1)School of Forestry and Resource Conservation, National Taiwan University, 1 Roosevelt Rd., Sec. 4,
Taipei 10617, Taiwan. 國立台灣大學森林環境暨資源學系,10617台北市羅斯福路四段1號。
2)Division of Silviculture, Taiwan Forestry Research Institute, 53 Nanhai Rd., Taipei 10066, Taiwan.
行政院農業委員會林業試驗所育林組,10066台北市南海路53號。
3)Division of Forest Utilization, Taiwan Forestry Research Institute, 53 Nanhai Rd., Taipei 10066,
Taiwan. 行政院農業委員會林業試驗所森林利用組,10066台北市南海路53號。
4)Department of Life Science, Institute of Ecology and Evolutionary Biology, National Taiwan
University, 1 Roosevelt Rd., Sec. 4, Taipei 10617, Taiwan. 國立台灣大學生命科學系;生態學與演 化生物學研究所,10617台北市羅斯福路四段1號。
5)Corresponding author, e-mail:linglong@ntu.edu.tw 通訊作者。
Received November 2005, Accepted January 2006. 2005年11月送審 2006年1月通過。
Anatomical Characteristics of Artificially Induced
Tension Wood in Seedlings of Honduras Mahogany
Ching-Ju Tsai,
1)Ching-Te Chien,
2)Chin-Mei Lee,
3)Shiang-Jiuun Chen,
4)Ling-Long Kuo-Huang
4,5)【
Summary】
Seedlings of Honduras mahogany (Swietenia macrophylla King) were laid horizontally for 4 and 10 mo to induce reaction wood. Gelatinous fibers occurred on the upper side of the trunks of the treated seedlings, and the longer the treatment, the higher the extent of the gelatinous fibers. More-abundant axial parenchymatous cells and starch grains were observed in the opposite wood than in the reaction wood. Such anatomical characteristics suggest that there are distinct patterns of regulation between the cambium zones of the reaction wood and opposite wood. Starch storage in axial parenchymatous cells may play a role in the active or passive processes involved in the bio-chemical regulation and source-to-sink transportation.
Key words: gelatinous fibers, Honduras mahogany, reaction wood, starch grains, Swietenia
macro-phylla.
Tsai CJ, Chien CT, Lee CM, Chen SJ, Kuo-Huang LL. 2006. Anatomical characteristics of
artificially induced tension wood in seedlings of Honduras mahogany. Taiwan J For Sci 21(2):147-54.
研究報告
大葉桃花心木苗木人為誘導的抗張材之解剖特徵
蔡謦竹
1)簡慶德
2)李金梅
3)陳香君
4)黃玲瓏
4,5)INTRODUCTION
The formation of reaction wood is re-lated to stem reorientation in response to en-vironmental factors. In order to maximize ex-posure to sunlight, the leaning trunks of most dicotyledons are able to recover to a vertical position by producing tension wood, a wood with high tensile stresses, on the upper side of the trunk, whether the cause is natural or ar-tificial (Wardrop 1964, Fisher and Stevenson 1981). One of the characteristics of tension wood is gelatinous fibers which are named for their unusual inner wall layer called the gelatinous layer (G-layer). The G-layer is known to contain high cellulose, low lignin contents, and small microfibril angles (MFAs) (Scurfield 1973, Dickison 2000). These differ-ences in chemical composition and structure give tension wood with particular properties in comparison to normal wood, notably high longitudinal shrinkage. The growth stress and G-layer of tension wood may cause log split-ting and lumber distortion during the cutsplit-ting and drying processes, respectively (Dinwood-ie 1966, Washusen et al. 2003a).
Swietenia macrophylla King (Honduras
mahogany or Caoba) is a member of the ma-hogany family (Meliaceae). It is an important commercial species with a desirable figure and good working properties. Many studies of the natural generation, dendroecology, genetic structure, and gene flow have been conducted on this species (Dünisch et al. 2003, Lemes et
al. 2003). However no report has investigated reaction wood formation. In this work, arti-ficially induced reaction wood of S.
macro-phylla seedlings was anatomically studied.
MATERIALS AND METHODS
Experiments were conducted at Hengc-hun in southern Taiwan. Several 1~2-yr-old seedlings of S. macrophylla were collected from a nursery of the Taiwan Forestry Re-search Institute. The seedlings were initially grown in an upright orientation to obtain normal wood as a control. Eight seedlings were then laid horizontally to induce different levels of reaction wood (Fig. 1A-C). Before cutting, the upper sides of the trunks were marked. The basal curved parts of the trunks of 4 seedlings treated for 4 months were dis-sected into 3 segments (Fig. 1B, C). Three additional segments were collected from the upper trunks of the 4 seedlings treated for 10 mo (Figs. 1C, 4A). The eccentricity (Ec) is defined as the eccentric distance (De) divided by the short diameter (Ds) (Japan Material Society 1982).
