667
A
nguillicola crassus (Kuwahara, Niimi andItagaki 1974) is an endemic swimbladder parasite of the Japanese eel Anguilla japonica in East Asia (Kuwahara et al. 1974, Nagasawa et al. 1994). Eels are infected by ingestion of 3rd-stage juveniles (L3 larvae) present in copepods, a crustacean that serves as an intermediate host (Kennedy and Fitch 1990, Ooi et al. 1997), or by preying on small paratenic fish hosts containing L3 larvae (Kirk 2003). Adult male and female nematodes copulate in the lumen of the eel’s swimbladder.
The Swimbladder Parasite Anguillicola crassus in Native Japanese Eels
and Exotic American Eels in Taiwan
Yu-San Han1,*, Ya-Ting Chang1, Horst Taraschewski2, Su-Ling Chang3, Che-Chun Chen4, and
Wann-Nian Tzeng1
1Institute of Fisheries Sciences, College of Life Science, National Taiwan University, Taipei 106, Taiwan 2Zoological Institute, Ökologie/Parasitologie, University of Karlsruhe, Karlsruhe 76131, Germany
3Tungkang Biotechnology Research Center, Fisheries Research Institute, Council of Agriculture, Pingdong 928, Taiwan 4Department of Aquatic Biosciences, National Chiayi University, Chiayi 600, Taiwan
(Accepted April 30, 2008)
Yu-San Han, Ya-Ting Chang, Horst Taraschewski, Su-Ling Chang, Che-Chun Chen, and Wann-Nian Tzeng (2008) The swimbladder parasite Anguillicola crassus in native Japanese eels and exotic American eels in Taiwan. Zoological Studies 47(6): 667-675. To understand differences in infection patterns of the swimbladder parasite Anguillicola crassus between habitats and eel species in Taiwan, the prevalence and intensity of the parasite were examined based on specimens collected from wild and cultured Japanese eel Anguilla japonica and from exotic cultured American eel A. rostrata in the Kaoping River and culture ponds in southwestern Taiwan in 2006-2007. The prevalence of Aco. crassus in wild Japanese eels was lower in winter compared with summer/autumn, varying 33%-58%, with a mean intensity of 1.5-4.4. The prevalence and intensity were size-dependent and increased with eel size. In cultured Japanese eels, the prevalence and mean intensity varied greatly at 3%-68% and 1.0-29.0, respectively. In cultured American eels, the prevalence and intensity were very high in ponds without drug treatment. In contrast to wild eels, the mean intensity of larval and adult worms showed a size-dependent decreasing trend in cultured eels. The mean body mass of Aco. crassus in American eels was significantly larger than that in Japanese eels. The external morphology, condition factor, and hepatosomatic index showed no significant differences between infected and uninfected groups, indicating a low pathogenic effect of Aco. crassus on these 2 eel hosts. Our results showed that both native Japanese eels and naive American eels are highly susceptible to Aco. crassus, but it causes little pathogenicity under good pond management. http://zoolstud.sinica.edu.tw/Journals/47.6/667.pdf
Key words: Anguillicola crassus, Anguilla japonica, Anguilla rostrata, Infection, Aquaculture.
Females passively release eggs which leave the swimbladder via the pneumatic duct, pass down the intestine and hatch in the water as a motile 2nd-stage juvenile (L2 larvae), although some hatch in the swimbladder (Kirk et al. 2000). L2 larvae attach to the substratum and undulate to stimulate predation by intermediate hosts and grow to L3 larvae while waiting to enter the definitive eel hosts (Thomas and Ollevier 1993).
In the early 1980s, Aco. crassus was acci-dentally introduced into Europe, possibly through
* To whom correspondence and reprint requests should be addressed. Tel: 886-2-33663726. E-mail:yshan@ntu.edu.tw
importation of infected Japanese eels from Taiwan into Germany in 1980 (Koops and Hartmann 1989). Aco. crassus was first detected in 1982 in European eel A. anguilla in northern Germany and then rapidly spread in Europe (Kirk 2003).
