a less retention period in the reactor. Therefore, the flow rate and tested ACFs were adjusted to
1 lpm and 0.05 g, respectively, in the later experiments.
Fig. 4 demonstrates the effects of the different metal catalysts, such as Au and Mn, via the PVD process on the ozone removal effi-ciency under the same conditions. The ozone inlet concentration was 200 ppm, the total flow rate was 1 lpm, and the ACF/PVD packed weight was 0.05 g. As shown (Fig. 4), the Au 8 nm and the Mn 8 nm ACFs outperformed the original ACF on processing effi-ciency. Under the operating conditions, both ozone decomposition and anti-decay characteristics of Au-ACF were superior to those of Mn-ACF, and the above phenomenon tallied with the result of Hao et al. [20]. The performance of Au catalyst in ozone removal was considerably better than that of transition metal catalyst, due to the active phase structure and reaction mechanism of Au.
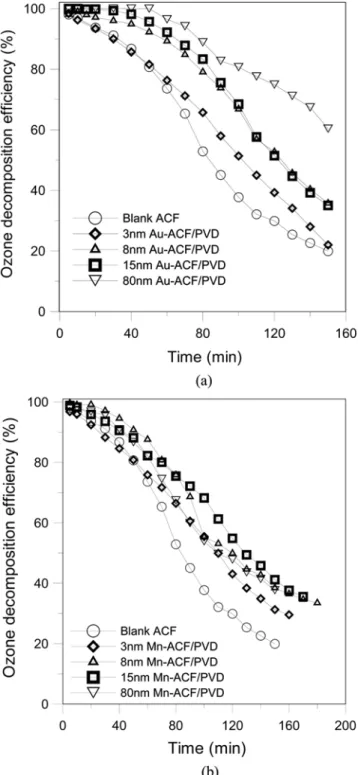
Fig. 5 illustrates the efficiencies of Au-ACF/PVD and Mn-ACF/ PVD with various thickness of metal film for ozone removal. (The ozone inlet concentration is 200 ppm, the total flow rate is 1 lpm, and the ACF/PVD packed weight is 0.05 g). As shown (Fig. 5), the increase of the thickness of Au film on ACF (from 3 to 80 nm) would elevate the decomposition efficiency of ozone in direct pro-portion. By contrast, the thickness of Mn film on ACF did not sig-nificantly influence its ozone decomposition efficiency. What is note-worthy is that the ozone decomposition efficiency of the 80 nm Mn-ACF/PVD was lower than that of 8 and 15 nm Mn-ACF/PVDs; this phenomenon may be a result of the 80 nm Mn film on ACF inhibiting the activated surface area of ACF.
Table 1 indicates the surface area of tested metal-ACF/PVD meas-ured by a surface area analyzer (Micromeritics model ASAP2020, USA); every surface area of coated metal-ACF/PVD was larger than that of the blank ACF, 966 m2/g. Although the difference be-tween the surface areas of coated ACF was not conspicuous, the surface area was inferred to be the one of influence factors on the ozone removal. The increasing surface area of Au-ACF/PVD, in proportion to the coated thickness, contributed to the reaction of ozone decomposition on ACF. When the Mn coated thickness was between 3 and 15 nm, the surface area of Mn-ACF/PVD was in-creased with the thickness. However, when the Mn coated thick-ness was up to 80 nm, the surface area turned to decline. From the data of Table 1, for the above condition it was presumed that Mn coated thickness of up to 80 nm covers the activated surface area, and causes the ozone decomposition decay.
There should be other influence factors existing, which are the reaction mechanisms, the thin-film characteristics of coated metal, and so on. However, those factors could not be confirmed at pres-ent, and they will be investigated in the future studies.
Fig. 6 shows the effects of reaction temperatures between 25 and 100oC on the decomposition efficiency of ozone via the original ACF and Au-ACF/PVD, respectively. The inlet flow had an ozone inlet concentration of 200 ppmv, and a flow rate of 1 lpm. The
Fig. 5. (a) Effects of metal-coated thickness via PVD on ozone re-moval efficiency (ozone flowrate: 1 lpm, ozone concentra-tion: 200 ppm, ACFs packed weight: 0.05 g). (b) Effects of metal-coated thickness via PVD on ozone removal efficiency (ozone flowrate: 1 lpm, ozone concentration: 200 ppm, ACFs packed weight: 0.05 g).