Ec = De/Ds =|Dg - Da︱/Ds;
where Dg is the distance between the rim of
the lower side and the geometric center, and
Da is the distance between the rim of the
Small wood blocks (1×1×2 mm3)
containing tension wood or opposite wood were processed through fixation (in 2.5% glutaraldehyde in 0.1 M sodium phosphate buffer), postfixation (in 1% OsO4 in 0.1 M
sodium phosphate buffer), and dehydration (in an acetone series), and were then embedded in Spurr’s resin (Spurr 1969). Semi-thin sec-tions (1 μm) were made using an ultramicro-tome (Ultracut E) and were stained with 1% toluidine blue in borax buffer. Free-hand or microtome sections (20~30 µm in thickness) were made from the wood blocks, and were stained with safranin O and Herzburg reagent. Typical G-layers of the gelatinous fibers in the tension wood were recognizable by their morphology and their reaction with different stains. Sections were stained with iodine-po-tassium iodine to identify starch grains (Gahan 1984). Sections stained with zinc-chlor-iodide (Krishnamurthy 1999) with a few drops of hydrochloric acid were used for morphomet-ric analysis. The lignified secondary wall stained light yellow, the G-layer orange, and the starch grains blackish-brown. All sec-tions were examined using a Leica Diaplan Microscope. Images (n = 50) were captured with a Nikon Coolpix 995 digital camera. The
percent areas of lignified cell walls, G-layers, cell lumens, and starch grains were calculated using the image analysis software ImagePro Plus.
RESULTS AND DISCUSSION
Swietenia macrophylla forms a diffuse
porous wood (Fig. 2A). Axial parenchyma-tous cells appeared regularly in the vicinity of vessel elements or were sometimes grouped along the initial or terminal part of the annual rings that usually refer to apotracheal banded (or marginal) parenchyma (Carlquist 2001). Starch grains were found in the ray and axial parenchymatous cells (Fig. 2B), however they often spread out during the free-hand section-ing procedure.
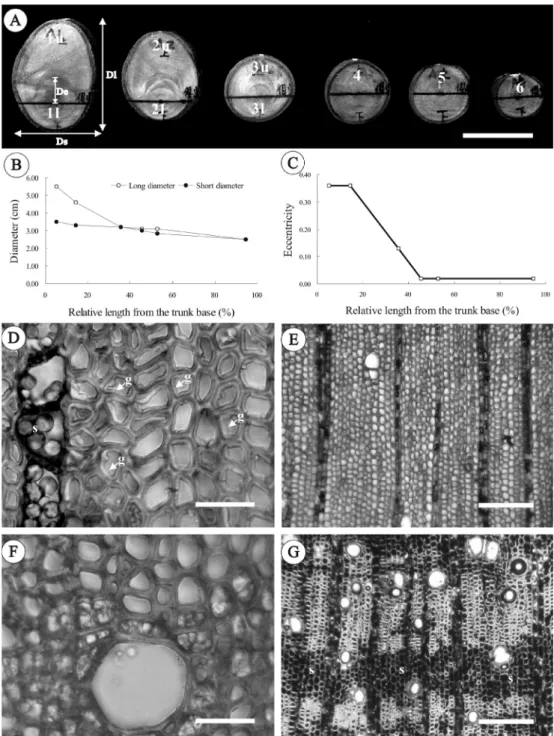
All trunks of seedlings laid horizontally for 4 or 10 mo exhibited radial growth pro-motion on the upper side. The discs cut from the curved parts of these trunks were more or less oval in shape (Figs. 3A, 4A, B), and the eccentricity decreased from the base to the apex of the seedling (Fig. 4C). To maintain normal angles with the vertical trunk, it is known that sufficient amounts of compression wood are continuously formed on the lower
Fig. 1. One to 2-yr-old seedlings of Swietenia macrophylla laid horizontally to induce tension wood. (A) At 0 mo; (B) after 4 mo, the trunk had bent upwards; (C) after 10 mo, the trunk had almost bent back to the vertical position. Cross-sections cut from segments 1~3 in both (B) and (C) were used for calculating the area percentages shown in Fig. 5. Segments 1~6 in (C) were the sites cut for measuring eccentricity.
sides of branches of gymnosperms (Westing 1965). The adaxial promotion of the growth of tension wood in the horizontally laid trunks of S. macrophylla seemed to follow a similar pattern.