Aco. crassus has now spread to eastern states
of North America (Fries et al. 1996, Barse et al. 2001) and also to North and East Africa (Sasal et al. 2008). Based on historical records, eel populations, including European, American, and Japanese eels, have declined to approximately 1%-10% of population levels in the 1980s (Dekker et al. 2003). Declines in eel recruitment have probably been caused by a combination of factors including overfishing, pollution, global climatic and oceanic changes, and loss of habitat (Naismith and Knights 1990, Tzeng et al. 1995, Dekker et al. 2003, Tesch 2003). The decline in European eel populations might be partly due to infection by Aco. crassus because of its high pathogenicity (Molnár et al. 1993, Würtz and Taraschewski 2000, Kirk 2003, Taraschewski 2006). Studies indicate that Aco. crassus does not usually cause serious pathological damage to Japanese eels as it tends to occur at low intensities (Egusa 1992). In Europe when Aco. crassus invades a new habitat, it can often rise to 100% prevalence in the eel population. The severity of pathogenicity to European eels and associated high infection levels may reflect a lack of adaptations for resistance acquired after a long host-parasite co-evolutionary period (Würtz and Taraschewski 2000, Kirk 2003). Laboratory infections have revealed a stronger resistance against Aco. crassus by Japanese eels than by European eels (Knopf and Mahnke 2004). An enhanced humoral immune response against A. crassus in Japanese eels was also found in comparison to European eels (Nielsen 1999). The success of Aco. crassus as a colonizer outside Asia may be attributed to its adaptability to a wide range of intermediate and paratenic hosts (Kennedy et al. 1992, Kirk 2003, Moravec et al. 2005) as well as salinities (Kirk et al. 2000).
In Europe, research activities related to Aco.
crassus are far more intensive than in Asia and
America. Aco. crassus was first documented in American eel populations in North America in 1995 (Johnson et al. 1995). Aco. crassus was found in the middle portion of eastern North America followed by a rapid increase in distribution (Barse and Secor 1999, Barse et al. 2001). The infectivity and pathogenicity of Aco. crassus in its American eel host, however, are less well understood. In Taiwan, the Japanese eel is a commercially
important species for aquaculture. Due to a shortage of local Japanese eel elvers for culture, culturists have imported American eel elvers from North America to meet their needs since 1969 (Li 1997). This situation gave us an opportunity to investigate the infection and pathogenicity of
Aco. crassus in native Japanese eels and exotic
American eels in Taiwan as well as the effect of different aquatic environments on the infection patterns of Aco. crassus.
MATERIALS AND METHODS
Wild Japanese eels (n = 533) were collected from the Kaoping River estuary in southwestern Taiwan from June 2006 to Feb. 2007. The sampling locations and methods used for eel capture were described in detail by Han and Tzeng (2006). Cultured Japanese eels (n = 257) were purchased from 1 pond in Yonglin, 6 ponds in Budai (run by the same farmer), and 1 pond in Syuejia all of southwestern Taiwan in Sept. 2006. Cultured American eels (n = 102) were purchased from ponds in Yiwu and Yonglin, southwestern Taiwan in Mar. and Aug. 2007, respectively. Eels were transported to the laboratory and killed by decapitation, followed by a parasitological examination. The length and weight of the eels were measured to the nearest 1.0 mm and 0.1 g, respectively. The condition factor (body weight (g)/ total length (mm)3 × 106) and hepatosomatic index (HSI, liver weight (g)/ body weight (g) × 100) of the eels were calculated as described by Han et al. (2003).
Adult parasites were removed from the swimbladder lumen of the eels by forceps. Their body weight (to the nearest 0.1 mm), sex, and number were recorded. The walls of the swimbladders were checked for larval numbers by squashing them between 2 Perspex plates. The prevalence, mean intensity, and abundance of parasites in eels were calculated as described by Bush et al. (1997).
Differences in mean total length, body weight, and HSI among eel groups were tested by one-way analysis of variance (ANOVA). Dependence of parasite prevalence on the total length of eel stocks was examined by the Chi-squared test of homogeneity using a X2 contingency table.
The condition factor in relation to total length of eels between infected and uninfected groups was first tested for homogeneity of regression slopes, followed by comparison of the adjusted
means using analysis of covariance (ANCOVA) as implanted in the SPSS 10 software. Differences in worm body weights among eel groups were analyzed using the Mann-Whitney U-test. Significance was accepted at p < 0.05.
RESULTS Eel data
Total length, body weight, HSI, and the condition factor of the investigated Japanese and American eels are summarized in table 1. For cultured Japanese and American eels, each batch of samples showed a more-uniform size distribution because of grading procedures or size selection during the course of culturing. Batches of cultured eels with the smallest and largest mean total lengths came from the Budai ponds BD2 and BD5, respectively (Table 1).