Table 1. Surface area of different metal-coated thickness via PVD on ACF Coated thickness (nm) Coated metal 3 8 15 80 Au Surface area (m2/g) 1,017 1,049 1,057 1,071 Mn 1,971 1,993 1,035 1,003
sults suggest a strong obverse correlation between decomposition efficiency and the operation temperature. The correlation may be attributed to the fact that the increasing temperature enhanced the catalysis reaction of ozone on the surface of ACF. Consequently, insufficient reactive energy could be provided at the normal atmo-spheric temperature; for the above reason, both the overall effi-ciency of blank ACF and Au-ACF/PVD falls presently. Regardless of reaction temperatures, Au-ACF/PVD exhibits better catalysis than blank ACF.
CONCLUSION
This study utilized the activated carbon fiber (ACF), modified with Au and Mn metals, via the PVD process (ACF/PVD) to di-minish ozone. The results indicate that the ozone removal effi-ciency of the ACF/PVD is better than that of the original ACF, and ACF modified with metal catalyst via impregnation process (ACF/ impregnation). The disparity observed in the efficiency of ozone removal by the ACF/impregnation is possibly a result of applying nitrate solution, and heating practice during the impregnation pro-cess. Those could potentially damage the structure of the ACF. The ozone removal efficiency of the different metal-coated ACF/PVD, in the order of superiority, is gold (Au) and manganese (Mn),
re-spectively. The increase of Au-coated thickness (3 to 80 nm) on ACF/PVD will enhance the ozone removal efficiency. However, when the Mn-coated thickness on ACF/PVD is larger than 15 nm, the ozone removal efficiency displays a declining trend. For the above condition it is presumed that Mn coated thickness of up to 80 nm inhibits the activated surface area and causes the ozone decompo-sition decay.
ACKNOWLEDGMENTS
This work was financially supported by the National Science Coun-cil, Taiwan through grant numbers NSC 96-2628-E-264-001-MY3, NSC 95-2221-E-264-011 and NSC-95-2221-E-264-013.
REFERENCES
1. J. W. Choi, H. K. Song, W. Lee, K. K. Koo, C. Han and B. K. Na, Korean J. Chem. Eng., 21, 398 (2004).
2. Y. S. Mok and I. S. Nam, Korean J. Chem. Eng., 21, 976 (2004). 3. S. J. Yoa, Y. S. Cho and J. H. Kim, Korean J. Chem. Eng., 22, 364
(2005).
4. J. E. Lee, B. S. Jin, S. H. Cho, S. H. Han, O. S. Joo and K. D. Jung, Korean J. Chem. Eng., 22, 536 (2005).
5. K. J. Choi, S. G. Kim, C. W. Kim and J. K. Park, Korean J. Chem. Eng., 23, 399 (2006).
6. R. Bascom, R. Naclerio, T. K. Fitzgerald, A. Kagey-Sobotka and D. Proud, Am. Rev. Respir. Dis., 142, 594 (1990).
7. S. C. Oh, S. H. Sohn, Y. K. Yeo and K. S. Chang, Korean J. Chem. Eng., 16, 144 (1999).
8. B. Dhandapani and S. T. Oyama, Appl. Catal. B-Environ., 11, 129 (1997).
9. C. Subrahmanyam, D. A. Bulushev, A. Renken, and L. Kiwi-Min-sker, Appl. Catal. B-Environ., 61, 98 (2005).
10. T. A. Metts and S. A. Batterman, Chemosphere, 62, 34 (2006). 11. G. Marbán, R. Antuña and A. B. Fuertes, Appl. Catal. B-Environ.,
41, 323 (2003).
12. D. A. Bulushev, I. Yuranov, E. I. Suvorova, P. A. Buffat and L. Kiwi-Minsker, J. Catal., 224, 8 (2004).
13. Y. Takeuchi and T. Itoh, Sep. Technol., 3, 168 (1993). 14. P. Serp, P. Kalck and R. Feurer, Chem. Rev., 102, 3085 (2002). 15. V. Caps and S. C. Tsang, Catal. Today, 61, 19 (2000).
16. M. Sasaki, H. Hamada and T. Ito, Appl. Catal. A-Gen., 207, 191 (2001).
17. A. M. Gaffney, S. Chaturvedi, M. B. Clark, S. Han, D. Le, S. A. Rykov and J. G. Chen, J. Catal., 229, 12 (2004).
18. C. K. O’Sullivan and G. G. Guilbault, Biosens. Bioelectron., 14, 663 (1999).
19. C. L. Chang and T. S. Lin, React. Kinet. Catal. Lett., 86, 91 (2005). 20. Z. P Hao, D. Cheng, Y. Guo and Y. Liang, Appl. Catal. B-Environ.,
33, 217 (2001). Fig. 6. Effects of reaction temperature on ozone removal efficiency
(ozone flowrate: 1 lpm, ozone concentration: 200 ppm, ACFs packed weight: 0.05 g).