Manuscript Number:
Title: Isoflurane for spinal anesthesia in the rat Article Type: Research Paper
Keywords: Intrathecal Injection; Isoflurane; Lidocaine; Spinal Anesthesia Corresponding Author: Associate Professor Yu-Wen Chen, Ph.D.
Corresponding Author's Institution: China Medical University First Author: Ching-Hsia Hung, PhD
Order of Authors: Ching-Hsia Hung, PhD; Chin-Chen Chu, MD, PhD; Yu-Chung Chen, MS; Yu-Wen Chen, Ph.D.; Huei-Jyun Hong, BS; Jhi-Joung Wang, MD, PhD
Abstract: Although isoflurane, a non-water soluble agent, has been known to block Na+ currents, its spinal anesthetic effect was not exposed. The aim of this experiment was to evaluate to evaluate the local anesthetic effect of isoflurane in spinal anesthesia. After intrathecal injection of isoflurane on rats, the spinal anesthetic effect in motor function, proprioception and nociception were evaluated.
Lidocaine, a common used local anesthetic, was used as control. Isoflurane acted like lidocaine and produced dose-related spinal blockades of motor function, proprioception and nociception. Although isoflurane [27.6 (25.4 - 30.0)] had less potency when compared with lidocaine [1.0 (0.9 - 1.1)] (P < 0.001) in spinal anesthesia, it caused a much longer duration of spinal blockades than lidocaine at equianesthetic doses (P < 0.001). Our results showed that when compared with lidocaine, isoflurane produced a less potency but much longer duration in spinal anesthesia.
Suggested Reviewers: Kuang-I Cheng kuaich@kmu.edu.tw Juei-Tang Cheng jtcheng@mail.ncku.edu.tw Yuk-Man Leung ymleung@mail.cmu.edu.tw Bor-Tsang Wu wusletter@mail.cmu.edu.tw Ray-Yau Wang rywang@ym.edu.tw Kuo-Sheng Liu lanceliu@mail.chna.edu.tw Dong-Zi Shao dzshao@ms33.hinet.net
Dear Editors:
Enclosed please find an original manuscript entitled" Isoflurane
for spinal anesthesia in the rat " by Drs. Hung, Chu, Chen, Chen, Hong,
and Wang, which we wish to submit to you for consideration of
publication in NEUROSCIENCE LETTERS.
The manuscript has been submitted solely to this journal and has
not previously been published in any form in another publication of any
type with the exception of preliminary reports in abstract form.
We look forward to receiving your correspondence in near future.
Sincerely yours,
Yu-Wen Chen, Ph.D.
Associate Professor
Department of Physical Therapy, China Medical University
No.91 Hsueh-Shih Road, Taichung, Taiwan
Email address:
cywhwok@mail.cmu.edu.tw
Tel: 886-4-22053366 ext 7327
Fax: 886-4-22065051
Highlights
> Intrathecal injection of isoflurane produced spinal blockades. > Isofluranehad less
potency when compared with lidocaine. > Isoflurane caused a much longer duration
than lidocaine at equianesthetic doses. > Isoflurane produced a longer duration of
1
Isoflurane for spinal anesthesia in the rat
Ching-Hsia Hung, Ph.D.,1,2 Chin-Chen Chu, M.D., Ph.D.,2 Yu-Chung Chen, M.S.,3 Yu-Wen Chen, Ph.D.,2,4,* Huei-Jyun Hong, B.S.,4 Jhi-Joung Wang, M.D., Ph.D.2
1
Institute & Department of Physical Therapy, National Cheng Kung University, Tainan, Taiwan
2
Department of Medical Research, Chi-Mei Medical Center, Tainan, Taiwan
3
Division of Physical Therapy, Department of Physical Medicine and Rehabilitation, Cheng Hsin General Hospital, Taipei, Taiwan
4
Department of Physical Therapy, China Medical University, Taichung, Taiwan
*Address correspondence and reprint requests to: Yu-Wen Chen, PhD, Department of Physical Therapy, China Medical University, No.91 Hsueh-Shih Road, Taichung, Taiwan
Tel: 886-4-22053366 ext 7327 Fax: 886-4-22065051
Abstract
Although isoflurane, a non-water soluble agent, has been known to block Na+ currents,
its spinal anesthetic effect was not exposed. The aim of this experiment was to
evaluate to evaluate the local anesthetic effect of isoflurane in spinal anesthesia. After
intrathecal injection of isoflurane on rats, the spinal anesthetic effect in motor
function, proprioception and nociception were evaluated. Lidocaine, a common used
local anesthetic, was used as control. Isoflurane acted like lidocaine and produced
dose-related spinal blockades of motor function, proprioception and nociception.