Infection patterns of Aco. crassus in wild and cultured eels
A l l p a r a s i t e s i d e n t i f i e d i n t h e e e l s’ swimbladders were Aco. crassus according to
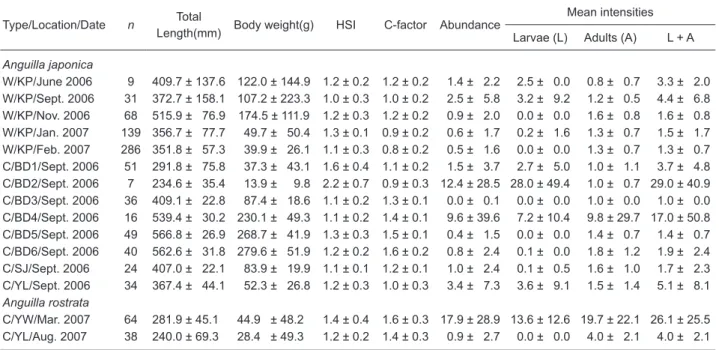
the key of Moravec and Taraschewski (1988). The prevalence of Aco. crassus in wild Japanese eels ranged 33%-58%, and were lower in winter compared to summer/autumn (Fig. 1). Prevalences of Aco. crassus varied 3%-68% in cultured Japanese eels and 24%-69% in cultured American eels (Fig. 1). The abundance and mean intensity of Aco. crassus were generally low in wild Japanese eels, but were highly variable in both cultured Japanese and American eels (Table 1).
The relationship between eel size and prevalence indicated that wild Japanese eels smaller than 40 cm had significantly lower prevalences than those larger than 40 cm (χ2 =
17.39, d.f. = 3, p < 0.001) (Table 2). The mean intensities of larval and adult worms showed size-dependent increases in wild Japanese eels. Prevalences varied among size classes in cultured Japanese and American eels (Table 2). Mean intensities of larval and adult worms in cultured Japanese and American eels, however, showed size-dependent decreasing and increasing tendencies (Table 2).
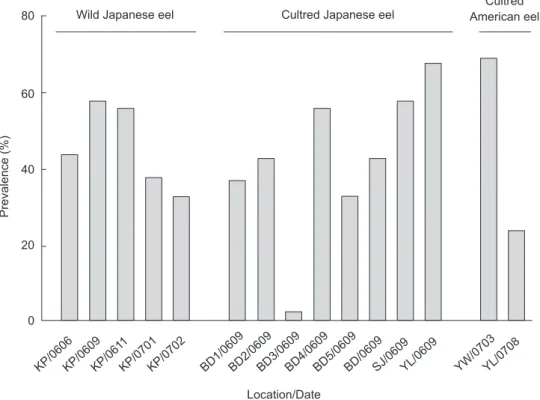
Frequency distributions of adult and larval
Aco. crassus per eel approached exponential
decay in wild and cultured Japanese eels but showed a negative binomial distribution in cultured American eels (Fig. 2). Percentages of seriously
Table 1. Sample size, mean total length (± S.D.), body weight, hepatosomatic index (HSI), condition factor (C-factor), and mean intensity of Anguillicola crassus in wild (W) and cultured (C) eels. KP, Kaoping; BD, Budai; SJ, Syuejia; YL, Yonglin; YW, Yiwu
Type/Location/Date n Length(mm)Total Body weight(g) HSI C-factor Abundance Mean intensities Larvae (L) Adults (A) L + A
Anguilla japonica W/KP/June 2006 9 409.7 ± 137.6 122.0 ± 144.9 1.2 ± 0.2 1.2 ± 0.2 1.4 ± 2.2 2.5 ± 0.0 0.8 ± 0.7 3.3 ± 2.0 W/KP/Sept. 2006 31 372.7 ± 158.1 107.2 ± 223.3 1.0 ± 0.3 1.0 ± 0.2 2.5 ± 5.8 3.2 ± 9.2 1.2 ± 0.5 4.4 ± 6.8 W/KP/Nov. 2006 68 515.9 ± 176.9 174.5 ± 111.9 1.2 ± 0.3 1.2 ± 0.2 0.9 ± 2.0 0.0 ± 0.0 1.6 ± 0.8 1.6 ± 0.8 W/KP/Jan. 2007 139 356.7 ± 177.7 49.7 ± 50.4 1.3 ± 0.1 0.9 ± 0.2 0.6 ± 1.7 0.2 ± 1.6 1.3 ± 0.7 1.5 ± 1.7 W/KP/Feb. 2007 286 351.8 ± 157.3 39.9 ± 26.1 1.1 ± 0.3 0.8 ± 0.2 0.5 ± 1.6 0.0 ± 0.0 1.3 ± 0.7 1.3 ± 0.7 C/BD1/Sept. 2006 51 291.8 ± 175.8 37.3 ± 43.1 1.6 ± 0.4 1.1 ± 0.2 1.5 ± 3.7 2.7 ± 5.0 1.0 ± 1.1 3.7 ± 4.8 C/BD2/Sept. 