國 立 交 通 大 學
生物科技研究所
碩士論文
利用飽和定點突變方法研究酵母菌氧化鯊烯環化酵素內
的假設活性區胺基酸對於催化環化
/重組反應的影響
Site-Saturated Mutagenesis Approach to Investigate the
Putative Active-Site Residues from Saccharomyces
cerevisiae Oxidosqualene-Lanosterol Cyclase that
Influences the Cyclization/Rearrangement Reactions
研 究 生 : 李文暄
指導教授 : 吳東昆 博士
Site-Saturated Mutagenesis Approach to Investigate the Putative
Active-Site Residues from Saccharomyces cerevisiae
Oxidosqualene-Lanosterol Cyclase that Influences the
Cyclization/Rearrangement Reactions
研究生:李文暄 Student: Wen-Hsuan Li
指導教授:吳東昆 博士 Advisor: Dr. Tung-Kung Wu
國 立 交 通 大 學
生物科技研究所
碩士論文
A Thesis
Submitted to Department of Biological Science and Technology
College of Science
National Chiao Tung University
in partial Fulfillment of the Requirements
for the Degree of
Master
in
Biological Science and Technology
July, 2007
Hsinchu, Taiwan, Republic of China
利用飽和定點突變方法研究酵母菌氧化鯊烯環化酵素內的假設活性區胺
基酸對於催化環化
/重組反應的影響
學生: 李文暄 指導教授: 吳東昆 博士 國立交通大學 生物科技研究所碩士班 摘要 在酵母菌以及哺乳類動物中,氧化鯊烯環化酵素(OSC)催化(3S)-2,3-氧化鯊烯 ((3S)-2,3-oxidosqulaene)進行環化/重組反應而產生羊毛硬脂醇。這個複雜的環化/重組 反應的機制包含了氧化鯊烯上的環氧基(epoxide)被質子化而起始環化反應以及一連 串的氫化基、甲基的重組和最後高度專一性的去質子化步驟。我們利用飽和定點突變 的方法來探討酵母菌中氧化鯊烯環化酵素的兩個胺基酸, Tyr99 以及 Trp443 的功能以 及在酵素催化反應的過程中所扮演的角色。在 OSCTyr99 的功能性分析中,當其殘基被 突變成某些帶極性側鏈的胺基酸時可以有功能性補充的作用而支持其氧化鯊烯環化 酵素(OSC)被突變的酵母菌存活下來,並且形成產物的多樣性。經由產物的分析我們 分 離 出 了 之 前 未 被 發 表 過 兩 個 新 的 三 環 碳 陽 離 子 中 間 產 物 , 分 別 是 (13αH)-isomalabarica-14Z,17E,21-trien-3β-ol 以 及 (13αH)-isomalabarica-14E,17E,21- trien-3β-ol。 經由實驗結果我們推測 Tyr99 在環化機制中可能是穩定椅形-船型 (chair-boat) 結 構 所 形 成 的 6-6-5 三 環 馬 可 尼 可 夫 碳 -14 陽 離 子 (6-6-5 tricyclic Markovnikov C-14 cation) 以 及 控 制 接 下 來 碳 -15 脫 氫 反 應 的 立 體 化 學 (stereochemical)。在分子模擬的分析中,Tyr99 相對於碳-陽離子的空間位置不同於 His234 和 Phe445,進而影響了酵素與其碳陽離子中間體之間的反應位向和靜電作用 因此導致反應最後選擇不同位置進行去質子化的結果。而量子力學的計算結果顯示出 酵素催化反應裡所產生的種種效應都對在三環馬可尼可夫碳陽離子的去質子化步驟 中立體化學特性有重大影響。另ㄧ方面,關於 OSCTrp443的飽和定點突變分析中,其camelliol C。而 achilleol A 和 camelliol C 這兩個單環產物証明了其生成是由於不完 整的環化反應被迫終止在碳-10 而形成碳陽離子中間體,再經由不同位置的去質子化 反應所造成。綜合產物比例以及分子模擬的分析,推測 OSCTrp443的功能可能是在於 影響受質反反應前的摺疊構形以及穩定環氧基被質子化隨即 A 環形的中所產生的碳 陽離子。最後,我們的實驗結果可以說明藉由飽和定點突變方法以及產物分離/分析 的實驗可以更深入了解氧化鯊烯環化酵素的結構、功能以及機制之間的關係。
Site-Saturated Mutagenesis Approach to Investigate the Putative
Active-Site Residues from Saccharomyces cerevisiae
Oxidosqualene-Lanosterol Cyclase that Influences the
Cyclization/Rearrangement Reactions
Student: Wen-Hsuan Li Advisor: Dr. Tung-Kung Wu
Institute of Biological Science and Technology Naitonal Chiao Tung Unversity
Abstract
Oxidosqualene-lanosterol cyclase (ERG7) catalyzes the cyclization/rearrangement of (3S)-2,3-oxidosqualene to lanosterol in yeast and mammals. The complex cyclization/rearrangement reaction mechanism consists of initial epoxide protonation, four successive cationic cyclizations, four consecutive hydride or methyl group rearrangements, and a final highly specific deprotonation step. Site-saturated mutagenesis experiments were carried out on the Tyr99 and Trp443 positions of OSC in Saccharomyces cerevisiae to investigate their putative functional role in the catalytic cyclization. For functional analysis of OSCTyr99, some polar side-chain group substitutions genetically complemented yeast viability and produced spatially related product diversity. Product isolation and characterization revealed two novel tricyclic intermediates, (13αH)-isomalabarica-14Z,17E,21-trien-3β-ol and (13αH)-isomalabarica-14E,17E,21- trien-3β-ol, for the first time. These results suggested that the functional role of the Tyr99 in affecting both the chair-boat 6-6-5 tricyclic Markonikov C-14 cation stabilization and the stereochemistry of the protons at the C-15 position for subsequent deprotonation reaction. Homology modeling analysis, results revealed that Tyr99 residue is located spatially differently from that of His234 and Phe445 to the common C-14 cation which
affects the orientation or electrostatic interaction between the enzyme and its cationic intermediate, and results in abstracting of a proton from a different position or orientation. The quantum mechanical calculation results suggested that the energetics of stereochemical control during the tricyclic Markovnikov cation deprotonation step could be affected by the inclusion of these enzymatic effects. The site-saturated mutagenesis applying on the OSCTrp443 revealed that two truncated monocyclic intermediates, achilleol A and camelliol C, were concomitantly produced from the Trp443Ala and Trp443Lys mutants. The formation of achilleol A and camelliol C were identified as evidence for premature truncation of C-10 cationic intermediates following the proton abstraction from different position. The product profile coupling with homology modeling analysis showed that the functional role of Trp443 of OSC might influence the substrate prefolding and stabilizing the epoxide protonation and A-ring formation. Finally, these results further exemplify the potential of site-saturated mutagenesis coupled with product isolation/characterization in elucidating the structure-function-mechanism relationships of the oxidosqualene cyclase.
謝 誌
忙碌而充實的、充滿酸甜苦辣的兩年研究生活,在完成這份論文後即將劃下句 點。在這段時間裡,所有我遇到的人、事、物,都帶來很大的衝擊,讓我成長、也讓 我改變了許多。這短短的兩年在我人生中扮演著關鍵性的時期,這段日子也是我受到 最多幫助的時候。要感謝的人太多了,可是我還是要ㄧㄧ列出來: 感謝吳東昆老師: 謝謝您在兩年前給我機會,願意讓沒有生科背景的我加入這間 正在成長茁壯的實驗室。在這裡我學習獨立思考的能力,養成積極的處事態度,十分 感謝您在這兩年研究上的指導。從老師身上我也學到許多做人做事的道理,對我的人 生有很大的啟發。 感謝袁俊傑老師、林敬堯老師、刁維光老師: 謝謝您們在百忙之中抽空審閱、修 改我的論文、親臨指導我的口試,並且不吝惜的給予許多寶貴的建議,使我收穫良多。 感謝清華大學貴儀中心的彭菊蘭小姐在NMR 光譜分析上的幫忙。 感謝程翔學長: 謝謝你在這段時間裡所有的幫助,沒有你,我的實驗會很不順 利,我的論文也不會完整。謝謝你像大哥哥ㄧ樣關心我的實驗、我的生活。你真的是 ㄧ位不可多得的好學長。 感謝媛婷學姊: 在我剛進來對所有都很陌生的時候,謝謝妳總是很有耐心、不厭 其煩的教導我、給我鼓勵,也感謝那段有妳陪伴熬夜通銀染的日子。 感謝已經畢業的美婷學姊在我初入實驗室時,教我很多實驗的技巧,也分享許多 她的碩班的心得以及在最後口試前的加油打氣。 感謝實驗室的博班學長們,晉豪學長:謝謝你在 GC-MS 的幫助,還有幫我解決 很多電腦上的問題。文鴻、晉源、裕國學長:謝謝你們在這段日子裡像大哥哥般的照 顧,讓我覺得在這間實驗室的女生都很幸福,跟你們一起打球、跑步也很開心。Thanks to my lovely friend, Mili: you are so kind and cute. Thanks for your concern, listening, and being my English teacher. I’ll always miss you.
