b
Editor-in-ChiefLuc Pieters, Antwerp, Belgium
Senior Editor
Adolf Nahrstedt, Münster, Germany
Review Editor
Matthias Hamburger, Basel, Switzerland
Editors
Wolfgang Barz, Münster, Germany Rudolf Bauer, Graz, Austria
Veronika Butterweck, Gainesville FL, USA João Batista Calixto, Florianopolis, Brazil Thomas Efferth, Mainz, Germany Jerzy W. Jaroszewski, Copenhagen, Denmark
Ikhlas Khan, Oxford MS, USA Wolfgang Kreis, Erlangen, Germany Irmgard Merfort, Freiburg, Germany Kurt Schmidt, Graz, Austria Thomas Simmet, Ulm, Germany Hermann Stuppner, Innsbruck, Austria Yang-Chang Wu, Taichung, Taiwan Yang Ye, Shanghai, China
Editorial Offices
Claudia Schärer, Basel, Switzerland Tess De Bruyne, Antwerp, Belgium
Advisory Board
Giovanni Appendino, Novara, Italy John T. Arnason, Ottawa, Canada Yoshinori Asakawa, Tokushima, Japan Lars Bohlin, Uppsala, Sweden
Gerhard Bringmann, Würzburg, Germany Reto Brun, Basel, Switzerland
Mark S. Butler, S. Lucia, Australia Ihsan Calis, Ankara, Turkey
Salvador Cañigueral, Barcelona, Spain Hartmut Derendorf, Gainesville, USA Verena Dirsch, Vienna, Austria Jürgen Drewe, Basel, Switzerland Roberto Maffei Facino, Milan, Italy Alfonso Garcia-Piñeres, Frederick MD, USA Rolf Gebhardt, Leipzig, Germany
Clarissa Gerhäuser, Heidelberg, Germany Jürg Gertsch, Zürich, Switzerland Simon Gibbons, London, UK De-An Guo, Shanghai, China Leslie Gunatilaka, Tucson, USA Solomon Habtemariam, London, UK Andreas Hensel, Münster, Germany Werner Herz, Tallahassee, USA Kurt Hostettmann, Geneva, Switzerland Peter J. Houghton, London, UK Jinwoong Kim, Seoul, Korea Gabriele M. König, Bonn, Germany Ulrich Matern, Marburg, Germany Matthias Melzig, Berlin, Germany Dulcie Mulholland, Guildford, UK Eduardo Munoz, Cordoba, Spain Kirsi-Maria Oksman-Caldentey, Espoo,
Finland
Ana Maria de Oliveira, São Paulo, Brazil Nigel B. Perry, Dunedin, New Zealand Joseph Pfeilschifter, Frankfurt, Germany Peter Proksch, Düsseldorf, Germany Thomas Schmidt, Münster, Germany Volker Schulz, Berlin, Germany Hans-Uwe Simon, Bern, Switzerland Leandros Skaltsounis, Athens, Greece Han-Dong Sun, Kunming, China
Benny K. H. Tan, Singapore, R. of Singapore Ren Xiang Tan, Nanjing, China
Deniz Tasdemir, London, UK
Nunziatina de Tommasi, Salerno, Italy Arnold Vlietinck, Antwerp, Belgium Angelika M. Vollmar, München, Germany Heikki Vuorela, Helsinki, Finland Jean-Luc Wolfender, Geneva, Switzerland De-Quan Yu, Beijing, China
Publishers
Georg Thieme Verlag KG Stuttgart · New York Rüdigerstraße 14 D-70469 Stuttgart Postfach 30 11 20 D-70451 Stuttgart Thieme Publishers 333 Seventh Avenue New York, NY 10001, USA www.thieme.com
Reprint
© Georg Thieme Verlag KG Stuttgart · New York Reprint with the permission of the publishers only
Planta Medica
b
Introduction
!
Scutellariae Radix (SR), the roots of Scutellaria baicalensis GEORGI (Labiatae), is a Chinese medi-cine in popular use and has been reported to ex-hibit various beneficial bioactivities such as anti-inflammation [1, 2], antihepatitis [3], antitumor [4, 5] and antivirus effects [6, 7]. More recently, SR has been reported to inhibit H1N1 virus repli-cation in mice [8]. The major flavonoids in SR in-clude baicalin, baicalein, wogonoside, and wogo-nin (structures shown in l"Fig. 1), which have been shown to exhibit anti-inflammatory [9, 10], antioxidative [11, 12], antiallergic [13], antiviral [14], and anticarcinogenic activities [15, 16]. Despite numerous in vitro bioactivities of SR and its flavonoid constituents reported thus far, their in vivo effects remain unclear due to the lack of in-formation concerning the pharmacokinetics and tissue distribution of the flavonoid constituents. Our previous study has reported that the free
form of baicalein was transiently present in blood circulation and the glucuronides/sulfates of baica-lein were the major forms after single-dose ad-ministrations of baicalin or baicalein to rats [17]. In addition, our human study has also shown that the glucuronides/sulfates of baicalein and wogo-nin were the major forms of flavonoids in urine after SR administration [18]. This study further investigated the pharmacokinetics and tissue dis-tribution of flavonoids and their metabolites fol-lowing repeated dosing of an SR decoction in rats.