2006 7 234.6 ± 135.4 13.9 ± 9.8 2.2 ± 0.7 0.9 ± 0.3 12.4 ± 28.5 28.0 ± 49.4 1.0 ± 0.7 29.0 ± 40.9 C/BD3/Sept. 2006 36 409.1 ± 122.8 87.4 ± 18.6 1.1 ± 0.2 1.3 ± 0.1 0.0 ± 0.1 0.0 ± 0.0 1.0 ± 0.0 1.0 ± 0.0 C/BD4/Sept. 2006 16 539.4 ± 130.2 230.1 ± 49.3 1.1 ± 0.2 1.4 ± 0.1 9.6 ± 39.6 7.2 ± 10.4 9.8 ± 29.7 17.0 ± 50.8 C/BD5/Sept. 2006 49 566.8 ± 126.9 268.7 ± 41.9 1.3 ± 0.3 1.5 ± 0.1 0.4 ± 1.5 0.0 ± 0.0 1.4 ± 0.7 1.4 ± 0.7 C/BD6/Sept. 2006 40 562.6 ± 131.8 279.6 ± 51.9 1.2 ± 0.2 1.6 ± 0.2 0.8 ± 2.4 0.1 ± 0.0 1.8 ± 1.2 1.9 ± 2.4 C/SJ/Sept. 2006 24 407.0 ± 122.1 83.9 ± 19.9 1.1 ± 0.1 1.2 ± 0.1 1.0 ± 2.4 0.1 ± 0.5 1.6 ± 1.0 1.7 ± 2.3 C/YL/Sept. 2006 34 367.4 ± 144.1 52.3 ± 26.8 1.2 ± 0.3 1.0 ± 0.3 3.4 ± 7.3 3.6 ± 9.1 1.5 ± 1.4 5.1 ± 8.1 Anguilla rostrata C/YW/Mar. 2007 64 281.9 ± 45.1 44.9 ± 48.2 1.4 ± 0.4 1.6 ± 0.3 17.9 ± 28.9 13.6 ± 12.6 19.7 ± 22.1 26.1 ± 25.5 C/YL/Aug. 2007 38 240.0 ± 69.3 28.4 ± 49.3 1.2 ± 0.2 1.4 ± 0.3 0.9 ± 2.7 0.0 ± 0.0 4.0 ± 2.1 4.0 ± 2.1
infected samples with the number of larvae > 7 in wild Japanese eels, cultured Japanese eels, and American eels were 0.4%, 2.8%, and 23.5%, and samples with a no. of adults of > 7 were 0.0%,
0.3%, and 24.5%, respectively (Fig. 2). Mean body mass values of Aco. crassus, regardless of being male or female, were significantly larger in American eels than in Japanese eels, and no
Table 2. Relationship between size and infection in wild and cultured eels. TL, total length (mm); n, sample size; P, prevalence (%)
TL < 300 300 ≤ TL < 400 400 ≤ TL < 500 TL ≥ 500
Wild Anguilla japonica
n 100 277 96 60
P (%) 30.3 33.7 52.1 51.7
Larval mean intensity 0.03 ± 0.18 0.34 ± 1.99 0.40 ± 1.21 1.56 ± 4.41 Adult mean intensity 1.23 ± 0.56 1.22 ± 0.67 1.50 ± 0.91 1.56 ± 1.09 Cultured Anguilla japonica
n 49 44 61 103
P (%) 44.9 50.0 25.0 40.8
Larval mean intensity 6.18 ± 16.38 2.73 ± 7.96 1.92 ± 3.62 1.63 ± 5.42 Adult mean intensity 1.05 ± 1.25 1.27 ± 1.16 1.77 ± 1.69 3.37 ± 12.30 Cultured Anguilla rostrata
n 75 25 -
-P (%) 42.7 76.0 -
-Larval mean intensity 14.40 ± 12.65 9.68 ± 12.38 - -Adult mean intensity 10.60 ± 22.59 18.16 ± 17.14 - -80 60 40 20 0 Prevalence (%)
Wild Japanese eel Cultred Japanese eel American eelCultred
KP/0606KP/0609KP/061 1
KP/0701KP/0702 BD1/0609BD2/0609BD3/0609BD4/0609BD5/0609BD/0609SJ/0609YL/0609 YW/0703YL/0708
Location/Date
Fig. 1. Prevalence of Anguillicola crassus in wild (n = 533) and cultured (n = 257) Japanese eels and cultured (n = 102) American eels. KP, Kaoping; BD, Budai; SJ, Syuejia; YL, Yonglin; YW, Yiwu. Sampling date: yy/mm.