感謝ㄧ起奮鬥的同窗好友,OSC 組的好搭檔,晧宇:你懂得很多,跟你一起討論 實驗很開心,也謝謝你在實驗上的幫忙; 大景、怡親:跟你們一起熬夜做實驗、吃宵夜、 寫論文的日子很開心,謝謝你們常常鼓勵我、還有一起搞笑。 感謝即將升上碩二的學弟妹: 文祥、采婷、小高,謝謝你們在過去ㄧ年裡所有的 幫助。也謝謝2007 年剛加入 Wu lab 的夥伴: 聖慈、禕婷、天昶、亦諄在口試其間的 幫忙。 感謝我最親愛的家人:老爸、老媽、老妹修惠,謝謝你們的支持,讓我可以在這 個遠離家鄉、高消費水準的新竹,無後顧之憂的完成我的學業,有你們的我真的很幸 福。也感謝時常關心我的摯友: 俊涵、嘉珮、姿穎、康康、莉芬姊、君如。 感謝ㄧ路走來始終如一的繹翔:很多時候、很多事,如果沒有你真的不知該怎麼 度過,你給了我好多也教了我好多,謝謝你。 感謝上天賜給我的這一切,感謝所有曾經幫助過我的人,希望將來我可以有機會 回報。
Table of Contents
Abstract (Chinese) ………. I
Abstract (English) ………... III
Acknowledgement... V
Table of Contents ………. VI
List of Figures ………... IX
List of Tables ……...………. XI
Chapter1 Introduction ……….. 1
1.1 Triterpenoids and sterol synthesis
……….... 11.2 The overview of oxidosqualene cyclase family
………... 51.2.1 The products specificity …………..………...….. 5
1.2.2 The substrate conformation and cyclization ………... 6
1.2.3 The historic hypothetical models based on the OSC cyclization mechanism ………...………...…..… 9
1.2.4 Oxidosqualene-lanosterol cyclase (OSC) ………...… 11
1.2.5 Cycloartenol synthase (CAS) ………... 19
1.2.6 Squalene-hopene cyclase (SHC) …………...………... 23
1.3 The amino acid sequence alignment of (oxido-)squalene cyclase
….... 271.4 Research goal
………. 31Chapter2 Materials and methods ………... 35
2.1 Materials
……….…. 352.2.1 The construction of recombinant plasmids ...………...…. 41
2.2.2 Preparation of competent cell (CBY57 and TKW14C2) ..…………... 44
2.2.3 Plasmid shuffle and counter selection (expression mutated ERG7 gene in yeast strain CBY57) ………..………...………… 44
2.2.4 Ergosterol complementation (expression mutated ERG7 in yeast strain TKW14C2) ..………...……….. 45
2.2.5 Extracting lipids and column chromatography ………...……… 46
2.2.6 Acetylating modification and argentic column chromatography ... 46
2.2.7 AgNO3-impregnated silica gel chromatography ..………... 47
2.2.8 Deacetylation reaction of the modified compounds …..…………... 47
2.2.9 GC-MS column chromatography condition ………..………...…. 48
2.2.10 Molecular modeling Quantum mechanical calculations protocol …... 48
2.2.11 Quantum mechanical calculations protocol …..………...…. 49
Chapter3 results and discussion ………...…….. 50
3.1 Functional analysis of ERG7
Tyr99within S. cerevisiae
………. 503.1.1 Generation of site-saturated mutants of ERG7Tyr99X …...…………... 50
3.1.2 The characterization and identification of novel products ………..…….. 53
3.1.3 Proposed cyclization/rearrangement pathways of TKW14C-2 expressing ERG7Y99X ………..………... 62
3.1.4 Analysis of the ERG7Y99X mutants in the OSC homology modeling ...… 64
3.1.5 The analysis of product energy profile ………..….. 67
3.2 Functional analysis of ERG7
W443within S. cerevisiae
………... 693.2.1 Generation of site-saturated mutants of ERG7Trp443X ………..…….. 69 3.2.2 Lipid extraction, column chromatography and product
characterization ...71
3.2.3 Proposed cyclization/rearrangement pathways of TKW14C2 expressing ERG7W443X …...……….…... 73
3.2.4 Analysis of the ERG7W443X in the OSC homology modeling …... 74
Chapter4 Conclusions ... 77
Cahpter5 Future works ... 80
Chapter6 References ... 81
Appendix 1... 85
Appendix 2 ... 86
Appendix 3 ... 87
List of Figures
Fig. 1.1 Examples of triterpenoid structure ... 1
Fig. 1.2 Outline of sterol biosynthetic pathway ... 3
Fig. 1.3 The product specificity of the cyclase is species-dependent ... 5
Fig. 1.4 Cyclization of oxidosqualene to the protosteryl and dammarenyl cations ... 7
Fig. 1.5 Mechanism of cyclization of 2,3-oxidosqualene to complexly cyclic products via different cation intermediates ... 8
Fig. 1.6 Johson model for ocidosqualene cyclization ... 10
Fig. 1.7 Griffin’s hypothesis model for involvement of aromatic residues in cyclization of OS to protosteryl cation ... 10
Fig. 1.8 Crystal structure of human OSC ... 12
Fig. 1.9 Mechanism of cyclization 2,3-oxidosqualene to lanosterol ... 13
Fig. 1.10 H. sapiens OSC (turquoise) with lanosterol (yellow) ... 14
Fig. 1.11 Trp387, Phe444 and Trp581 are able to stabilize the cyclization intermediates cation C-6 and C-10 through cation-π interaction ... 16
Fig. 1.12 Substrate analogues and products catalyzed by S. cerevisiae OSC which are suggestive of a five-membered C-ring intermediate ... 16
Fig. 1.13 The products diversity from site-saturated mutant of His234, Tyr510 and Trp232 ... 18
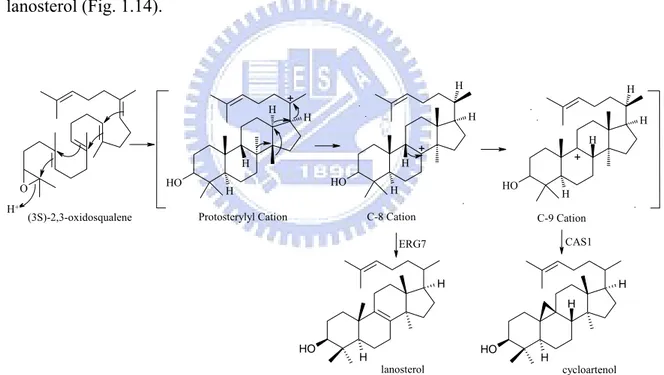
Fig. 1.14 Cyclization of oxidosqualene by oxidosqualene-lanosterol cyclase and cycloartenol synthase ... 19
Fig. 1.15 Conservation pattern between CAS1 and ERG7 ... 20
Fig. 1.16 Products formed by cycloartenol synthase mutants ... 22
Fig. 1.17 Polycyclization Mechanism of squalene by prokaryotic squalene-hopene cyclase (SHC) ... 23
Fig. 1.18 Overall structure of A. acidocaldarius squalene-hopene cyclase (SHC) ... 24
Fig. 1.19 The placements and functions of crucial amino acids inside the central cavity of SHC ... 25
Fig. 1.21 Aligned QW motifs of squalene and oxidosqualene cyclase ... 30
Fig. 1.22 The complementary results of 12 Alanine mutants ... 33
Fig. 1.23 Overall experimental flowchart of this study ... 34
Fig. 2.1 QuickChange Site-Directed Mutagenesis Kit ... 43
Fig. 2.2 The acetylation modification ... 47
Fig. 3.1 The strategy of ergosterol complement selection ... 51
Fig. 3.2 Separation of lanosterol fraction on AgNO3-impregenated TLC ... 53
Fig. 3.3 GC analysis of NSL extracts derived from ERG7Y99Thr ... 54
Fig. 3.4 Electron-impact mass spectra of two novel products and acetylated form derived from ERG7Y99Thr ... 55
Fig. 3.5 The major features of compound2 in 1H NMR and DEPT spectrum ... 57
Fig. 3.6 The structure of compound 2 ... 58
Fig. 3.7 Bond connectivity and stereochemistry of (13αH)-isomalabarica-14Z, 17E, 21-trien-3β-olestablished by HMBC/HSQC and NOEs spectrums ... 59
Fig. 3.8 The structure of compound 1 ... 60
Fig. 3.9 Bond connectivity and stereochemistry of (13αH)-isomalabarica-14E, 17, 21-trien-3β-ol established by HMBC/HSQC and NOEs spectrums ... 61
Fig. 3.10 Proposed cyclization/rearrangement pathway occurred in the ERG7Y99X site-saturated mutants ... 63