Materials and Methods
!
Animals
Male Sprague-Dawley rats were supplied by the National Laboratory Animal Center (Taipei, Tai-wan) and kept in the animal center of the China Medical University (Taichung, Taiwan). The ani-mal study adhered to“The Guidebook for the Care and Use of Laboratory Animals (2002)”, published by the Chinese Society of Animal Science, Taiwan, ROC. The animal protocol was approved by the
In-Abstract
!
Scutellariae Radix (root of Scutellaria baicalensis, SR) contains numerous flavonoids such as baica-lin, baicalein, and wogonin. This study investi-gated the pharmacokinetics and tissue distribu-tion of flavonoids and their metabolites in rats after repeated dosing of a SR decoction. Sprague-Dawley rats were orally administered SR at 2 g/kg for seven doses. After the 7th dose, blood samples were withdrawn at specific times and organs, in-cluding the liver, kidney, lung, and brain, and col-lected. The concentrations of baicalein and wogo-nin in the serum and various tissues were assayed by HPLC before and after hydrolysis with glucuro-nidase and sulfatase. Baicalein and wogonin were
not detected in the serum, and the molecules found were their glucuronides/sulfates. In tissues, the free forms of baicalein and wogonin appeared in the liver, kidney, and lung in addition to their glucuronides/sulfates. Baicalein was the major form in the lung, whereas baicalein glucuro-nides/sulfates were the major forms in the liver and kidney. Wogonin was the major form in the liver, kidney, lung, and traces of wogonin glucuro-nides/sulfates were detected in the kidney and liver. Neither baicalein and wogonin nor their glu-curonides/sulfates were detected in the brain. In conclusion, the glucuronides/sulfates of baicalein and wogonin were exclusively present in the cir-culation, whereas their free forms appeared in the lung, liver, and kidney.
* These authors contributed equally to the study.
Flavonoid Pharmacokinetics and Tissue
Distribution after Repeated Dosing of the Roots
of
Scutellaria baicalensis in Rats
Authors Yu-Chi Hou1*, Shiuan-Pey Lin1*, Shang-Yuan Tsai1, Miau-Hwa Ko2, Yun-Chia Chang1, Pei-Dawn Lee Chao1
Affiliations 1School of Pharmacy, College of Pharmacy, China Medical University, Taichung, Taiwan
2Department of Anatomy, College of Medicine, China Medical University, Taichung, Taiwan
Key words l" Scutellaria baicalensis l" Labiatae l" pharmacokinetics l" tissue distribution l" baicalein l" wogonin received Feb. 26, 2010 revised July 16, 2010 accepted Sept. 20, 2010 Bibliography DOI http://dx.doi.org/ 10.1055/s-0030-1250433 Published online October 18, 2010
Planta Med 2011; 77: 455–460 © Georg Thieme Verlag KG Stuttgart · New York · ISSN 0032‑0943 Correspondence
Prof. Dr. Pei-Dawn Lee Chao School of Pharmacy China Medical University No. 91 Hsueh-Shih Road Taichung Taiwan 40402 Republic of China Phone: + 88 64 22 03 10 28 Fax: + 88 64 22 03 10 28 pdlee@mail.cmu.edu.tw
This
is
a
cop
y
of
the
author
ʼs
per
sonal
reprint
This
is
a
cop
y
of
the
author
ʼs
per
sonal
reprint
b
stitutional Animal Care and Use Committee (IACUC) of the ChinaMedical University, Taiwan.
Rats weighing 380–450 g were housed in a 12-h light-dark cycle, constant temperature environment prior to the study. All rats were fasted 12 h before dosing and fasting continued for another 3 h.
Chemicals and reagents
Baicalin (purity 99 %), baicalein (purity 98 %), and wogonin (purity 98 %) were purchased from Wako Chemical Co. Sulfatase (type H-1, 14 400 units/g, from Helix pomatia, containing 574 000 unit/g of β-glucuronidase),β-glucuronidase (type B-1, 666 400 units/g, from bovine liver), ethyl paraben, and propyl paraben were purchased from Sigma Chemical Co. Hydrochloric acid was obtained from Merck. Acetonitrile, methanol, and ethyl acetate were LC grade and purchased from Mallinckrodt Baker, Inc. L-(+)-Ascorbic acid was obtained from Riedel-deHaen. Milli-Q plus water (Millipore) was used throughout the study.
Instrumentation
The HPLC apparatus included one pump (LC-10A; Shimadzu), a UV spectrophotometric detector (SPD-10A; Shimadzu), an auto-matic injector (SIL-10A; Shimadzu), and an Apollo®100 RP-18e
column (5 µm, 4.6 × 250 mm; Alltech Associates, Inc.).