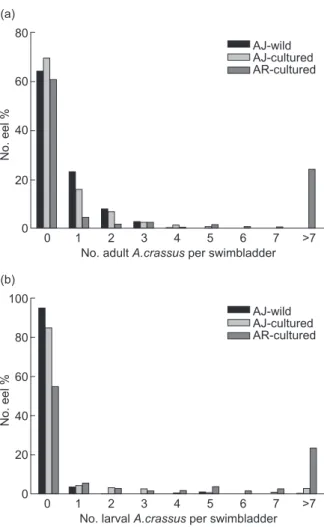
significant differences were found between wild and cultured Japanese eels (Fig. 3).
Comparison of morphometric characteristics between infected and uninfected eels
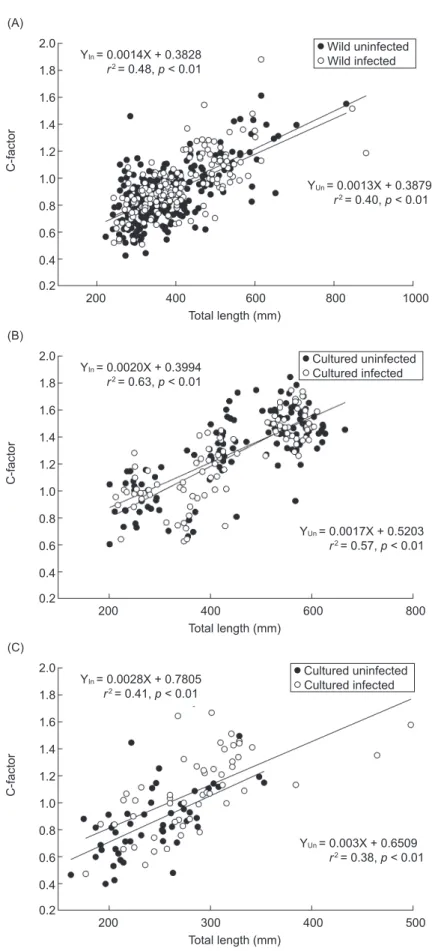
Infected eels showed a normal external morphology and activity with no observable disorders. The total length, body weight, and HSI showed no significant differences between infected and uninfected eel groups among wild and cultured Japanese eels or in American eels. Condition factors showed significant size-dependent increases (Fig. 4). The regression slopes and adjusted means (ANCOVA, wild Japanese eels:
p = 0.302; cultured Japanese eels: p = 0.782;
and cultured American eels: p = 0.102) showed no significant differences between infected and uninfected eel groups (Fig. 4).
DISCUSSION
In a previous study in 2000-2003 (Münderle et al. 2006), the prevalence of Aco. crassus in eels from the Kaoping River ranged 51%-62% in spring and autumn but dropped to 21% in winter. In this study, the prevalence of Aco. crassus in the same location showed similar trends, with higher prevalences in summer/autumn than in winter. Seasonal changes in prevalences of Aco. crassus were also observed in cultured Japanese eels in Japan (Egusa et al. 1969) and Korea (Kim et al. 1989), which might reflect the lower abundances of intermediate and/or paratenic fish hosts in the cold season, or reduced feeding activity of the eel hosts. On the other hand, the prevalence and mean intensity of both larval and adult-stage worms showed size-dependent increases in wild Japanese eels. This suggests that the eel accumulates parasites during its lifetime. However, the intensity of larval stage worms showed size-dependent decreases in both cultured Japanese and American eels. The reason might be that the host regulates repeated infections by Aco. crassus to some extent (Knopf and Lucius 2008), especially when the infection intensity is already high, such as in cultured eels.
Under aquaculture conditions, the mean intensity of Aco. crassus infection was at least 2-fold higher than that in the wild. The higher intensity of infection in cultured eels is probably related to the higher density of eels and greater chances of contact with the parasite in controlled environments like ponds. Due to the absence or
Fig. 2. Frequency distribution of adult (a) and larval (b)
Anguillicola crassus in wild and cultured Japanese eels (AJ)
and cultured American eels (AR). 80 60 40 20 0 No. eel % 0 1 2 3 4 5 6 7 >7
No. adult A.crassus per swimbladder AJ-wild AJ-cultured AR-cultured (a) 100 60 40 20 0 No. eel % 0 1 2 3 4 5 6 7 >7
No. larval A.crassus per swimbladder AJ-wild AJ-cultured AR-cultured (b)
80
Fig. 3. Comparison of the mean body mass of male and female Anguillicola crassus among wild and cultured Japanese eels and cultured American eels. *p < 0.05, compared to wild Japanese eels. 300 200 150 100 0 Body mass (mg) Male Female
Wild Japanese eel Cultured Japanese eel Cultured American eel 250
50
*
Fig. 4. Regression of total length on the condition factor (C-factor) between uninfected and infected groups of wild (A) and cultured (B) Japanese eels and cultured (C) American eels.