Fig. 3.11 ERG7 residues form a putative π-electron pocket ... 64
Fig. 3.12 The product energy profile from quantum mechanical calculations ... 67
Fig. 3.13 Electron-impact mass spectra of two monocyclic triterpenoid products derived from ERG7W443Lys ... 71
Fig. 3.14 S. cerevisiae OSC ERG7W443X mutants convert oxidosqualene to monocyclic triterpenoid products: Camelliol C and Achilleol A ... 73
Fig. 3.15 Local views of the homology modeled Asp456, Trp443, Lay448, and Phe445 positions in S. cerevisiae ERG7 structure based on the X-ray structure of lanosterol-complexed human OSC and determined by using the Insight II Homology program ... 75
List of Tables
Tab. 1.1 Product profiles of A. thaliana cycloartenol synthase Ile481, Tyr410 and
His477 mutants ... 22
Tab. 2.1 Primer design for site-saturated mutagenesis ... 41
Tab. 2.2 QuickChange Site-Directed Mutagenesis Kit PCR composition ... 41
Tab. 2.3 QuickChange Site-Directed Mutagenesis PCR program ... 42
Tab. 2.4 QuickChange Site-Directed Mutagenesis PCR products diegestion ... 42
Tab. 3.1 The results of ERG7Y99X ergosterol complement selection ... 52
Tab. 3.2 The products profile of S. cerevisiae TKW14C2 expressing the ERG7Y99X site-saturated mutagenesis ... 56
Tab. 3.3 NMR assignments for (13αH)-isomalabarica-14Z, 17E, 21-trien-3β-ol for dilute CDCl3 solution ... 58
Tab. 3.4 NMR assignments for (13αH)-isomalabarica-14E, 17E, 21-trien-3β-ol for dilute CDCl3 solution ... 60
Tab. 3.5 The genetic selection results of S. cerevisiae TKW14C2 expressing the ERG7W443X site-saturated mutagenesis ... 70
Tab. 3.6 The products profile of S. cerevisiae TKW14C2 expressing the ERG7W443X site-saturated mutagenesis ... 72
Chapter 1: Introduction
1.1 Triterpenoids and sterol biosynthetic pathways
Terpenoids are ubiquitous in all forms of life. This family of natural products
encompasses an enormous variety of chemical structures and equally diverse array of biological functions. Triterpenoids usually have a tetracyclic or pentacyclic core structure designated as A, B, C, D (and E) rings, with the C30H50O formula (1) (Fig. 1.1). However,
monocyclic, bicyclic, tricyclic, and hexacyclic triterpenoids have also been isolated from various sources.[1-3] Although sterols and steroids are subsets of triterpenoids, having the same general ring structure and biosynthetic origin, different transformations such as alkylations or methylations are occurred in either the ring structure or sidechain. Sterols are characterized by the presence of an alcohol moiety at C-3 position and are usually found in membranes. Oxidosqualene (2) is converted to the different triterpene skeletons in various organisms. Lanosterol (3) is the first cyclized intermediate product in the biosynthesis of cholesterol (4) in animals and ergosterol (5) in fungi. The lanosterol equivalent in plants is cycloartenol (6). The direct cyclization of squalene (7) via squalene-hopene cyclase leads to the production of bacterial sterol surrogates such as diplopterol (8). [1]
O H R O O H H H O H H O H H O H H H OH A B C D 1 2 3 4 5 6 7 8
Sterols are vital constituents of cell membranes. Cholesterol is the plasma membrane sterol in animals and also serves as the metabolic precursor of steroid including estrogens and androgens. In plants, campesterol, stigmasterol, and β–sitosterol are the most common sterol components of membranes. In bacteria, sterols are usually absent, but some bacteria and protozoan produce pentacyclic triterpenes such as hopene and 3-deoxytriterpenoids which are regarded as sterol surrogates in these organisms.[1] As each of these compounds serves an indispensable role, it is evident why a complete understanding of the enzymes directing their biosynthesis is necessary.
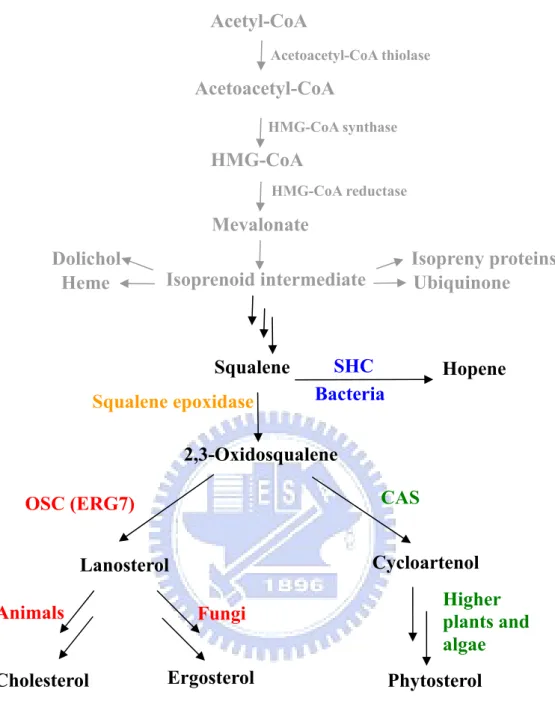
In general, the sterol biosynthetic pathway is from acetyl-CoA initially converted to isoprene units, which is subsequently condensed to form a linear molecule with 30 carbons, squalene, that cyclized to form hopene by squalene-hopene cyclase in bacteria. Squalene epoxidase is responsible for the epoxidation of squalene to 2,3-oxidosqualene which is further converted to lanosterol, the first cyclized precursor leading to cholesterol and ergosterol in animals or fungi via oxidosqualene cyclase, the product of the ERG7 gene (Fig. 1.2).
Figure 1.2 Outline of sterol biosynthetic pathway.
One of the most relevant triterpene derivatives is cholesterol. Current treatments for the hypercholesterolemia was targeting at the step catalyzed by HMG-CoA reductase, which is the rate-limiting step in the early part of the pathway. However, this reaction is not the first committed step in the biosynthesis of cholesterol. Mammals require HMG-CoA reductase for the biosynthesis of isoprenoid intermediates, and its inhibition can not only effect the cholesterol pathway, but also a variety of other pathways including
Phytosterol Higher plants and algae Ergosterol Cholesterol Animals Fungi
Heme Isoprenoid intermediate
Dolichol Isopreny proteins
Ubiquinone Mevalonate HMG-CoA Acetyl-CoA Squalene Hopene 2,3-Oxidosqualene Cycloartenol Lanosterol SHC Bacteria CAS OSC (ERG7) Squalene epoxidase Acetoacetyl-CoA Acetoacetyl-CoA thiolase HMG-CoA synthase HMG-CoA reductase
protein prenylation as well as ubiquinone and dolichol biosynthesis.[4,5] Among the downstream of cholesterol biosynthesis, cyclization of lanosterol for example via oxidosqualene cyclase toward the hypercholesterolemia, might provide insight of the development of a safer, more effective treatment.
1.2 The overview of oxidosqualene cyclase family
1.2.1 Products specificity
Oxidosqualene cyclases (OSCs) are a unique family of eukaryotic enzymes that catalyze the cyclization of acyclic (3S)-2,3-oxidosqualene into over 100 cyclic triterpene alcohols (C30H50O) and triterpene diols (C30H52O2) identified in nature.[3] The cyclization
of oxidosqualene and squalene to polycyclic triterpenoids has fascinated and captivated scientists for more than fifty years.