Preparation of SR decoction
The crude drugs of SR were purchased from a Chinese drugstore in Taichung, Taiwan. The plant material was identified by Dr. Yu-Chi Hou. A voucher specimen (CMCSB1) was deposited in the Graduate Institute of Chinese Pharmaceutical Sciences, China Medical University. Six liters of water was added to 300 g of crude drugs and heated on a gas stove. After boiling, gentle heating was continued until the volume reduced to about 3 L and then the mixture was filtered while hot. The filtrate was further concen-trated with gentle boiling until the volume reduced to 600 mL; it was immediately divided into aliquots (40 mL each) and frozen at −30°C for later use.
Quantitation of flavonoids in SR decoction
and the acid hydrolysate
SR decoction (3.0 mL) was added with MeOH (7.0 mL), vortexed and centrifuged. After appropriate dilution, 100 µL of the super-natant was mixed with methanol containing 20 µg/mL of ethyl paraben as the internal standard, and 20 µL was subjected to HPLC analysis. The mobile phase consisted of acetonitrile (A)– 0.1 % phosphoric acid (B) and programmed in a gradient manner as follows: A/B: 30/70 (0–12 min), 50/50 (14–26 min), and 70/30 (28–33 min). The detection wavelength was set at 270 nm, and the flow rate was 1.0 mL/min.
Acid hydrolysis of 2.5 mL of SR decoction was conducted with 2.5 mL of 2.4 N HCl, and the reaction was carried out in a water bath at 100 °C for 8 h, which had been determined by a time study. The hydrolysate was then added with sufficient water to make 5.0 mL before HPLC analysis. The concentration of wogonin glyco-side was calculated by subtracting the wogonin concentration in the original decoction from that in the decoction hydrolysate.
Drug administration, blood, and tissue collection
In the pharmacokinetic study, the SR decoction was given at doses of 2.0 g/4 mL/kg via gastric gavage thrice daily for seven doses to six rats. After an overnight fasting, the blood samples were collected before and at 10, 30, 60, 180, 240, 480 min after the 7th dose. At each time point, 0.7 mL of blood was collected via cardiac puncture. The blood samples were collected in micro-tubes and centrifuged at 10 000 g for 10 min to obtain serum, which was stored at− 30 °C before analysis.
In the tissue distribution study, the dosage regimen was the same as that described above for the pharmacokinetic study. Before the 7th dose of SR, five rats were fasted overnight and a blood sample was withdrawn at 10 min after dosing; the rats were then sacri-ficed. Following complete systemic perfusion with cold saline, various organs including the liver, lung, kidney, and brain were removed, washed with normal saline, blotted dry with filter pa-per and then accurately weighed. The tissues were homogenized in saline solution (70 mg/mL), and the homogenates were stored at− 30 °C until analysis.
Quantitation of baicalein, wogonin,
and their metabolites in serum
The concentrations of the free forms and conjugated metabolites of baicalein and wogonin in serum were determined before and after treatments with sulfatase andβ-glucuronidase, respective-ly. For the determination of glucuronides/sulfates, 100 µL of se-rum sample was mixed with 50 µL of sulfatase (1000 unit/mL in pH 5 acetate buffer, containing 39 861 unit/mL of β-glucuroni-dase) and 25 µL of ascorbic acid (200 mg/mL) and incubated at 37 °C for 10 min under anaerobic conditions and away from light. After hydrolysis, the serum was acidified with 25 µL of 0.1 N HCl and partitioned with 200 µL of ethyl acetate (containing 1.0 µg/ mL of propyl paraben as the internal standard). The ethyl acetate layer was evaporated under N2gas to dryness and reconstituted
with an appropriate volume of the mobile phase, then 20 µL was subjected to HPLC analysis. The mobile phase consisted of aceto-nitrile/0.1 % phosphoric acid (50 : 50, v/v). The detection wave-length was set at 270 nm, and the flow rate was 1.0 mL/min. For the determination of glucuronides, 100 µL of serum sample was subjected to the method described above except for the ad-dition of 50 µL ofβ-glucuronidase (1000 unit/mL in pH 5 acetate buffer) and incubation at 37 °C for 1 h. For the assay of free forms of baicalein and wogonin, 100 µL of serum sample was subjected
Fig. 1 Chemical structures of baicalein, wogonin, baicalin, and
wogono-side.
This
is
a
cop
y
of
the
author
ʼs
per
sonal
reprint
This
is
a
cop
y
of
the
author
ʼs
per
sonal
reprint
b
to the method described above except for the addition ofen-zyme–free buffer and without incubation. Due to the consider-able amount ofβ-glucuronidase contained in the sulfatase used in this study, the treatment with sulfatase hydrolyzed both sul-fates and glucuronides. The difference of released aglycones be-tween treatments with glucuronides/sulfates and β-glucuroni-dase afforded the estimated concentration of sulfates.
For the calibrator preparation, 100 µL of serum was mixed with various concentrations of standard solutions of baicalein and wo-gonin and added with 50 µL of pH 5 acetate buffer. The later pro-cedure followed that described above for serum samples. The cal-ibration graph was plotted by linear regression of the peak area ratios (baicalein and wogonin to internal standard) against con-centrations of baicalein and wogonin.