Although isoflurane [27.6 (25.4 – 30.0)] had less potency when compared with
lidocaine [1.0 (0.9 – 1.1)] (P < 0.001) in spinal anesthesia, it caused a much longer
duration of spinal blockades than lidocaine at equianesthetic doses (P < 0.001). Our
results showed that when compared with lidocaine, isoflurane produced a less potency
but much longer duration in spinal anesthesia.
Isoflurane, an inhaled anesthetic agent, is commonly used in clinical anesthesia, and
its pharmacokinetic has been studied in healthy human volunteers and animals [7, 11,
15]. Important actions of inhaled anesthetics are associated with altered activity of
neuronal ion channels, particularly the fast synaptic neurotransmitter receptors such as
GABAA, nicotinic acetylcholine, and glutamate receptors [1, 20]. There is also
growing evidence that anesthetics affect neuronal ion channels by binding directly to
protein sites [1, 10, 19]. For instance, isoflurane at concentrations that occur during
clinical anesthesia inhibited both tetrodotoxin-resistant (TTX-r) Nav1.8 and
tetrodotoxin-sensitive (TTX-s) Nav [10]. Blockade of Na+ currents, which is one of
the major mechanisms of local anesthesia, produces spinal anesthesia, cutaneous
analgesia, and sciatic nerve block [5, 16].
Recently, it has been shown that subcutaneous injection of the three inhaled
anesthetics (halothane, isoflurane, and enflurane), like local anesthetics (lidocaine and
procaine), elicited a concentration-dependent, cutaneous analgesic effect on rat skin
[6]. However, to the best of our knowledge, no study of isoflurane in spinal anesthesia
has been reported to date. Spinal anesthesia is a relatively easy practice, which
produces adequate surgical conditions via injecting a small dose of local anesthetics,
giving a wide popularity to this practice. Dr. August Bier in 1899 first described
surgical goal [12]. The aim of this study was to investigate whether isoflurane
produced spinal blockades of motor, proprioception, and nociception, as well as the
spinal block effect of lidocaine. Lidocaine, a commonly used local anesthesia, was
used as a control.
Male Sprague-Dawley rats (300 ± 25 g) were obtained from the National
Laboratory Animal Centre, Taipei, Taiwan, and then they were housed in groups of
three, with food and water freely available until the time of testing. The climate-
controlled room was maintained at 22 ˚C with approximately 50% relative humidity
on a 12-h light/dark cycle (6:00 AM – 6:00 PM). The experimental protocol was
approved according to the Institutional Animal Care and Use Committee of China
Medical University, Taiwan, and conformed to the recommendations and policies of
the International Association for the Study of Pain (IASP).
AERRANE (Isoflurane, USP) were purchased from Baxter Healthcare of Puerto
Rico (Guayama, PR 00784, USA). Lidocaine base and sesame oil were purchased
from Sigma Chemical Co. (St. Louis, MO, USA). Isoflurane and lidocaine were
freshly prepared in sesame oil as solution before intrathecal injections.
Three specific experiments were performed. In experiment 1, the time courses of
isoflurane (60, 40, 30, 20, and 10 %), vehicle (sesame oil), and lidocaine (2.98, 2.17,
each drug) in Figs. 2 and 3. In experiment 2, at equianesthetic doses, the block effect
of 60% isoflurane in spinal anesthesia was compared with 2.98% lidocaine (n=8 rats
for each dose of each drug) in Table 1. In experiment 3, on equipotent doses (ED25,
ED50 and ED75), the block duration of isoflurane was compared with that of lidocaine
(n=8 rats for each dose of each drug) in Fig. 4.