Eccentricity of the stem can be used to show the significance of growth strain from a macroscopic view, while the anatomical structure, i.e. the distribution of gelatinous fibers, can represent these facts from a more-microscopic field of view (Washusen et al. 2003b). In the trunks of seedlings laid hori-zontally for 4 mo, the axial fibers in the ten-sion wood were mostly G-fibers (Figs. 3B, 5A), while those in the opposite wood were mainly normal fibers with a few G-fiber in thin layers (Figs. 3D, 5A). In the trunks of those treated for 10 mo, the area percentage of the G-layers was closely related to the ec-centricity of the trunks (Figs. 4C, 5B). Almost all of the G-fibers were found in the sections of the upper side of the eccentric or basal part of the trunks (Figs. 4D, 5B), but not in those
sections of the lower side (Figs. 4E, 5B), while equal amounts of G-fibers were found in all sections from the almost upright parts of the trunks (Fig. 5B).
Gelatinous fibers shorten at matura-tion and induce longitudinal tension strains in tension wood areas. When the seedlings receive the gravitational signal, the xylem mother cells follow different pathways of dif-ferentiation. That is, more xylem mother cells develop into gelatinous fibers in the reaction cambium, but into axial parenchymatous cells in the opposite cambium. The process of cell differentiation during wood formation is a complicated research topic involving many biochemical pathways and genetic regulators.
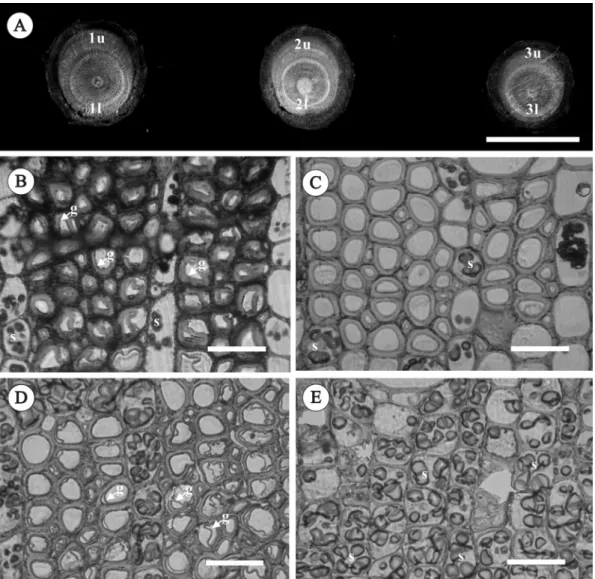
The distribution of carbon sources in the plant tissue is functionally allocated. It is in-teresting to note that in the trunks of seedlings laid horizontally for 4 mo, starch grains were found in most ray and axial parenchymatous cells of opposite wood (Fig. 3D, E), while in tension wood (upper side), starch grains were
Fig. 2. Normal wood structure of the basal part of seedlings. (A) Transverse section of the trunk showing the diffuse porous wood with vesicentric scanty and apotracheal banded parenchyma. (B) Starch grains stained with iodine solution occurring in the ray and axial parenchymatous cells. ap, axial parenchymatous cells; rp, ray parenchyma; s, starch grain; v, vessel. Bars = 200 µm in (A) and 30 µm in (B).
Fig. 3. Tension and opposite wood structures of seelings laid horizontally for 4 mo. (A) Discs cut from the curved parts of a seedling. Transverse sections of the upper (B, C) and lower sides (D, E) of the trunk representing the distributions of G-fibers and starch grains. g, gelatinous fiber; l, lower side; s, starch grain; u, upper side. Bars = 1 cm in (A) and 30 µm in (B-E).
only found in ray parenchymatous cells (Fig. 3B, C). Based on the morphometric analysis, we found that on average, about 60~70% of the starch grains were found in wood of the lower side (Fig. 5A). However, in the basal trunks of seedlings laid horizontally for 10 mo where severe tension wood was formed, most starch grains (about 90%) were found in
wood on the lower side, while in the almost upright parts of the trunk, starch grains were found equally in both the lower and upper sides (Figs. 4D-G, 5B).
Formation of gelatinous fibers is be-lieved to involve additional energy costs and carbon sources compared with those of oppo-site wood. Physiologically, starch grains can
Fig. 4. Tension and opposite wood structures of seedling laid horizontally for 10 mo. (A) Discs cut from the base to the apex of the seedling. (B) Diameters and (C) eccentricity of each disc decreased from the base to the apex of the seedling. Transverse sections of the upper (D, E) and lower sides (F, G) of the trunk representing the distributions of G-fibers and starch grains. De, eccentric distance; Dl, long diameter; Ds, short diameter; g,
gelatinous fiber; l, lower side; s, starch grain; u, upper side. Bars = 3 cm in (A), 30 µm in (D, F), and 200 µm in (E, G).