Quantitation of baicalein, wogonin,
and their metabolites in tissue homogenates
The concentrations of the free forms and conjugated metabolites of baicalein and wogonin in tissue were determined before and after treatments with sulfatase orβ-glucuronidase, respectively. Treatment withβ-glucuronidase resulted in the hydrolysis of glu-curonides, whereas sulfatase hydrolyzed both the sulfates and glucuronides because the sulfatase in use contained a consider-able amount ofβ-glucuronidase.
Tissue homogenate (300 µL) was mixed with 150 µL of pH 5 ace-tate buffer, sulfatase (1000 unit/mL in pH 5 aceace-tate buffer, con-taining 39 861 unit/mL ofβ-glucuronidase) or β-glucuronidase (1000 unit/mL), and 300 µL of ascorbic acid (200 mg/mL) and in-cubated at 37 °C for 1 h under anaerobic conditions and away from light. After hydrolysis, the serum was acidified with 50 µL of 0.1 N HCl and partitioned with 800 µL of ethyl acetate (contain-ing 0.3 µg/mL of propyl paraben as the internal standard). The ethyl acetate layer was evaporated under N2gas to dryness and
reconstituted with 50 µL of mobile phase, then 20 µL was sub-jected to HPLC analysis. The mobile phase for kidney samples, consisting of acetonitrile (A) and 0.1 % phosphoric acid (B), was programmed in a gradient manner as follows: A/B: 35/65 (0– 20 min), 45/55 (23–35 min), 70/30 (40–45 min), 35/65 (50– 60 min); for lung and liver samples, the mobile phase was A/B: 38/62 (0–30 min), 70/30 (41–51 min), and 38/62 (55–60 min). The detection wavelength was set at 270 nm, and the flow rate was 1.0 mL/min. The difference of released aglycones between treatments with sulfatase/glucuronidase andβ-glucuronidase af-forded the estimated concentration of sulfates.
For the calibrator preparation, 300 µL of tissue homogenate was mixed with various concentrations of standard solutions of bai-calein and wogonin, and added with 150 µL of pH 5 acetate buffer. The later procedure followed that described above for tissue sam-ples. The calibration graphs were plotted by linear regression of the peak area ratios (baicalein and wogonin to internal standard) against concentrations of baicalein and wogonin.
Data analysis
The peak serum concentration (Cmax) and the time to peak
con-centration (tmax) were obtained from experimental observation.
The area under the serum concentration-time curve (AUC0-t)
was calculated using the trapezoidal rule to the last point. The pharmacokinetic parameters were analyzed by a noncompart-ment model using WinNonlin (version 1.1 SCI software; Statisti-cal Consulting, Inc.). Paired Studentʼs t-test and one-way ANOVA with the Scheffe test were used for statistical comparisons of the
pharmacokinetic parameters and tissue distribution of various flavonoid forms in each organ, respectively.
Results
!
The adopted quantitation method of flavonoids in the SR decoc-tion largely followed that in a previous study with minor modifi-cations [18]. The concentrations of baicalin, baicalein, and wogo-nin in the SR decoction were 43.1, 17.6, and 5.7 µmol/mL, respec-tively. After acid hydrolysis, the concentration of wogonoside was calculated, being 12.8 µmol/mL. It showed that the abundances of baicalin and wogonoside were 2.5- and 2.2-fold of the corre-spondent aglycones, respectively. A dose of SR decoction con-tained 77.0 mg/kg of baicalin, 19.0 mg/kg of baicalein, 6.4 mg/kg of wogonin, and 8.2 mg/kg of wogonoside.