Before intrathecal injections and behavioral tests, animals were handled to
minimize stress-induced analgesia and to be familiarized with the experiments. The
agents were intrathecally injected into conscious rats as previously described [3, 14].
In brief, a 27-gauge needle attached to a 50-μL syringe (Hamilton, Reno, Nevada)
was inserted into the midline of the lumbar 4-5 (L4-5) intervertebral space and 25-μL
of drugs was injected. Rats were then observed for paralysis of two hind limbs,
meaning for spinal blockades. Rats that displayed unilateral blockades were excluded
from the experiment and sacrificed by using an overdose of isoflurane. All animals
were injected intrathecally one time in this study. After the experiment, rats were
sacrificed by using an overdose of isoflurane.
For consistency, one experimenter who was blinded to the drugs and doses used,
was responsible for handling all the rats and behavioral evaluations. Motor function,
proprioception, and nociception were assessed as previously described [2, 12]. In brief,
right hind limb of each rat on a digital scale. A force less than 20 g [4] was interpreted
as a 100% motor block or 100% maximal possible effect (MPE), and the pre-injection
control value was considered a 0% motor block or 0% MPE.
The % possible effect (PE) is calculated via the equation:
% PE = 100% (Gm–Gt) ÷ (Gm–20)
where Gm is the peak muscle force (g) of each rat before drug injections and Gt is the
peak muscle force (g) of each rat after drug injections. The maximum value of % PE
is the %MPE.
The nociception was graded as 4 (normal or 0% MPE), 3 (25% MPE), 2 (50%
MPE), 1 (75% MPE), and 0 (absent or 100% MPE) according to the withdrawal reflex
or vocalization elicited via pinching the lateral metatarsus of the two hind limbs, the
dorsal part of the mid-tail, and a skin fold on each rat's back at 1 cm from the
proximal part of the tail. Proprioceptive evaluation was based on the postural
reactions and resting posture (‘tactile placing’ and ‘hopping’). A predominantly
proprioceptive block causes a delayed hopping followed by greater lateral hops to
prevent the animal from falling. In the case of full blockade, there would be no
hopping maneuvers. The functional deficit was graded as 3 (normal or 0% MPE), 2
impaired or 100% MPE).
After animals were intrathecal injected with different doses of isoflurane and
lidocaine (n = 8 for each dose of each drug), the % MPE of each dose of each drug
were obtained. The % MPE of each dose of each drug was then fitted by using SAS
Nonlinear (NLIN) Procedures (version 9.1, SAS Institute, Cary, NC), and the value of
ED50, defined as the dose that elicited 50% spinal blockades, were gotten [12, 17].
The ED25 and ED75 of drugs were obtained via the same curve-fitting (SAS NLIN
Procedures) that was used to derive the ED50[17]. Drug potencies were compared via
the ED50, constructed from the % MPE of each dose of each drug.
The blockade duration, defined as the interval from drug injection to full
recovery, caused by each drug (n = 8 rats for each dose of each drug) was evaluated at
equipotent doses (ED25, ED50, and ED75). In this study, we also evaluated the %MPE,
complete blockade time, time to full recovery, area under curves (AUCs) of motor,
proprioception and nociception for 60% isoflurane and 2.98% lidocaine. The AUCs of
spinal blockades of drugs were obtained via Kinetica v 2.0.1 (MicroPharm
International, USA).
Data were presented as mean±S.E.M. or ED50 value with 95% confidence
interval (95% CI) and were analyzed by the Student’s t-test. The differences in
HSD test. SPSS for Windows (version 17.0) was used for all statistical analyses.
Statistical significance was set at P < 0.05.