These polyolefin are stereoselectively cyclized and skeletally rearranged in a single enzyme-catalyzed reaction to yield tetracyclic and pentacyclic triterpenoids including hopene (6-6-6-6-5 pentacycles) in bacterial, lanosterol (6-6-6-5 tetracycles) in animals and fungi, cycloartenol (6-6-6-5 tetracycles), lupeol (6-6-6-6-5 pentacycles) and β–amyrin (6-6-6-6-6 pentacycles) in higher plants (Fig. 1.3).[3]
O 2 3 6 10 15 18 2 3 19 1 4 5 7 8 9 10 11 13 14 12 15 16 18 20 21 22 24 23 6 O H 26 27 29 30 28 25 2 3 19 1 4 5 7 8 9 10 11 13 14 12 15 16 18 20 21 22 24 23 6 O H 27 29 30 28 25 26 O H O H O H OH (3S)-2,3-Oxidosqualene Lanosterol Cycloartenol Dammaradienol β Amyrin Lupenol Hopene Hopanol Bacterial Squalene
Animals and Fungi higher plants
higher plants higher plants higher plants Bacterial
1.2.2 Substrate conformation and cyclization
The cyclization of oxidosqualene reaction is initiated by epoxide protonation, followed by cation-olefin mediated cyclization, hydride/methyl group rearrangement, and terminated either by deprotonation or water addition.[1] These enzymes catalyze what are possibly the most complex multiple-steps chemical transformations found in nature. As many as sixteen bonds are broken and sixteen new bonds formed in the course of cyclization.[6] In addition, these enzymes display remarkable regiospecificity and stereoselectivity during the reaction. In some case, several different stereocenters are established in the product molecule.[6] Furthermore, the chemical transformations catalyzed by oxidosqualene cyclase proceed through highly reactive cationic intermediates. The short-lived cation intermediates are protected and stabilized within the active site cavity of the oxidosqualene cyclases. Despite being surrounded by numerous nucleophiles, either form the amino acid sidechains or from the protein backbone, these cations are not quenched prematurely.[7]
The different substrate prefolded conformation results in different intermediate cations formation. The species-dependent cyclization products can be grouped into two types, protosterol and dammarenyl cation. The conformation of the two major intermediate cations is different at the formation of B-ring. In protosterol cation pathway, a six-membered B-ring boat form was produced, whereas the chair form six-membered B-ring was found in the dammarenyl cation pathway (Fig. 1.4).[1]
O 2 3 6 10 15 18 O O O H HO (3S)-2,3-Oxidosqualene chair-chair-chair chair-boat-chair Enz-AH+ Enz-AH +
Protosteryl Cation Dammarenyl Cation
20
20
+ +
Figure1.4 Cyclization of oxidosqualene to the protosteryl and dammarenyl cations
The protosteryl cation contains A/B-trans, 9/10-syn, B/C-trans ring junction stereochemistry, requiring the substrate to adopt the energically less favorable chair-boat-chair conformation.[8-12] It is accepted that “chair-boat-chair” conformation of the substrate, oxidosqualene, is triggered to give a protosterol C-20 cation, following a series of 1,2-methyl and hydride shifts and final a different position proton elimination to yield either lanosterol or cycloartenol. On the other hand, the “all-chair” dammarenyl cation conformation undergoes similar rearrangement or ring expansion and final elimination to give dammaradienol, lepenol,α-amyrin or β-amyrin via different cation intermediates (Fig. 1.5).[6]
O O H O H O O H O H O H O H O H O O H H H H O H H O H H O H O H O H Lanosterol Cycloartenol 20 Dammaradienol Dammarenyl Cation + Baccharenyl Cation Lupenol Lupenyl Cation + Germanicyl Cation β -Amyrin 12 13 α -Amyrin (3S)-2,3-oxidosqualene Chair-Boat-Chair Chair-Chair-Chair 20 + Enz-AH+ 6 10 14 15 18 19 23 22 Enz-AH 6 10 14 15 18 19 23 22 + 20+ Protosterylyl Cation 17 18 19 20 + + +19
Figure1.5 Mechanism of cyclization of 2,3-oxidosqualene to complexly cyclic products via different cation intermediates.
1.2.3 The historic hypothetical models based on the OSC cyclization
mechanism
Insight into the cyclization of lanosterol came when Rittenberg and Bloch used
previously published isotopic data, along with new data of their own to show how the carbons of cholesterol could be derived from squalene in 1937-1942.[13-14] In 1953, Woodward and Bloch first proposed a hypothesis concerning the course of cyclization of squalene followed by rearrangement to lanosterol.[15] However, the isotopic feeding experiment showed that the oxidosqualene rather squalene was the direct precursor of lanosterol. Afterward, the mechanistic and evolutionary aspects have elicited intense chemical and biochemical interest.
Bloch and Cornforth also used the isotopic feeding experiments to obtain the shifts of 1,2-methyl and hydride during lanosterol formation.[16-17] Furthermore, Corey and van Tamelen demonstrated the more detail intermediates information during lanosterol biosynthesis in the enzyme active site via many substrate analogue compounds feeding experiments.[18-20] Further, Barton proved that OSC in eukaryote only accepted the (3S)- and not the (3R)-enantiomer of oxidosqualene as a substrate.[21]
Lots of theoretical models of the cyclization mechanism have also been proposed in the absence of the information about the enzyme itself. Ourisson proposed the essential elements for enzyme-catalyzed cyclization/rearrangement reaction. An acidic site, a basic site, and many nucleophilic cations stabilizing sites are highly conserved in the cyclases. The cyclase enzymes might be derived from a common ancestor, primitive squalene cyclase to oxidosqualene cyclase in higher organisms. This hypothesis opened an evolutionary study of these cyclase enzymes.[22-24]
In the light of substrate specificity and stereochemistry, Johnson proposed a model for the cyclization mechanism in 1987 (Fig. 1.6).[25-26] This model supposes that the acid residues on the active site provide proton for epoxide group of oxidosqualene or
double-bond of squalene to initiate the reaction and subsequently proceed the cation-olefin polycyclization. A key concept in this model, which is proposed that a number of anionic sites in the cyclase enzyme would guide the cation generation and the formation of the proper ring system.[27]
O H Enz-AH 2 3 6 7 10 11 15 18 23 14 19 + B: O H Enz-AH 2 3 6 7 10 11 15 18 23 14 19 + B: O H Enz-AH 2 3 6 7 10 11 15 18 23 14 19 + B: O H Enz-AH 2 3 6 7 10 11 15 18 23 14 19 + B:
Figure 1.6 Johson model for ocidosqualene cyclization.
The unusual large amounts of tyrosines or tryptophans are highly conserved in most oxidosqualene cyclase known to date.[28] In 1992, this finding has led Griffin to propose an aromatic hypothesis for cyclase active site. In the aromatic hypothesis model, the “π-cation interaction” could be performed via the electron-rich indole of tryptophans, phenol group of tyrosine, or phenylalanine residues in the manner (Fig. 1.7). In such a model, aromatic residues play the role of the anions group just like in the Johnson et al. mechanism.
Figure 1.7 Griffin’s hypothesis model for involvement of aromatic residues in cyclization of OS to protosteryl cation
1.2.4 Oxidosqualene-lanosterol cyclase (OSC)
The formation of lanosterol, the required precursor of cholesterol in vertebrates and ergosterol in fungi, is catalyzed by oxidosqualene-lanosterol cyclase (or lanosterol synthase), the protein encoded from ERG7 gene (EC 5.4.99.7). The oxidosqualene cyclase gene was initially isolated from C. albicans by complementation of an ERG7 mutation in S.
cerevisiae.[29] This ERG7 gene contains an open reading frame encoding a 731-amino acid,
83kDa protein.
In order to understand the structure-function relationships of oxidosqualene cyclase-catalyzed reactions, both chemical and molecular biological studies have significantly contributed mechanistic insights into the oxidosqualene cyclase-catalyzed cyclization/rearrangement cascade. For example, cloning, sequencing, and site-directed mutagenesis studies of putative oxidosqualene cyclase genes have facilitated understanding of cyclase evolution and analysis of product diversity determining factors, perhaps the single most remarkable feature of cyclase enzymes.