2.0 1.2 1.0 0.8 0.2 C-factor Total length (mm) YIn = 0.0014X + 0.3828 r2 = 0.48, p < 0.01 1.6 0.4 1.8 1.4 0.6 200 400 600 800 1000 Wild uninfected Wild infected YUn = 0.0013X + 0.3879 r2 = 0.40, p < 0.01 (A) 2.0 1.2 1.0 0.8 0.2 C-factor Total length (mm) YIn = 0.0020X + 0.3994 r2 = 0.63, p < 0.01 1.6 0.4 1.8 1.4 0.6 200 400 600 800 Cultured uninfected Cultured infected YUn = 0.0017X + 0.5203 r2 = 0.57, p < 0.01 (B) 2.0 1.2 1.0 0.8 0.2 C-factor Total length (mm) YIn = 0.0028X + 0.7805 r2 = 0.41, p < 0.01 1.6 0.4 1.8 1.4 0.6 200 300 400 500 Cultured uninfected Cultured infected YUn = 0.003X + 0.6509 r2 = 0.38, p < 0.01 (C)
low availability of paratenic fish hosts in eel ponds, the dispersal of Aco. crassus totally depends on copepods and ostracods in the rearing water, which are swallowed with the feed by accident. Although intermediate hosts are abundant in culture ponds, the availability of Aco. crassus, however, seemed to differ among ponds. The reuse of pond water and customary size selection activities of eels every 1-2 mo during the culture course might result in diverse prevalences among ponds.
The frequency distribution of adult and larval worms exhibited an exponential decay in wild and cultured Japanese eels, which differed from the negative binominal distribution in cultured American eels. This reflects a high susceptibility to Aco.
crassus by American eels. Interestingly, some
batches of cultured Japanese eels possessed a similar prevalence (YL), mean larval intensity (BD2), and mean adult intensity (BD4) compared to cultured American eels (YW). This suggests that infection by Aco. crassus can also be very high in cultured Japanese eels under high infection pressures. On the other hand, infection of cultured American eels greatly differed between the 2 sampled ponds. This may be attributed to the regular use of the insecticide, Trichlorfon (Dipterex), in the pond with a low prevalence. Trichlorfon killed the intermediate hosts of Aco. crassus, thus efficiently interrupting its infection route. All other batches of cultured eels were collected from ponds without a history of drug application. In addition,
Aco. crassus adults collected from American eels
were considerably larger than worms dissected from Japanese eels, similar to the condition found in the European eels (Knopf and Mahnke 2004, Münderle 2005). This indicates that both naive American and European eels have poor defenses against Aco. crassus infection compared to native Japanese eels.
Since the swimbladder is important for buoyancy control, oxygen exchange, absorption, secretion, and acid-base homeostasis (Würtz et al. 1996, Tesch 2003), impairment of the swimbladder's function may result in aberrant migratory behavior in association with reduced swimming performance (Sprengel and Lüchten-berg 1991). Infected eels may also be more vulnerable to predation (Barse and Secor 1999) and capture by commercial trawlers (Sprengel and Lüchtenberg 1991). In the present study, except for a slight thickening and dispersed blood vessels of the swimbladder in heavily infected eels, we observed no other clinical signs in infected
Japanese or American eels. The condition factor, which represents the nutrition status, showed no significant differences between infected and uninfected groups. The HSI, an index that may reflect the presence of infection due to liver swelling in fish (Rahimian 1998), also showed no significant differences between infected and uninfected eels. This is supported by a study of European eels (Möller et al. 1991). Although economic losses of cultured European (Egusa 1969) and American eels (Ooi et al. 1996) after
Aco. crassus infections have been reported, the
parasite obviously did not seem to be a serious problem for cultured eels in the present study. Good pond management might prevent the possible pathogenesis in culture ponds.
Declines in eel resources seem to be a global trend, which occurred long before the invasion by Aco. crassus into Europe and North America (Johnson et al. 1995, Dekker et al. 2003, Taraschewski 2006). The possible effect of the Aco. crassus invasion on the long-term resources of European and American eels thus requires further study. In conclusion, we found diverse infection patterns of Aco. crassus among native wild and cultured Japanese eels and exotic American eels. The normal growth and insignificant pathogenesis in infected eels provide some information on eel culture. Many countries in South and Southeast Asia, Oceania, and Africa (South Africa, Mozambique, Madagascar, and Reunion) are presently establishing eel farms with local or imported eels (Taraschewski 2006). These projects should pay more attention first to preventing the invasion and spread of Aco. crassus and second to good pond management to alleviate any possible pathogenesis.