be converted into energy for cell metabolism or components for cell division in the cambi-um zone and for cell wall construction. These processes might be regulated by alterations in nutrition transportation to form gelatinous fibers combined with a source-to-sink move-ment of nutrients across the gradient. Oribe et al. (2001, 2003) described how the localiza-tion of starch grains in Albies sachalinensis is closely related to cambial reactivation, because they occur during dormancy of the cambium and disappear during activation. Carbohydrates across cambial region tissues, i.e., fructose, glucose (monosaccharides), and sucrose (disaccharide), and their metabolizing enzymes can be studied using tissue-specific microanalysis. It was found that sucrose was abundant in the phloem where it is trans-located to and assumed to be decomposed in the cambium zone and in the developing xylem to supply the energy for cell develop-ment, while simultaneously being converted to monosaccharides for cell wall components in Scots pine (Pinus sylvestris) (Uggla et al. 2001). Although evidence of starch hydrolyz-ing enzymes was not pursued in our study, the importance of carbohydrate during wood formation is obvious.
Anatomical research of tension wood formation is enriched by our studies as well as those of many others. To reveal the nature of growth regulation by tree species, tensile measurements should be combined with in-formation from anatomical research. At the same time, using biochemical and genomic methods to investigate the regulation of wood formation is also necessary to understand the processes of reaction wood formation. The combination of the data from tension strain measurements, anatomical ultrastructures, and biochemical and genetic regulations will shed new light on the research of tension wood formation.
ACKNOWLEDGEMENTS
The authors would like to thank Drs. Yan-San Huang and Chih-Lin Huang for their critical reading of the manuscript and valu-able suggestions.
LITERATURE CITED
Carlquist S. 2001. Comparative wood
anato-my: systematic, ecological, and evolutionary
Fig. 5. Area percentages of the distributions of starch grains, cell lumens, G-layers, and lignified cell walls. (A) Seedling laid horizontally for 4 mo exhibited a high content of G-layers (83 ) and a low content of starch grains (37 ) in upper-side wood. (B) In the basal part after 10 mo of treatment, a few starch grains (16 ) and all G-layers occurred in the fibers on the upper side of tree trunks, while in the almost upright parts of the seedling, equal areas of starch grains and G-layers occurred on either side of the tree trunk.
Dünisch O, Montóia VR, Bauch J. 2003.
Dendroecological investigations on
Swiete-nia macrophylla King and Cedrela odorata
L. (Meliaceae) in the central Amazon. Trees 17:244-50.
Fisher JB, Stevenson JW. 1981. Occurrence
of reaction wood in branches of dicotyle-dons and its role in tree architecture. Bot Gaz 142(1):82-95.
Gahan PB. 1984. Plant histochemistry and
cytochemistry: an introduction. London; Aca-demic Press. p 239-40.
Japan Material Society. 1982. Dictionary of
wood industry. Committee of Woody Mate-rial Department, editor. Kyoto: Wood Industry Publishers. p 573.
Krishnamurthy KV. 1999. Methods in cell
wall cytochemistry. Boca Raton, FL: CRC Press. p 59.
Lemes MR, Gribel R, Proctor J, Grattapa-glia D. 2003. Population genetic structure of
mahogany (Swietenia macrophylla King, Me-liaceae) across the Brazilian Amazon, based on variation at microsatellite loci: implications for conservation. Mol Eco 12(11):2875-83.
Oribe Y, Funada R, Kubo T. 2003.
Relation-ships between cambial activity, cell
differen-Scurfield G. 1973. Reaction wood: its
struc-ture and function. Sci 179:647-55.
Spurr AR.1969. A low viscosity epoxy resin
embedding medium for electron microscopy. J Ultrastr Res 26:31-43.
Uggla C, Magel E, Moritz T, Sundberg B. 2001. Function and dynamics of auxin and
car-bohydrates during earlywood/latewood transi-tion in Scots pine. Plant Physiol 125:2029-39.
Wardrop AB. 1964. The reaction anatomy of
arborescent angiosperms. In: Zimmerman MH, editor. The formation of wood in forest trees. New York and London: Academic Press, p 405-56.
Washusen R, Ilic J, Waugh G. 2003a.
Longi-tudinal growth strain and its relationship with the occurrence of gelatinous fibers in 1074- and 11-year-old Eucalyptus globulus Labill. Holz Roh-Werkst 61(4):299-303.
Washusen R, Ilic J, Waugh G. 2003b. The
relationship between longitudinal growth strain. Tree form and tension wood at the stem periphery of ten- to eleven-year-old Eucalyptus
globulus Labill. Holtzforschung 57(3):308-16.
Westing AH. 1965. Formation and function of
compression wood in gymnosperms. Bot Rev 31:381-480.