Detailed studies with nonnatural substrates have established the overall pathways of the enzymatic reactions involving: 1) binding of the polyolefinic substrate in a pre-folded conformation, 2) initiation of the reaction by protonation of a double bond (squalene) or an epoxide (2,3-oxidosqualene), 3) ring formation, 4) skeletal rearrangement by 1,2-methyl and hydride shifts, 5) termination by deprotonation or addition of water.[30]
Crystallization and structural characterization of the membrane protein SHC from
Alicyclobacillus acidocaldarius have provided a detailed model for determination of the
substrate entrance channel and catalytically important active-site residues.[2, 31-32]
In 2004, Thoma et al. have succeeded in determining the long-awaited structure of human OSC in complex with the reaction product lanosterol and it provided an important additional snapshot of the triterpene polycyclization cascades (Fig. 1.8a).[33]
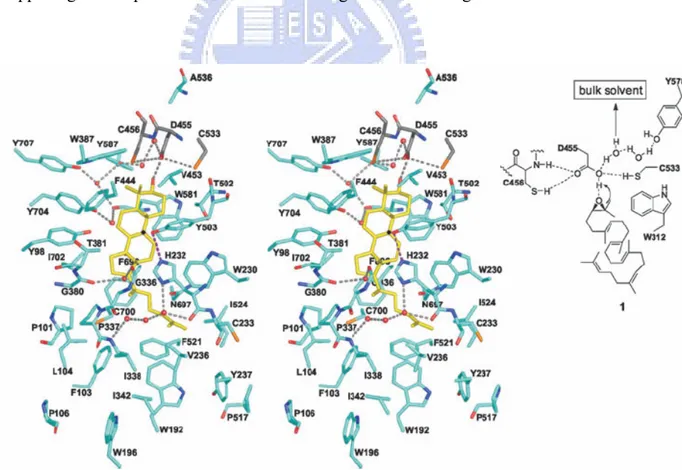
Figure 1.8 Crystal structure of human OSC (a) Ribbon diagram of human OSC. The C
and N termini and several sequence positions are labeled. The inner barrel helices are colored yellow. The bound inhibitor, Ro48-8071 (black), indicates the location of the active site. (b) The orientation of OSC relative to one leaflet of the membrane, Ro 48-8071 bind in the certral active-site cavity.[33]
Base on the observation of crystal structure of monotopic membrane protein human OSC, the membrane-inserted surface consists of a plateau in 25Å diameter and a channel that leads to the active-site cavity. This channel is supposed to allow the substrate oxidosqualene to enter the hydrophobic active site but a constriction site separates it from the active-site cavity. Either the sidechain conformation change in the residues Tyr237, Cys233, and Ile524, or the strained loops rearrangement from 516-524 or 697-699 would lead the substrate passing the constricted site of the channel then enter into the enzyme active site. (Fig. 1.8b).
b a
Cyclization mechanism:
The catalytic mechanism for the cyclization reaction of (3S)-2,3-oxidosqualene to lanosterol involved several steps and a series of discrete conformationally rigid, partially cyclized carbocationic intermediates. The formation of lanosterol is initiated in the pre-chair-boat-chair conformation of 2,3-oxidosqualene from an initial protonation of the epoxide moiety by Asp455 ( in human OSC numbering). Protonation of this epoxide ring would trigger a cascade of ring-forming reactions to the protosterol cation formation. Skeletal rearrangement of this intermediate cation through a series of 1,2-hydride and 1,2-methyl group would shift the cation to the C-8 (lanosterol numbering) cation production, and a final deprotonation step would lead to the product lanosterol generation (Fig. 1.9). O O H 15 O H 6 O H H H H 17 20 9 8 O Enz-AH 6 10 15 14 18 19 22 23 O H 10 H H H O H + + +
+ Above molecular plane
Below molecular plane
A ring + B ring C ring D ring (3S)-2,3-oxidosqualene Lanosterol anti-Markovnikov addition
Initiation
In early work to predict the catalytically important residues, Corey et al. set a series of alanine scanning site-directed mutations of the highly conserved amino acids from S.
cerevisiae ERG7. Complementation experiment results showed that His146, H234, and
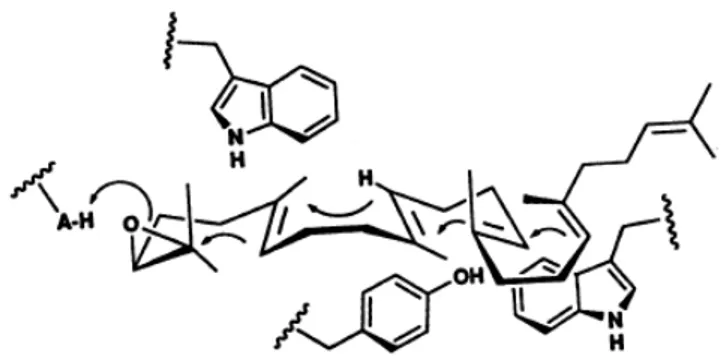
Asp456 were the catalytically essential residues. From this, a model was proposed that the protonated His146 would increases the acidity of Asp456 which acts as the proton donor for initiating cyclization.[34-35] In parallel, the X-ray structure of humanOSC also showed that Asp455 (Asp456 of S. cerevisiae ERG7) is hydrogen-bonding to Cys456 and Cys533, and it contributes to the required acidity for the protonation of epoxide. And then Asp455 can be reprotonated from a H-bonding network of bulk solvent (Fig. 1.10).[30,33] Based on experimental and theoretical studies it is widely accepted that initiation reaction was happening via the protonation and the following concerted A ring was also carried out.[36-40]
Figure 1.10 H. sapiens OSC (turquoise) with lanosterol (yellow): polar residues around
Ring formation
In early studies about the B-ring formation, Matsuda et al. found that Val454 is strictly conserved in the S. cerviase OSC and the corresponding residue Ile481 in A.
thaliana cycloartenol synthase was also considered as a catalytically important residue. A
series of Val454 mutants were substituted as the hydrophobic residues in decreasing the amino acid side chain size of steric bulk from isopropyl to methyl or hydrogen (Phe, Leu, Ile, Ala and Gly). The Ala and Gly mutants allow the monocyclic products formation. It was suggested that steric bulk contributed from the valine side chain might participate in prefolding of the oxidosqualene especially in the Δ10 olefin condensation in the B-ring formation.[41] On the basis of X-ray crystallographic analysis of human OSC structure, Thomal et al. suggested that highly conserved aromatic residues Trp387, Phe444, and Trp581 (which corresponds to Trp390, Phe445, and Trp 583 of S. cerevisiae ERG7) stabilize the intermediate tertiary cation at C-6 and C-10 through cation-π interaction. However, generation of truncated tricyclic and altered deprotonation products from the Phe445 site-directed mutagenesis from S. cerevisiae provides an evidence that Phe445 might affect the cationic stabilization at the C-14 position for C-ring formation and also at C-8/C-9 position for final deprotonation.[42]
Moreover, the induction of the energetically unfavorable boat conformation of the B-ring is thought to be generated via the optimally positioned Tyr98 (Tyr99 of S. cerevisiae ERG7) pushing the methyl group at C-8 (lanosterol numbering) below the molecular plane of oxidosqualene (Fig. 1.11).
Figure 1.11 Trp387, Phe444 and Trp581 are able to stabilize the cyclization intermediates cation C-6 and C-10 through cation-π interaction. Catalytic Asp455 is
activated by Cys456 and Cys533. The Tyr 98 side chain contributes the energetically unfavorable boat conformation of B-ring.
The concept of C-ring closure with subsequent ring expansion was initially suggested by Corey et al. based on the finding that the cyclization of 20-oxaoxidosqualene by the OSC of S. cerevisiae yields not only the expected 6-6-6-5 product but 6-6-5 fused-ring product additionally (Fig. 1.12).[2, 30, 43]
O O O O H H H O O H H H HO H H O H H + 12 19 +
Figure 1.12 Substrate analogues and products catalyzed by S. cerevisiae OSC which are suggestive of a five-membered C-ring intermediate.