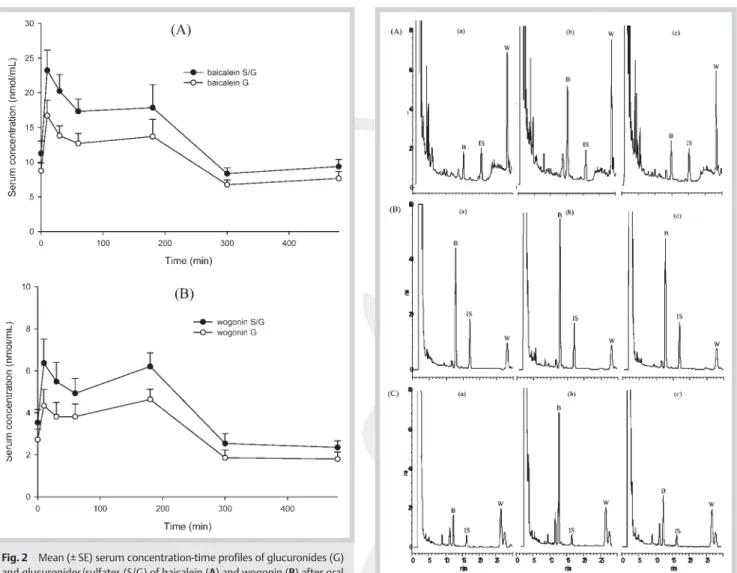
The calibration curves of baicalein and wogonin in serum were within the ranges of 0.3 to 20.0 and 0.2 to 10.0 µg/mL, respective-ly. Validation of this assay method indicated that all coefficients of variation for intra-run and inter-run analysis were less than 8.6 %, and the relative errors were below 15.0 %, indicating good precision and accuracy. The recoveries of baicalein and wogonin from serum were 90.2 ~ 93.4 % at 0.6, 2.5, and 10.0 µg/mL for bai-calein and 73.9 ~ 84.0 % at 0.3, 1.3, and 5.0 µg/mL for wogonin. In serum specimens, no free forms of baicalein and wogonin have been detected. The concentrations of the glucuronides and glucu-ronides/sulfates of baicalein and wogonin were determined indi-rectly after hydrolysis with glucuronidase and sulfatase/glucuro-nidase, respectively.l"Fig. 2 depicts the mean serum concentra-tion–time profiles of the glucuronides/sulfates and glucuronides of baicalein and wogonin in six rats. The mean serum levels of baicalein glucuronides/sulfates were much higher than those of wogonin glucuronides/sulfates at all time points throughout the study. Both the serum profiles of baicalein glucuronides/sulfates and wogonin glucuronides/sulfates exhibited a second peak, in-dicating possible enterohepatic circulation. The pharmacokinetic parameters were listed inl"Table 1. The Cmaxand AUC0-tof glu-curonides/sulfates of baicalein and wogonin were significantly higher than those of glucuronides, indicating the presence of sul-fates of baicalein and wogonin in serum. The systemic exposure of baicalein glucuronides/sulfates was significantly higher than that of wogonin glucuronides/sulfates.
The calibration ranges of baicalein (0.3–20.0 µg/mL) and wogonin (0.2–10.0 µg/mL) in various tissue homogenates had good linear-ities (r > 0.999). The method validation indicated that all coeffi-cients of variation in intra-run and inter-run analysis of various tissue homogenates were below 8.6 % and 9.7 %, and the relative errors were below 13.9 % and 11.0 % for baicalein and wogonin, respectively. The recoveries of baicalein and wogonin from the lung, liver, and kidney were 69 ~ 93 % and 91 ~ 113 %, respectively, at tested concentrations.
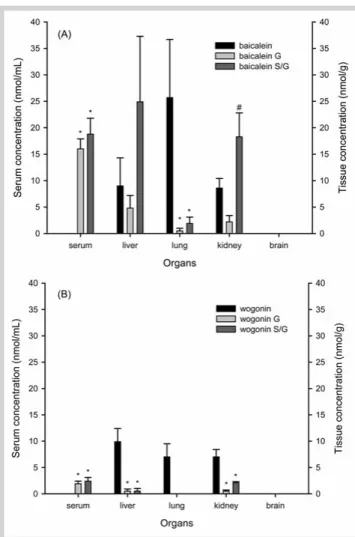
l"Fig. 3 shows typical chromatograms of baicalein and wogonin in the kidney, lung, and liver before (a) and after treatments with sulfatase/glucuronidase (b) and glucuronidase (c).l"Fig. 4 shows the concentrations of baicalein, wogonin, and their glucuronides and glucuronides/sulfates in serum and in various tissues, which had been collected at 10 min after the 7th dose of SR decoction in five rats. The free forms of baicalein and wogonin were found in the lung, kidney, and liver. The relative concentration of baicalein free form in various tissues was: lung > kidney, liver, whereas the concentration of wogonin was ranked as liver > lung, kidney. In regard to the distribution of baicalein glucuronides/sulfates, the
This
is
a
cop
y
of
the
author
ʼs
per
sonal
reprint
This
is
a
cop
y
of
the
author
ʼs
per
sonal
reprint
b
relative abundance was: liver > kidney >> lung, and all organscontained more sulfates than glucuronides. As for the distribu-tion of wogonin glucuronides/sulfates, the relative abundance was ranked as kidney > liver, and no wogonin glucuronides/sul-fates was detected in the lung. In addition, no traces of both free forms and glucuronides/sulfates of baicalein and wogonin were detected in all brains of five rats. Serum withdrawn at the time of tissue collection contained no free forms of baicalein and
gonin, and the major form was glucuronides of baicalein and wo-gonin.
Discussion
!
The quantitation methods of baicalein and wogonin in serum and various organ homogenates were established and validated in this study. The results indicated that the precision and accuracy of intra-run and inter-run analyses as well as their recoveries were satisfactory. After a 7-dose administration of SR decoction, the free forms of baicalein and wogonin were not detected in se-rum, suggesting that they did not enter the circulation per se. While the serum samples were treated with β-glucuronidase and sulfatase, the peaks of baicalein and wogonin emerged in the HPLC chromatogram, indicating that the glucuronides/sul-fates of baicalein and wogonin were present in the bloodstream. This result was in part consistent with our previous work report-ing the biological fates of baicalin and baicalein when they were administered as pure compounds in rats [18]. The Cmaxand AUC0-t
Fig. 3 HPLC chromatograms of a specimen of the kidney (A), lung (B),
and liver (C) collected at 10 min post-7th dose. a Before hydrolysis, b hy-drolyzed with sulfatase/glucuronidase, and c hyhy-drolyzed with glucuroni-dase. B: baicalein, W: wogonin, IS: internal standard.