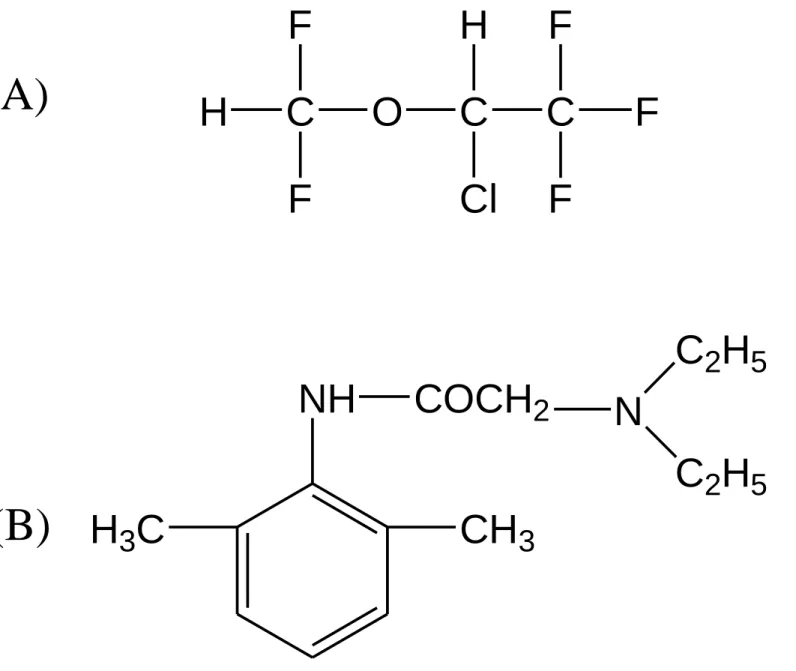
The structures of isoflurane and lidocaine are shown in Figure 1. Intrathecal
isoflurane, as well as lidocaine produced spinal blockades of motor function,
proprioception, and nociception in rats (Figs. 2 and 3). Isoflurane (60%) caused 100%
spinal blockades (% MPE) of motor function, proprioception, and nociception with
durations of actions of 53.8±4.2, 55.0±4.9, and 60.6±4.8 min, respectively (Fig. 2 and
Table 1). Lidocaine (2.98%) elicited 100% spinal blockades of motor function,
proprioception, and nociception with durations of actions of 26.3±3.6, 32.5±3.1, and
35.0±1.9 min, respectively (Fig. 3 and Table 1). To rule out the effect of vehicle,
intrathecal injections of sesame oil produced no spinal anesthetic effects (Figs. 2 and
3). There were no significant differences in efficacy between 60% isoflurane and
2.98% lidocaine in spinal blockades of motor function, proprioception, and
nociception (Figs. 2 and 3). However, complete block time, time to full recovery, and
AUC of spinal blockade of 60% isoflurane are significantly greater than those of
2.98% lidocaine in motor function, proprioception, and nociception (Table 1).
After intrathecal injections (5 doses in each group), the time courses of motor
function, proprioception, and nociception of isoflurane and lidocaine were constructed
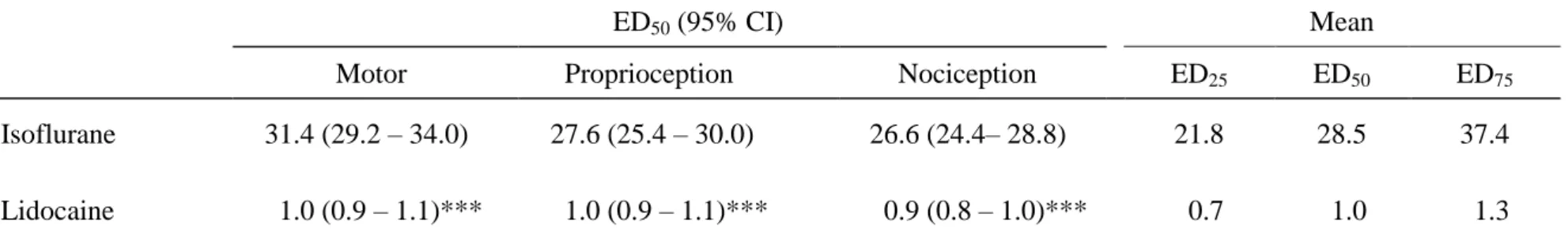
using SAS Nonlinear (NLIN) Procedures, are shown in Table 2. On the ED50 basis,
lidocaine was more potent than isoflurane in spinal anesthesia (Table 2; P < 0.001).