Observations of numerous partially cyclized 6-6-5 and 6-6-6-5 side products arising from the substrate-analogue studies have been considered to support the relevance of the C-14 cation intermediate and also provided further evidence for a five-membered C-ring closure (Markovnikov) followed by subsequent ring expansion. Steric pressure through the enzyme, however, might only play a secondary role for the prefolded C-ring conformation
formation, because the energetically favorable chair conformation is required for the C-ring formation.[7] Computational studies also provides further support for the 6-6-5 to 6-6-6 rearrangement pathway.[37] In addition, Thoma et al. showed that side chains of His232 and Phe696 (His234 and Phe699 in S. cerevisiae ERG7) are situated in well position for stabilizing the anti-Markovnikov secondary cation at C-14 with π–cation interactions during C-ring formation through the X-ray crystallographic analysis of human OSC protein.
Rearrangement and deprotonation
Due to the lack of an aromatic residue like Trp169 in SHC for stabilizing the long-lived secondary cation at C-17 for the six-membered E-ring cyclization of hopene, the end of OSC cyclization cascade is stopped at the formation of the five-membered D- ring. Skeletal rearrangement through 1,2-shift of hydride and methyl substituents (Fig. 1.9) convert the protosterol cation to lanosterol C-8 or C-9 cation. It was confirmed that the high π–electron density in the enzyme active site could stabilize the positive charge intermediate during the rearrangement. Thus, the enzyme’s role in skeletal rearrangement is thought to shift equilibrium between the protosteryl cation toward carbocations at the C-8 and C-9 position of lanosteryl cation.[7, 33]
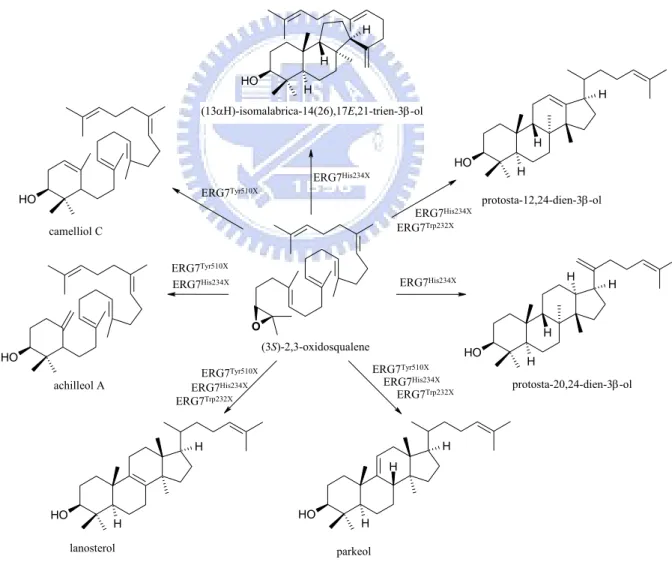
After skeletal rearrangement to C-8 and C-9 lanosterol cation the cyclization is terminated by proton removement. In the deprotonation step, OSC displays the specific catalytic selectivity for lanosterol synthesis which is the most thermodynamic stable deprotonation product (Saytzeff product) with a tetrasubstituted double bond. Thomal’s group confirmed that His 232 (H234 in S. cerevisiae ERG7) is the only basic residue in the proximity to the termination site,[2,7, 34-35] and His232 would also form a hydrogen bond with the hydroxy group of Tyr503 (Tyr510 in S. cerevisiae ERG7 ), which is in a better position to accept the proton from C-9 of the lanosterol cation than His232 itself. The
interpretation of His232 and Tyr 503 as the catalytic base dyad in OSC was supported by site-directed mutagenesis data (Fig. 1.13): 1) The site-saturated mutagenesis experiments of His234 residue in S. cerevisiae ERG7 generated multiple triterpene products including protosta-20,24-dien-3β-ol, protosta-12,24-diene-3β-ol and parkeol from various ERG7His234x mutants[44-45] and 2) the ERG7Tyr510Ala mutant in S. cerevisiae formed parkeol.[46,47] By the same token, the functional analysis of Trp232 in S. cerevisiae ERG7 illustrated that Trp232 might play a catalytic role in the influence of rearrangement process and determination of deprotonation position but does not intervene in the cyclizaiotn steps (Fig. 1.13).[48] O O H O H H H H O H H H H H O H H H H O H H H O H H H H O H achilleol A (13αΗ)-isomalabrica-14(26),17E,21-trien-3β-ol protosta-20,24-dien-3β-ol protosta-12,24-dien-3β-ol lanosterol parkeol camelliol C (3S)-2,3-oxidosqualene ERG7Tyr510X ERG7Tyr510X
ERG7Tyr510X ERG7Tyr510X
ERG7His234X
ERG7His234X
ERG7His234X
ERG7His234X
ERG7His234X ERG7His234X
ERG7Trp232X
ERG7Trp232X ERG7Trp232X
Figure 1.13 The products diversity from site-saturated mutant of His234, Tyr510 and Trp232.
1.2.5 Cycloartenol synthase (CAS)
Cycloartenol synthase (EC 5.4.99.8, from Arabidopsis thaliana) is a protosteryl-type oxidosqualene cyclase that initially cyclizes oxidosqualene to the protosteryl cation intermediate and then mediates a series of hydride and methyl shifts to form the C-9 cation intermediate. In the final step of the reaction, cycloartenol synthase induces cyclopropyl ring formation via abstracting a proton form C-19 to form cycloartenol. This reaction is very similar to that catalyzed by oxidosqualene-lanosterol cyclase. All steps in cycloartenol and oxidosqualene-lanosterol cyclase are identical with the exception of the final deprotonation reaction. Cycloartenol synthases form the cyclopropyl ring and abstract a proton from C-19, whereas lanosterol synthases remove a different proton and form lanosterol (Fig. 1.14). O HO H H H H O H H H H H O H H H H H O H H H O H H H H
(3S)-2,3-oxidosqualene Protosterylyl Cation + + C-8 Cation + C-9 Cation lanosterol ERG7 CAS1 cycloartenol H+
Figure 1.14 Cyclization of oxidosqualene by oxidosqualene-lanosterol cyclase and cycloartenol synthase.
Furthermore, because the cyclopropyl ring formation in the cycloartenol biosynthesis is thermodynamically unfavorable relative to the lanosterol formation, some of amino acid differences are probably specifically required for inducing cyclopropyl ring formation and excluding the formation of more energetically favored products by the cycloartenol
synthase.
Regarding the previous mutagenesis studies in cycloartenol synthase, Tyr410, His477, and Ile481 are catalytically important residues in AthCAS1.[6, 49-52] These residues are strictly conserved in cycloartenol synthase, but in animal or fungal oxidosqualene-lanosterol cyclase maintain Thr, Cys or Gln, and Val at the corresponding positions (Fig. 1.15). These residues synergize to promote the cycloartenol biosynthesis, and mutations at these positions would allow the lanosterol formation.
Figure 1.15 Conservation pattern between CAS1 and ERG7. Tyr410 (◆), His477 (*)
and Ile481 (▼) are strictly conserved in CAS1; Thr, Cys or Gln, Val is conserved at the
corresponding position in ERG7.
Ile481 is conserved in all cycloartenol synthase, whereas Val is present in oxidosqualene-lanosterol synthase (Fig. 1.15). The Ile481 γ-methyl might promote cycloartenol formation by preventing the rotation of the intermediate cation through steric interactions with C-2 and the two axial methyl groups at the A- ring. Removing the γ-methyl group with an Ile481Val mutation resulted in 25% lanosterol production in addition to cycloartenol and parkeol. Besides, Ile481 may also be involved in assisting for the proper substrate folding as well as cyclization reaction. Mutation of Ile481 to smaller residues (Ala and Gly) has led to achilleol A and camelliol C production (Fig. 1.16 and Tab. 1.1).[49]
Tyr410 and His 257 participate in an H-bonding network positioned near the C-19 methyl group for deprotonation reaction.[51] Tyr410 is present in all cycloartenol synthase,
but animal and fungal oxidosqualene-lanosterol synthase maintain Thr at the corresponding position (Fig. 1.15). The AthCAS1 Tyr410Thr mutant forms 65% lanosterol along with 9β-lanosta-7,24-dien-3β-ol and parkeol (Fig. 1.16 and Tab. 1.1). Removing the aromatic ring of Tyr410 decreases the steric bulk above the intermediate cation. Because the hydroxyl group in Thr is closer to the α–carbon then in Tyr, the polar groups of Tyr410Thr, Tyr532, and His257 were repositioned in the Tyr410Thr mutant. This combination of steric and electronic changes abolishes the cycloartenol synthesis and allows deprotonation of C-8/C-9 lanosterol cation to form lanosterol, parkeol and 9β-lanosta-7,24-dien-3β-ol.[50] His477 is not in the active site, but is a second-sphere residue that affects the product profile through interactions with the side chain of Tyr410.[51] His477 is strictly conserved in the known cycloartenol synthase, whereas oxidosqualene-lanosterol synthases maintain either Gln or Cys (Fig. 1.15). The AthCAS1 His477Gln mutant has the polar functionality moved toward C-11 and consequently resulting in more parkeol production than lanosterol.