Table 1 Pharmacokinetic parameters of glucuronides/sulfates (S/G) and
glu-curonides (G) of baicalein and wogonin after administration of seven doses of 2 g/kg of SR decoction to six rats.
Metabolites Parameters
Tmax Cmax AUC0 ~ 480 MRT0 ~ 480
Baicalein S/G 38.3 ± 28.3 26.6 ± 2.7 6 871.7 ± 618.3 194.4 ± 7.8
Baicalein G 116.7 ± 77.8 19.3 ± 2.0** 5 237.4 ± 463.3*** 202.1 ± 8.3
Wogonin S/G 66.7 ± 35.8 7.8 ± 0.8 2 077.5 ± 181.3 188.6 ± 5.8
Wogonin G 151.7 ± 28.3 5.4 ± 0.6* 1 548.5 ± 145.7*** 190.8 ± 7.2
Tmax(min): the time of maximum concentration; Cmax(nmol/mL): the maximum
con-centration; AUC0 ~ 480(nmol · min/mL): the area under concentration-time curve to the last time; MRT0 ~ 480(min): the mean residence time; data expressed as mean ± SE; * p < 0.05, ** p < 0.01, *** p < 0.001, compared to correspondent S/G
Fig. 2 Mean (± SE) serum concentration-time profiles of glucuronides (G)
and glucuronides/sulfates (S/G) of baicalein (A) and wogonin (B) after oral administration of seven doses of 2 g/kg SR decoction to six rats.
This
is
a
cop
y
of
the
author
ʼs
per
sonal
reprint
This
is
a
cop
y
of
the
author
ʼs
per
sonal
reprint
b
of baicalein glucuronides/sulfates were 3.3-fold of those ofwogo-nin glucuronides/sulfates, which could be explained by the rela-tive content of baicalin/baicalein to wogonoside/wogonin being 3.3-fold in the SR decoction.
Owing to the considerable amount of glucuronidase in the sulfa-tase (type H-1) used in this study, treatment of serum and tissue homogenates with this enzyme resulted in the hydrolysis of both sulfates and glucuronides. Through the comparison of the agly-cones released between treatments with sulfatase and glucuroni-dase, the concentrations of sulfates in serum and tissues could be estimated. By subtracting the AUC0-t of the glucuronides from
that of correspondent glucuronides/sulfates, it could be found that the systemic exposures of the sulfates of baicalein and wogo-nin were about one-third of their correspondent glucuronides. This fact furnishes the evidence that baicalin/baicalein and wogo-noside/wogonin were majorly metabolized to glucuronides and less to sulfates. However, the sulfoglucuronyl diconjugates of baicalein and wogonin could not be distinguished under this method.
In regard to tissue analysis, rats were sacrificed near the serum peak time of glucuronides/sulfates of baicalein and wogonin based on our pharmacokinetic data. The emergence of a
consid-erable concentration of the free forms of baicalein and wogonin in the lung, liver, and kidney may have been biotransformed from their glucuronides/sulfates in the blood circulation. This fact can be explained by the presence of deconjugation enzymes such as glucuronidase and sulfatase on the cell membranes of these or-gans [19–21]. Our finding of the free forms of baicalein and wo-gonin in the lung, liver, and kidney was different from an earlier work detecting no free form of quercetin, a popular dietary flavo-noid, in these organs [22]. This discrepancy may arise from the different catalytic capability of deconjugation enzymes in organs on the hydrolysis of various flavonoid glucuronides/sulfates. Contrary to the finding in serum, sulfates rather than glucuro-nides were found as the major conjugates of baicalein in the liver, kidney, and lung, indicating that the baicalein glucuronides in blood circulation might have transformed through deconjugation by glucuronidase and subsequent reconjugation by sulfotransfer-ase in these organs. The concentrations of baicalein glucuronides/ sulfates were highest in the liver, followed by the kidney and lung. Together with the fact that the lung contained the highest concentration of the baicalein free form, it provided evidence that the deconjugation reaction was higher and reconjugation lower in the lung when compared to the liver and kidney. These facts point to the likelihood that the biotransformation fate of baicalein glucuronides/sulfates varied from organ to organ. With regard to the distribution of wogonin and wogonin glucuro-nides/sulfates, the markedly higher concentrations of wogonin than wogonin glucuronides/sulfates in the liver, lung, and kidney indicated that the wogonin free form might have been trans-formed from wogonin glucuronides through deconjugation by glucuronidase in the organs. Traces of wogonin glucuronides/sul-fates present in the liver and kidney, but not in the lung, further implied that the deconjugation reaction was higher and reconju-gation lower in the lung when compared to the liver and kidney. Based on these findings, the reconjugation transformation of wo-gonin in the liver, kidney, and lung were apparently in lesser ex-tents than those of baicalein, leading us to suggest that the meta-bolic fates of flavonoids in organs were substrate-dependent. Neither the free forms nor the glucuronides/sulfates of baicalein and wogonin were detected in all brains of five rats, suggesting that these flavonoids might not enter the central nervous system after oral administration of the SR decoction. This result was dif-ferent from previous studies reporting the distribution of narin-genin, genistein, tangeretin, epicatechin, and puerarin in the brain [23–29]. We speculate that this discrepancy might arise from the residual blood in the brain, different routes of drug ad-ministration, or different biological fates of various flavonoids. Although various in vitro beneficial activities of baicalin, baica-lein, and wogonin have been reported in recent decades [11–16, 30], we suggest that the in vitro bioactivities of baicalin, a glucu-ronide of baicalein, may likely predict its in vivo effect in the vas-cular system, liver, or kidney, but not in the cerebral system. On the other hand, those in vitro bioactivities of baicalein and wogo-nin may predict their in vivo effects in the liver, lung, or kidney, but not in the vascular and cerebral systems.