On equianesthetic basis (ED25, ED50, and ED75), the block duration in motor function,
proprioception, and nociception caused by isoflurane (P < 0.001) was longer than that
caused by lidocaine (Fig. 4). The nociceptive block potency (26.6 [24.4– 28.8]) by
isoflurane was found to be greater than the motor one (31.4 [29.2 – 34.0]) in Table 2.
All rats recovered completely after intrathecal injections of drugs or vehicles.
In this report we showed that intrathecal isoflurane produces a spinal anesthetic
effect. Isoflurane has a weak potency but much longer duration when compares with
lidocaine in spinal anesthesia in rats.
Local anesthetics are well-known to produce spinal anesthesia through their Na+
channel blocking activities on the central nervous system [8, 16]. In this report, we
found that the inhaled isoflurane produced dose-dependent, spinal anesthesia, similar
to that of the local anesthetic lidocaine. Inhaled anesthetics are also known to have
Na+ channel blocking activities, not only on the peripheral nervous system [10], but
also on the central nervous system [21]. Accordingly, it is possible that inhaled
isoflurane may exert their spinal anesthetic effect through similar Na+ channel
blocking activities on the central nervous system, although more studies are needed to
Long-acting local anesthetics and analgesics currently used for surgery and
postoperative pain in clinical practice [13, 16]. The nociceptive blockade (AUC) of
isoflurane was approximately 1.7-folds greater than that of lidocaine at equivalent
doses. Furthermore, the block duration in motor, proprioception, and nociception
caused by isoflurane was longer than that caused by lidocaine at equianesthetic doses
(Fig. 4). Although 60% isoflurane displayed completely spinal anesthetic effects, it is
still higher than 2.98% lidocaine. Because isoflurane produced spinal anesthesia
through a local mechanism after intrathecal injection, this mechanism might also play
a role on the analgesic effect of inhaled anesthetics during general anesthesia.
In this study, sesame oil was used as a vehicle for inhaled isoflurane. Before this
study, several solvents (e.g., saline, intralipid, lecithin, sesame oil etc.) had been
tested for their potential suitability as vehicles for inhaled anesthetics. Among these
solvents, sesame oil showed the best solubility for inhaled isoflurane. Meanwhile, it
remains unclear whether the high concentration isoflurance affects the function of
spinal cord to modify the results of spinal anesthesia. However, all rats recovered
completely after intrathecal injections.
Bupivacaine in resemblance to the clinical impression is the drug of choice when
a more sensory-selective action over motor blockade [9, 18]. Intrathecal injection of
blockade (Figs. 2 and 4). Furthermore, we found that the potency (ED50) of isoflurane
in nociceptive blockade was more potent than that in motor blockade (Table 2). The
sensory/nociceptive blockade in isoflurane was almost 1.2-folds higher potency (ED50)
than the motor blockade. Bupivacaine is rarely noted the sensory/motor potency in
clinical practice because complete blockades are practiced. Further studies on sciatic
nerve block and related neural and cardiovascular toxicities will be warranted.
In conclusion, this preclinical study demonstrated that isoflurane is shown to
hold spinal (local) anesthetic properties. Although isoflurane is less potent to
lidocaine in spinal anesthesia, its anesthetic action is much more long-lasting than that
Acknowledgements
The financial support provided for this study was from the National Science
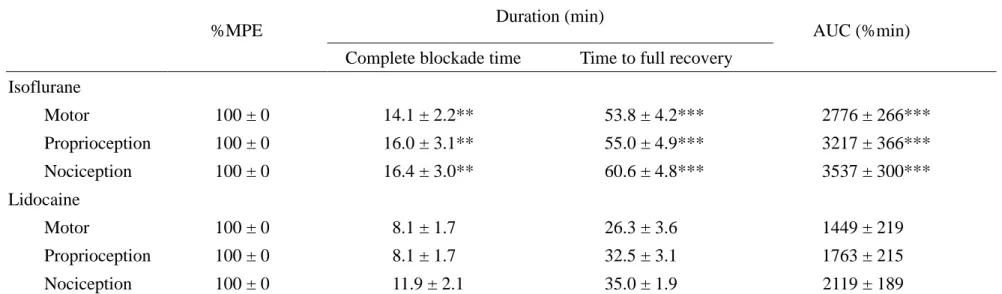
Table 1. Percent of maximal possible effect (%MPE), duration of drug action, and area under curve (AUC) values for motor,
proprioception, and nociception after intrathecal injection of 60% isoflurane or 2.98% lidocaine.