AthCAS1 His477Asn mutant forms lanosterol by positioning the basic group near the
C-9/C-8, but also produced parkeol due to close enough to C-11.[52]
The double mutant of CAS I481V/ Y410T formed lanosterol more accurately than either single mutant. However the triple mutant (His477Asn/Gln Ile481Val Tyr410Thr) didn’t promote the lanosterol synthesis because the hydroxyl group of Thr positioned too far to interact with the amide group of Asn or Gln residues. The His477Asn Ile481Val double mutant is the most accurate example for the enzyme mutation to generate lanosterol.[52] (Tab. 1.1)
O HO H H H H O H H H H H O H H H H H O H H H O H H H H O H H H H O H HO H O H H H
(3S)-2,3-oxidosqualene Protosterylyl Cation
+ + C-8 Cation + C-9 Cation lanosterol cycloartenol 9β-lanosta-7,24-dien-3β-ol Achilliol A Camelliol C Parkeol
Figure 1.16 Products formed by cycloartenol synthase mutants.
AthCAS1 mutants Cycloartenol Lanosterol Parkeol 9ß-Δ7-
Lanosterol Achilleol A Camelliol C
CAS1I481 99 - 1 - - - CAS1I481L 83 1 16 - - - CAS1I481V 55 24 21 - - - CAS1I481A 12 54 15 - 13 6 CAS1I481G 17 23 4 - 44 12 CAS1Y410T - 65 2 33 - - CAS1Y410C - 75 - 24 1 - CAS1H477N - 88 12 - - - CAS1H477Q - 22 73 5 - -
CAS1I481V/ Y410T - 78 < 1 22 - -
CAS1I481V/ H477N/ Y410T - 78 - 22 - -
CAS1I481V/ H477Q/ Y410T - 78 - 22 - -
CAS1I481V/ H477N - 99 1 - - -
CAS1I481V/ H477Q - 94 6 - - -
Table 1.1 Product profiles of A. thaliana cycloartenol synthase Ile481, Tyr410 and His477 mutants.
1.2.6 Squalene-hopene cyclase (SHC)
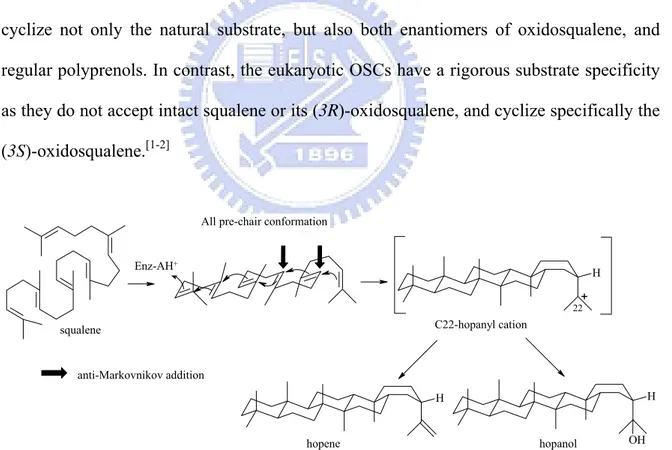
In bacteria, sterols are usually absent, but some bacteria and protozoans produce pentacyclic triterpenes which are regarded as sterol surrogates in these organisms.[1] The prokaryotic squalene-hopene cyclases (SHCs) catalyze a stereochemically and mechanistically simpler process than the eukaryotic oxidosqualene cyclase did. SHCs convert squalene into the pentacyclic hopene skeleton by adopting an all pre-chair conformation leading to C-22 hopanyl cation, then undergoes a deprotonation step to form hopene or alternatively the nucleophilic attack by water molecule to afford hopenol (Fig. 1.17). However, the skeletal rearrangement is not generally observed in the catalytic pathways of SHCs.
Furthermore, the bacteria SHCs displayed very low substrate specificity. They can cyclize not only the natural substrate, but also both enantiomers of oxidosqualene, and regular polyprenols. In contrast, the eukaryotic OSCs have a rigorous substrate specificity as they do not accept intact squalene or its (3R)-oxidosqualene, and cyclize specifically the (3S)-oxidosqualene.[1-2] H H H OH squalene + C22-hopanyl cation 22 hopene hopanol
All pre-chair conformation
anti-Markovnikov addition Enz-AH+
Figure 1.17 Polycyclization Mechanism of squalene by prokaryotic squalene-hopene cyclase (SHC).
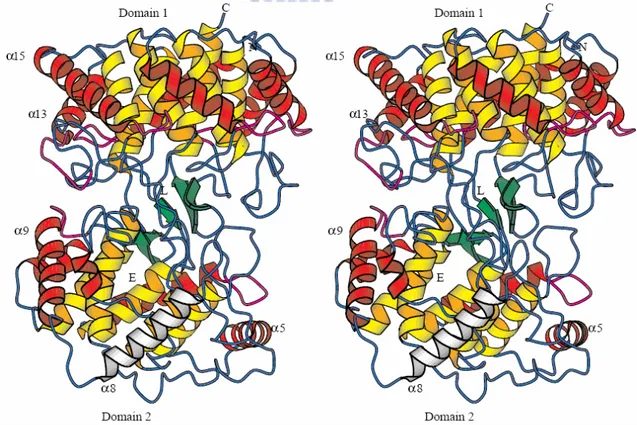
In 1997, Wendt et al. reported the X-ray analysis of A. acidocaldarius SHC, which is the first three-dimensional crystal structure among the many know triterpene synthase, revealing an α–helix-rich dumbbell-shaped homodimer containing a large central cavity as the putative active site.[31,53-55]
Squalene-hopene cyclase is a dimeric membrane protein, and it penetrates but does not completely pass through the bacterial membrane. Substrate squalene, embedded in the membrane, enters the cyclase active site through a hydrophobic channel connecting with the membrane surface. A nonpolar “plateau” flanked the channel entrance in the vicinity of α-helix 8, would likely comprise the membrane association motif (Fig. 1.18).