Knowing where a compound accumulates in the human body makes it reasonable to predict where most of its benefits will likely occur, though in practice it is hard to do so. We provide here the pharmacological concentrations of baicalein, wogonin, and their glucuronides/sulfates in the blood, lung, liver and kid-ney of rats. In conclusion, the glucuronides/sulfates of baicalein and wogonin were exclusively present in the blood circulation, whereas their free forms appeared in the lung, liver, and kidney.
Fig. 4 Concentration of free form, glucuronides (G), and glucuronides/
sulfates (S/G) of baicalein (A) and wogonin (B) in serum and various tissues after oral administration of seven doses of 2 g/kg SR decoction to five rats.
* P < 0.05 compared to correspondent free form;#p < 0.05 compared to
correspondent G.
This
is
a
cop
y
of
the
author
ʼs
per
sonal
reprint
This
is
a
cop
y
of
the
author
ʼs
per
sonal
reprint
b
Acknowledgements
!
This work was supported by NSC95-2320-B039-001, NSC 96-2320-B‑039-037-MY3 (National Science Council), CCMP98-RD-104 (the Committee on Chinese Medicine and Pharmacy, Depart-ment of Health, Executive Yuan, ROC), and 063, CMU96-233 (China Medical University).
References
1 Kim EH, Shim B, Kang S, Jeong G, Lee JS, Yu YB, Chun M. Anti-inflamma-tory effects of Scutellaria baicalensis extract via suppression of im-mune modulators and MAP kinase signaling molecules. J
Ethnophar-macol 2009; 126: 320–331
2 Yoon SB, Lee YJ, Park SK, Kim HC, Bae H, Kim HM, Ko SG, Choi HY, Oh MS, Park W. Anti-inflammatory effects of Scutellaria baicalensis water ex-tract on LPS-activated RAW 264.7 macrophages. J Ethnopharmacol
2009; 125: 286–290
3 Tseng YP, Wu YC, Leu YL, Yeh SF, Chou CK. Scutellariae Radix suppresses hepatitis B virus production in human hepatoma cells. Front Biosci
(Elite Ed) 2010; 2: 1538–1547
4 Ikemoto S, Sugimura K, Yoshida N, Yasumoto R, Wada S, Yamamoto K, Kishimoto T. Antitumor effects of Scutellariae Radix and its compo-nents baicalein, baicalin, and wogonin on bladder cancer cell lines.
Urology 2000; 55: 951–955
5 Kumagai T, Müller CI, Desmond JC, Imai Y, Heber D, Koeffler HP. Scutel-laria baicalensis, a herbal medicine: anti-proliferative and apoptotic ac-tivity against acute lymphocytic leukemia, lymphoma and myeloma
cell lines. Leuk Res 2007; 31: 523–530
6 Wu JA, Attele AS, Zhang L, Yuan CS. Anti-HIV activity of medicinal herbs:
usage and potential development. Am J Chin Med 2001; 29: 69–81
7 Ma SC, Du J, But PP, Deng XL, Zhang YW, Ooi VE, Xu HX, Lee SH, Lee SF. Antiviral Chinese medicinal herbs against respiratory syncytial virus. J Ethnopharmacol 2002; 79: 205–211
8 Chu M, Chu ZY, Wang DD. The extract of compound Radix Scutellariae on mRNA replication and IFN expression of influenza virus in mice. Zhong Yao Cai 2007; 30: 63–65
9 Yang LP, Sun HL, Wu LM, Guo XJ, Dou HL, Tso MO, Zhao L, Li SM. Baicalein reduces inflammatory process in a rodent model of diabetic
retinopa-thy. Invest Ophthalmol Vis Sci 2009; 50: 2319–2327
10 Hsieh CJ, Hall K, Ha T, Li C, Krishnaswamy G, Chi DS. Baicalein inhibits IL-1beta- and TNF-alpha-induced inflammatory cytokine production from human mast cells via regulation of the NF-kappaB pathway. Clin Mol Allergy 2007; 5: 5
11 Shao ZH, Vanden Hoek TL, Qin Y, Becker LB, Schumacker PT, Li CQ, Dey L, Barth E, Halpern H, Rosen GM, Tuan CS. Baicalein attenuates oxidant stress in cardiomyocytes. Am J Physiol Heart Circ Physiol 2002; 282:
H999–H1006
12 Huang WH, Lee AR, Yang CH. Antioxidative and anti-inflammatory ac-tivities of polyhydroxyflavonoids of Scutellaria baicalensis GEORGI.