%MPE Duration (min) AUC (%min)
Complete blockade time Time to full recovery Isoflurane Motor 100 ± 0 14.1 ± 2.2** 53.8 ± 4.2*** 2776 ± 266*** Proprioception 100 ± 0 16.0 ± 3.1** 55.0 ± 4.9*** 3217 ± 366*** Nociception 100 ± 0 16.4 ± 3.0** 60.6 ± 4.8*** 3537 ± 300*** Lidocaine Motor 100 ± 0 8.1 ± 1.7 26.3 ± 3.6 1449 ± 219 Proprioception 100 ± 0 8.1 ± 1.7 32.5 ± 3.1 1763 ± 215 Nociception 100 ± 0 11.9 ± 2.1 35.0 ± 1.9 2119 ± 189
Values are meanS.E.M.; n = 8, each group. Of note, all of the rats showed complete blockade (100%MPE) of any function tested. Symbols (***, **) indicate P < 0.01 and P < 0.001, respectively, when isoflurane compared with lidocaine.
Table 2. The 50% effective dose (ED50) values of isoflurane and lidocaine with 95% confidence interval (95% CI) on spinal
blockades of motor, proprioception, and nociception in rats.
ED50 (95% CI) Mean
Motor Proprioception Nociception ED25 ED50 ED75
Isoflurane 31.4 (29.2 – 34.0) 27.6 (25.4 – 30.0) 26.6 (24.4– 28.8) 21.8 28.5 37.4 Lidocaine 1.0 (0.9 – 1.1)*** 1.0 (0.9 – 1.1)*** 0.9 (0.8 – 1.0)*** 0.7 1.0 1.3 The ED50s of isoflurane and lidocaine (%) were obtained from Figs. 2 and 3 by SAS Nonlinear (NLIN) Procedures. CI = confidence
15
Legends to figures
Fig. 1. The chemical structures of isoflurane (A) and lidocaine (B).
Fig. 2. Time courses of spinal blockade (% PE) by isoflurane (60-10%) and sesame
oil in rats. Neurological evaluation was constructed after drug injection. Data are presented as mean±S.E.M.; each group, n=8.
Fig. 3. Time courses of spinal blockade (% PE) by lidocaine (0.54-2.98%) and sesame
oil in rats. Neurological evaluation was constructed after drug injection. Data are presented as mean±S.E.M.; each group, n=8.
Fig. 4. Full recovery time of action of isoflurane and lidocaine on spinal blockades of
motor, proprioception, and nociception at equipotent doses of ED25, ED50, and ED75
(n = 8 at each testing point). Values are expressed as meanS.E.M. The differences in duration were evaluated by using 2-way ANOVA followed by pairwise Tukey's HSD test.
16
References
[1] J.A. Campagna, K.W. Miller, S.A. Forman, Mechanisms of actions of inhaled anesthetics, The New England journal of medicine 348 (2003) 2110-2124. [2] Y.W. Chen, Y.C. Chen, C.N. Lin, C.C. Chu, M.T. Lin, J.J. Wang, C.H. Kao,
The spinal anaesthetic effect of dextromethorphan, dextrorphan, and 3-methoxymorphinan, Eur. J. Pharmacol. 569 (2007) 188-193.
[3] Y.W. Chen, C.C. Chu, Y.C. Chen, J.J. Wang, C.H. Hung, The dose-dependent study of verapamil and diltiazem on spinal anesthesia in the rat, Neurosci. Lett. 482 (2010) 76-80.