Acknowledgments: This study was financially supported by the National Science Council of Taiwan (NSC95-2313-B-002-019 and NSC96-2313-B-002-038-MY3). The authors are grateful to all previous students and research assistants for their help with the field and laboratory work.
REFERENCES
Barse AM, SA McGuire, MA Vinores, LE Elermann, JA Weeder. 2001. The swimmbladder nematode Anguillicola crassus in American eels (Anguilla rostrata) from middle and upper regions of Chesapeake bay. J. Parasitol. 87: 1366-1370. Barse AM, DH Secor. 1999. An exotic nematode parasite of
the American eel. Fisheries 24: 6-10.
Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 83: 575-583.
Dekker W, JM Casselman, DK Cairns, K Tsukamoto, D Jellyman, H Lickers. 2003. Worldwide decline of eel resources necessitates immediate action: Québec declaration of concern. Fisheries 28: 28-30.
Egusa S. 1992. Nematode diseases. In S Egusa, ed. Infectious diseases of fish. Rotterdam/Brookfield: A.A. Balkema, pp. 643-657.
Egusa S, K Kira, H Wakabayashi. 1969. On the occurrence of Anguillicola globiceps Yamaguti, a swimbladder roundworm, in pond cultured eels. Fish Pathol. 4: 52-58. Fries LT, DJ Williams, SK Johnson. 1996. Occurrence of
Anguillicola crassus, an exotic parasite swim bladder
nematode of eels, in the southeastern United States. Trans. Am. Fish. Soc. 125: 794-797.
Han YS, IC Liao, YS Huang, JT He, CW Chang, WN Tzeng. 2003. Synchronous changes of morphology and gonadal development of silvering Japanese eel Anguilla japonica. Aquaculture 219: 783-796.
Han YS, WN Tzeng. 2006. Sex ratio as a means of resource assessment for the Japanese eel Anguilla japonica: a case study in the Kaoping River of Taiwan. Zool. Stud. 45: 255-263.
Johnson SK, TF Loraine, J Williams, DG Huffman. 1995. Presence of the parasitic swimbladder nematode,
Anguillicola crassus, in Texas aquaculture. World
Aquacult. 26: 35-36.
Kennedy CR, DJ Fitch. 1990. Colonization, larval survival and epidemiology of the nematode Anguillicola crassus, parasitic in the eel, Anguilla anguilla, in Britain. J. Fish Biol. 36: 117-131.
Kennedy CR, P Nie, J Kaspers, J Paulisse. 1992. Are eels (Anguilla anguilla L.) planktonic feeders? Evidence from parasite communities. J. Fish Biol. 41: 567-580.
Kim YG, EB Kim, JY Kim, SK Chun. 1989. Studies on a nematode. Anguillicola crassus parasitic in the air bladder of the eel. J. Fish Pathol. 2: 1-18.
Kirk RS. 2003. The impact of Anguillicola crassus on European eel. Fisheries Manag. Ecol. 10: 385-394. Kirk RS, CR Kennedy, JW Lewis. 2000. Effect of salinity on
hatching, survival and infectivity of Anguillicola crassus (Nematoda: Dracunculoidea) larvae. Dis. Aquat. Organ. 40: 211-218.
Knopf K, R Lucius. 2008. Vaccination of eels (Anguilla japonica and Anguilla anguilla) against Anguillicola crassus with irradiated L3. Parasitology 135: 633-640.
Knopf K, M Mahnke. 2004. Differences in susceptibility of the European eel (Anguilla anguilla) and the Japanese eel (Anguilla japonica) to the swim-bladder nematode
Anguillicola crassus. Parasitology 129: 491-496.
Koops H, F Hartmann. 1989. Anguillicola-infestations in Germany and in German eel imports. J. Appl. Ichthyol. 5: 41-45.
Kuwahara A, A Niimi, H Itagaki. 1974. Studies on a nematode parasitic in the air bladder of the eel. I. Description of
Anguillicola crassus n. sp. (Philometridea, Anguillicolidae).
Jpn. J. Parasitol. 23: 275-279.
Li LS. 1997. Aquaculture. Kaohsiung, Taiwan: Chien-Cheng Publishing, p. 84.
Möller H, S Holst, H Liichtenberg, F Petersen. 1991. Infection of eel Anguilla anguilla from the River Elbe estuary with two nematodes, Anguillicola crassus and Pseudoterranova
decipiens. Dis. Aquat. Organ. 11: 193-199.