Biosci Biotechnol Biochem 2006; 70: 2371–2380
13 Kimata M, Shichijo M, Miura T, Serizawa I, Inagaki N, Nagai H. Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated me-diator release from human cultured mast cells. Clin Exp Allergy 2000;
30: 501–508
14 Huang RL, Chen CC, Huang HL, Chang CG, Chen CF, Chang C, Hsieh MT. Anti-hepatitis B virus effects of wogonin isolated from Scutellaria
bai-calensis. Planta Med 2000; 66: 694–698
15 Chan FL, Choi HL, Chen ZY, Chan PS, Huang Y. Induction of apoptosis in prostate cancer cell lines by a flavonoid, baicalin. Cancer Lett 2000;
160: 219–228
16 Himeji M, Ohtsuki T, Fukazawa H, Tanaka M, Yazaki S, Ui S, Nishio K, Ya-mamoto H, Tasaka K, Mimura A. Difference of growth-inhibitory effect of Scutellaria baicalensis-producing flavonoid wogonin among human
cancer cells and normal diploid cell. Cancer Lett 2007; 245: 269–274
17 Lai MY, Hsiu SL, Tsai SY, Hou YC, Chao PD. Comparison of metabolic pharmacokinetics of baicalin and baicalein in rats. J Pharm Pharmacol
2003; 55: 205–209
18 Lai MY, Hsiu SL, Chen CC, Hou YC, Chao PD. Urinary pharmacokinetics of baicalein, wogonin and their glycosides after oral administration of
Scutellariae Radix in humans. Biol Pharm Bull 2003; 26: 79–83
19 OʼLeary KA, Day AJ, Needs PW, Sly WS, OʼBrien NM, Williamson G. Flavo-noid glucuronides are substrates for human liver beta-glucuronidase. FEBS Lett 2001; 503: 103–106
20 Pasqualini JR, Chetrite GS. Recent insight on the control of enzymes in-volved in estrogen formation and transformation in human breast can-cer. J Steroid Biochem Mol Biol 2005; 93: 221–236
21 Reed MJ, Purohit A, Woo LW, Newman SP, Potter BV. Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr Rev 2005; 26:
171–202
22 de Boer VC, Dihal AA, van der Woude H, Arts IC, Wolffram S, Alink GM, Rietjens IM, Keijer J, Hollman PC. Tissue distribution of quercetin in rats
and pigs. J Nutr 2005; 135: 1718–1725
23 Peng HW, Cheng FC, Huang YT, Chen CF, Tsai TH. Determination of nar-ingenin and its glucuronide conjugate in rat plasma and brain tissue by high-performance liquid chromatography. J Chromatogr B Biomed Sci
Appl 1998; 714: 369–374
24 Prasain JK, Jones K, Brissie N, Moore R, Wyss JM, Barnes S. Identification of puerarin and its metabolites in rats by liquid
chromatography-tan-dem mass spectrometry. J Agric Food Chem 2004; 52: 3708–3712
25 Datla KP, Christidou M, Widmer WW, Rooprai HK, Dexter DT. Tissue dis-tribution and neuroprotective effects of citrus flavonoid tangeretin in a
rat model of Parkinsonʼs disease. Neuroreport 2001; 12: 3871–3875
26 Coldham NG, Sauer MJ. Pharmacokinetics of [14C]genistein in the rat:
gender-related differences, potential mechanisms of biological action, and implications for human health. Toxicol Appl Pharmacol 2000; 164:
206–215
27 Chang HC, Churchwell MI, Delclos KB, Newbold RR, Doerge DR. Mass spectrometric determination of genistein tissue distribution in
diet-exposed Sprague-Dawley rats. J Nutr 2000; 130: 1963–1970
28 Abd El Mohsen MM, Kuhnle G, Rechner AR, Schroeter H, Rose S, Jenner P, Rice-Evans CA. Uptake and metabolism of epicatechin and its access to
the brain after oral ingestion. Free Radic Biol Med 2002; 33: 1693–
1702
29 Youdim KA, Shukitt-Hale B, Joseph JA. Flavonoids and the brain: interac-tions at the blood-brain barrier and their physiological effects on the central nervous system. Free Radic Biol Med 2004; 37: 1683–1693 30 Ono K, Nakane H. Mechanisms of inhibition of various cellular DNA and
RNA polymerases by several flavonoids. J Biochem 1990; 108: 609–613