[4] Y.W. Chen, C.C. Chu, Y.C. Chen, J.J. Wang, C.H. Hung, Isobolographic analysis of caramiphen and lidocaine on spinal anesthesia in rats, Neurosci. Lett. 469 (2010) 174-178.
[5] Y.W. Chen, J.J. Wang, T.Y. Liu, Y.C. Chen, C.H. Hung, Systemic
dextromethorphan and dextrorphan are less toxic in rats than bupivacaine at equianesthetic doses, Can. J. Anaesth. 58 (2011) 55-61.
[6] C.C. Chu, S.Z. Wu, W.L. Su, J.P. Shieh, C.H. Kao, S.T. Ho, J.J. Wang, Subcutaneous injection of inhaled anesthetics produces cutaneous analgesia, Can. J. Anaesth. 55 (2008) 290-294.
[7] A. Fassoulaki, C. Sarantopoulos, G. Karabinis, C. Derveniotis, Skin application of isoflurane attenuates the responses to a mechanical and an electrical stimulation, Canadian journal of anaesthesia = Journal canadien d'anesthesie 45 (1998) 1151-1155.
[8] H.A. Fozzard, P.J. Lee, G.M. Lipkind, Mechanism of local anesthetic drug action on voltage-gated sodium channels, Curr. Pharm. Des. 11 (2005) 2671-2686.
17
[9] S. Gurlit, S. Reinhardt, M. Mollmann, Continuous spinal analgesia or
opioid-added continuous epidural analgesia for postoperative pain control after hip replacement, Eur. J. Anaesthesiol. 21 (2004) 708-714.
[10] K.F. Herold, C. Nau, W. Ouyang, H.C. Hemmings, Jr., Isoflurane inhibits the tetrodotoxin-resistant voltage-gated sodium channel Nav1.8, Anesthesiology 111 (2009) 591-599.
[11] C.W. Honemann, J. Washington, M.C. Honemann, G.W. Nietgen, M.E. Durieux, Partition coefficients of volatile anesthetics in aqueous electrolyte solutions at various temperatures, Anesthesiology 89 (1998) 1032-1035. [12] C.H. Hung, J.J. Wang, Y.C. Chen, C.C. Chu, Y.W. Chen, Intrathecal
oxybuprocaine and proxymetacaine produced potent and long-lasting spinal anesthesia in rats, Neurosci. Lett. 454 (2009) 249-253.
[13] M.A. Khan, P. Gerner, G. Kuo Wang, Amitriptyline for prolonged cutaneous analgesia in the rat, Anesthesiology 96 (2002) 109-116.
[14] Y.M. Leung, B.T. Wu, Y.C. Chen, C.H. Hung, Y.W. Chen, Diphenidol
inhibited sodium currents and produced spinal anesthesia, Neuropharmacology 58 (2010) 1147-1152.
[15] C.C. Lu, S.T. Ho, J.J. Wang, C.S. Wong, O.Y. Hu, S.Y. Chang, C.Y. Lin, Pharmacokinetics of isoflurane: uptake in the brain, Pharmacology 69 (2003) 102-107.
[16] H.A. McLure, A.P. Rubin, Review of local anaesthetic agents, Minerva. Anestesiol. 71 (2005) 59-74.
[17] S. Minkin, K. Kundhal, Likelihood-based experimental design for estimation of ED50, Biometrics 55 (1999) 1030-1037.
18
sodium channels by the enantiomers of bupivacaine, Anesthesiology 93 (2000) 1022-1033.
[19] W. OuYang, H.C. Hemmings, Jr., Isoform-selective effects of isoflurane on voltage-gated Na+ channels, Anesthesiology 107 (2007) 91-98.
[20] W. Ouyang, K.F. Herold, H.C. Hemmings, Jr., Comparative effects of halogenated inhaled anesthetics on voltage-gated Na+ channel function, Anesthesiology 110 (2009) 582-590.
[21] W. Ouyang, G. Wang, H.C. Hemmings, Jr., Isoflurane and propofol inhibit voltage-gated sodium channels in isolated rat neurohypophysial nerve terminals, Mol. Pharmacol. 64 (2003) 373-381.