Molnár K, F Baska, G Csaba, R Glávits, C Székely. 1993. Pathological and histopathological studies of the swimbladder of eels Anguilla anguilla infected by
Anguillicola crassus (Nematoda: Dracunculoidea). Dis.
Aquat. Organ. 15: 41-50.
Moravec F, KS Nagasawa, M Miyakawa. 2005. First record of ostracods as natural intermediate hosts of Anguillicola
crassus, a pathogenic swimbladder parasite of eels Anguilla spp. Dis. Aquat. Organ. 66: 171-173.
Moravec F, H Taraschewski. 1988. Revision of the genus
Anguillicola Yamaguti, 1935 (Nematoda: Anguillicolidae)
of the swimbladder of eels, including descriptions of two new species, Anguillicola novaezelandiae sp. n. and Anguillicola papernai sp. n. Folia. Parasit. (Ceske Budejovice) 35: 125-156.
Münderle M. 2005. Ökologische, morphometrische und genetische Untersuchungen an Populationen des invasiven Schwimmblasennematoden Anguillicola crassus aus Europa und Taiwan. PhD Dissertation, University of Karlsruhe, Karlsruhe, Germany, p. 279.
Münderle M, H Taraschewski, B Klar, CW Chang, JC Shiao, KN Shen, JT He, SH Lin, WN Tzeng. 2006. Occurrence of Anguillicola crassus (Nematoda: Dracunculoidea) in Japanese eels Anguilla japonica from a river and an aquaculture unit in southwest Taiwan. Dis. Aquat. Organ. 71: 101-108.
Nagasawa K, YG Kim, H Hirose. 1994. Anguillicola crassus and A. globiceps (Nematoda: Dracunculoidea) parasitic in the swimbladder of eels (Anguilla japonica and A. anguilla) in East Asia: a review. Folia. Parasit. 41: 127-137. Naismith IA, B Knights. 1990. Modeling of unexploited and
exploited populations of eels, Anguilla anguilla (L.), in the Thames Estuary. J. Fish Biol. 37: 975-986.
Nielsen ME. 1999. An enhanced humoral immune response against the swimbladder nematode, Anguillicola crassus, in the Japanese eel, Anguilla japonica, compared with the European eel, A. anguilla. J. Helminthol. 73: 227-232. Ooi HK, CC Lin, MC Chen, WS Wang. 1997. Eucyclops
euacanthus (Copepoda: Cyclopidae) is an intermediate
host of Anguillicola crassus (Nematoda: Dracunculoidea) in Taiwan. Taiwan J. Vet. Med. Anim. Husb. 67: 135-138. Ooi HK, WS Wang, HY Chang, CH Wu, CC Lin, MT Hsieh.
1996. An epizootic of anguillicolosis in cultured American eel, Anguilla rostrata, in Taiwan. J. Aquat. Anim. Health 8: 163-166.
Rahimian H. 1998. Pathology and morphology of
Ichthyophonus hoferi in naturally infected fishes off the
Swedish west coast. Dis. Aquat. Organ. 34: 109-123. Sasal P, H Taraschewski, P Valade, H Grondin, S Wielgoss,
F Moravec. 2008. Parasite communities in eels of the Island of Reunion (Indian Ocean): a lesson in parasite introduction. Parasitol. Res. 102: 1343-1350.
Sprengel G, H Lüchtenberg. 1991. Infection by endoparasites reduces maximum swimming speed of European smelt
Osmerus eperlanus and European eel Anguilla anguilla.
Dis. Aquat. Organ. 11: 31-35.
Taraschewski H. 2006. Hosts and parasites as aliens. J. Helminthol. 80: 99-128.
Tesch FW. 2003. The eel. Oxford, UK: Blackwell Science. Thomas K, F Ollevier. 1993. Hatching, survival and penetration
efficiency of second- stage larvae of Anguillicola crassus (Nematoda). Parasitology 107: 211-217.
Tzeng WN, PW Cheng, FY Lin. 1995. Relative abundance, sex ratio and population structure of the Japanese eel
Anguilla japonica in the Tanshui River system of northern
Taiwan. J. Fish Biol. 46: 183-201.
Würtz J, H Taraschewski. 2000. Histopathological changes in the swimbladder wall of the European eel Anguilla
anguilla due to infection with Anguillicola crassus. Dis.
Aquat. Organ. 39: 121-134.
Würtz J, H Taraschewski, B Pelster. 1996. Changes in gas composition in the swimbladder of the European eel (Anguilla anguilla) infected with Anguillicola crassus (Nematoda). Parasitology 112: 233-238.