Figure 1.18 Overall structure of A. acidocaldarius squalene-hopene cyclase (SHC). N
and C: NH2- and COOH-termini; L: the position of the competitive inhibitor LDAO; E: the entrance of the active site channel; Color code: internal (yellow) and external (red) barrel helices; β structure (green); QW-motifs (purple); and helix α-8 in the suggested membrane-binding region (white).[31]
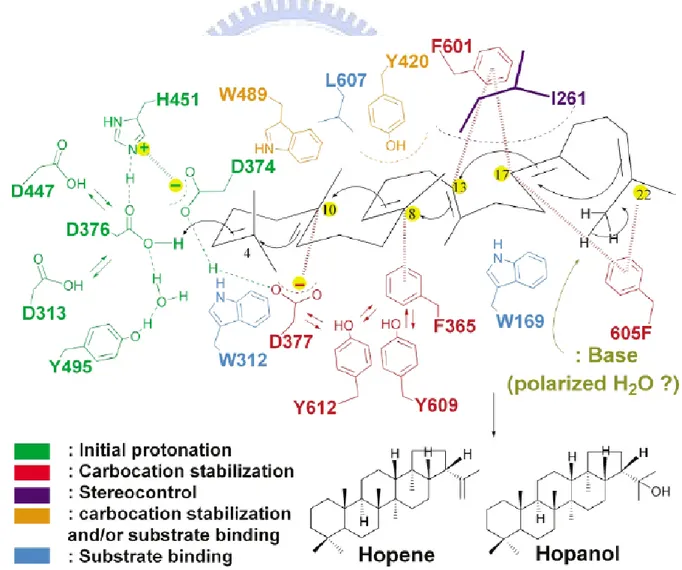
In 1996, Poralla’s group reported the mutagenesis of the short amino acid sequence from Asp374 to Ala379 (the DXDDTA motif) of A.acidicaldarius SHC. The polycyclization reaction is triggered via proton attack on the terminal double bond of squalene, donated by the DXDDTA motif.[53,56-57] Wentd et al. have proposed that Asp376 acts as proton donor on the terminal double bond to initiate the polycyclization and that H451 would enhance the acidity of Asp376. A negative countercharge on the hydrogen bond pair Asp374:Asp377 resides in an appropriate position to stabilize the C2 and C6 cations (squalene numbering). Three hydrogen bonds connecting Asp376 with bulk solvent would lead reprotonation while the cyclizing cation migrates away from the catalytic acid (Fig. 1.19).[2,30]
Figure 1.19 The placements and functions of crucial amino acids inside the central cavity of SHC.[54]
Crystal structures of SHC coupled with the site-directed mutational data have provided a wealth of mechanism information between the active site residues and the substrate.[2] The residues of Trp312 and Trp169 were suggested for binding with substrate, but Wendt et al. proposed these two residues could be assigned for the stabilization of C-4 cation and C-13 cation (hopene numbering) via cation-π interaction (Fig. 1.19).[2,55] The function of Tyr420 is likely to be assigned for stabilization of the C-8 cation despite Tyr609 being positioned around the C-8 cation (Fig. 1.19). Because mutating Tyr420 to Ala and Gly afforded bicyclic and tricyclic product, Tyr420 may additionally play a structural role in the folding process of the hopene skeleton in B- or C-ring formation.[53] Trp489 has been proposed to work for stabilizing the C-10 cation through cation-π interaction during A-ring formation.[54]
Phe605 seems well positioned to stabilize the terminal cation resulting form E-ring formation in SHC, and it is supported by the conservation of Phe605 among all known squalene cyclase as well as the absence of this residue in the OSC, where only four rings are formed.[55]
For the terminal cyclization reaction, the hopanyl cation is then quenched by the putative water molecule that either deprotonates C23 or adds to C23 as a hydroxyl group. Based on molecular dynamics simulations, Reinert et al. suggested that the cyclizing squalene contracts from both sides towards its center. The water molecule has never been observed in any SHC structure, indicating that it has no defined binding site. However, after the hopenyl cation has been formed, there is enough space in the cavity to accommodate a hydrogen-bonding water network which is in turn fixed by Glu45 and Gln262.[58]
1.3 The amino acid sequence alignment of (oxido-)squalene
cyclase
Although S. cerevisiae and human OSC and SHC only shared sequence identity of about 40%, the catalytic cyclization reactions by SHC or OSC family show enormously similar structural and stereochemical control and parallel mechanism. High product specificity in cyclases is believed to be achieved through several factors: (1) by enforcing substrates to occupy prefolded conformations, (2) by progression of reaction via rigidly held, partially cyclized carbocationic intermediates, and (3) by stabilization of intermediate carbocations by cation-π interactions of frequently occurring aromatic residues in the active site, thus preventing early truncation of the cyclization cascade by deprotonation or nucleophilic addition of solvent molecules.[7]
During the past decade, advances in molecular biology, molecular modeling, and protein crystallography have provided enormous information for understanding this remarkable cyclization. Especially, X-ray structural information of SHC and human OSC examined a perspective view of the active site, coupling with site-directed mutagenesis, many of functional important residues had been identified.
In order to understand a consequence of functional, structural, or evolutionary relationships between the cyclases sequence, we used Clustal W to produce multiple sequence alignment of the following enzymes: H. sapoens OSC: P48449, S. cerevisiae OSC: P38604, A. thaliana CAS: P38605, A. acidocaldarius SHC: P33247, which were identified from Protein Data Bank (PDB) in NCBI (Fig. 1.20).
Hs_OSC MTEGTCLRRRGGPYKTEPATDLGR--WRLNCERGR---QTWTYLQDERAGREQTG 50 At_CAS -MWKLKIAEGGSPWLRTTNNHVGRQFWEFDPNLGTPEDLAAVEEARKSFSDNRFVQKHSA 59 Sc_OSC -MTEFYSDTIG---LPKTDPRLWR---LRTDELGR---ESWEYLTPQQAANDPPS 45 Aa_SHC ---MAEQLVEAP-- 9 Hs_OSC LEAYALGLDTKNYFKDLP---KAHTAFEGALN-GMTFYVGLQAED-GHWTGDY 98 At_CAS DLLMRLQFSRENLISPVLPQVKIEDTDDVTEEMVETTLKRGLDFYSTIQAHD-GHWPGDY 118 Sc_OSC TFTQWLLQDPK-FPQPHPERNK----HSPDFSAFDACHN-GASFFKLLQEPDSGIFPCQY 99 Aa_SHC ---AYARTLDRAVEYLLSCQKDE-GYWWGPL 36 Hs_OSC GGPLFLLPGLLITCHVARIP---LPAGYREEIVRYLRSVQL-PDGGWGLHIEDKSTVFGT 154 At_CAS GGPMFLLPGLIITLSITGALNTVLSEQHKQEMRRYLYNHQN-EDGGWGLHIEGPSTMFGS 177 Sc_OSC KGPMFMTIGYVAVNYIAGIE---IPEHERIELIRYIVNTAHPVDGGWGLHSVDKSTVFGT 156 Aa_SHC LSNVTMEAEYVLLCHILDRVD----RDRMEKIRRYLLHEQR-EDGTWALYPGGPPDLDTT 91 Hs_OSC ALNYVSLRILGVGPDDPD--LVRARNILHKKGGAVAIPSWGKFWLAVLNVYSWEGLNTLF 212 At_CAS VLNYVTLRLLGEGPNDGDGDMEKGRDWILNHGGATNITSWGKMWLSVLGAFEWSGNNPLP 237 Sc_OSC VLNYVILRLLGLPKDHPV--CAKARSTLLRLGGAIGSPHWGKIWLSALNLYKWEGVNPAP 214 Aa_SHC IEAYVALKYIGMSRDEEP--MQKALRFIQSQGGIESSRVFTRMWLALVGEYPWEKVPMVP 149 Hs_OSC PEMWLFPDWAPAHPSTLWCHCRQVYLPMSYCYAVRLSAAEDPLVQSLRQELYVEDFASID 272 At_CAS PEIWLLPYFLPIHPGRMWCHCRMVYLPMSYLYGKRFVGPITSTVLSLRKELFTVPYHEVN 297 Sc_OSC PETWLLPYSLPMHPGRWWVHTRGVYIPVSYLSLVKFSCPMTPLLEELRNEIYTKPFDKIN 274 Aa_SHC PEIMFLGKRMPLNIYEFGSWARATVVALSIVMSRQPVFPLPERARVP--ELYETDVPPRR 207 Hs_OSC WLAQRNNVAPDELYTPHSWLLRVVYALLN---LYEHHHS-AHLRQRAVQKLYEHIVAD 326 At_CAS WNEARNLCAKEDLYYPHPLVQDILWASLHKIVEPVLMRWPG-ANLREKAIRTAIEHIHYE 356 Sc_OSC FSKNRNTVCGVDLYYPHSTTLNIANSLVV---FYEKYLRNRFIYSLSKKKVYDLIKTE 329 Aa_SHC RGAKGG---GGWIFDALDRALHG---YQKLSVHPFRRAAEIRALDWLLERQ 252 Hs_OSC DRFTKSISIGPISKTINMLVRWYVDGPASTAFQEHVSRIPDYLWMGLDGMKMQGTNGSQI 386 At_CAS DENTRYICIGPVNKVLNMLCCWVED-PNSEAFKLHLPRIHDFLWLAEDGMKMQGYNGSQL 415 Sc_OSC LQNTDSLCIAPVNQAFCALVTLIEEGVDSEAFQRLQYRFKDALFHGPQGMTIMGTNGVQT 389 Aa_SHC AGDGSWGGIQPP-WFYALIALKILDMTQHPAFIKGWEGLELYGVELDYGGWMFQASISPV 311 Hs_OSC WDTAFAIQALLEAGGHHRPEFSSCLQKAHEFLRLSQVPDNPP-DYQKYYRQMRKGGFSFS 445 At_CAS WDTGFAIQAILATN--LVEEYGPVLEKAHSFVKNSQVLEDCPGDLNYWYRHISKGAWPFS 473 Sc_OSC WDCAFAIQYFFVAGLAERPEFYNTIVSAYKFLCHAQFDTECV---PGSYRDKRKGAWGFS 446 Aa_SHC WDTGLAVLALRAAG---LPADHDRLVKAGEWLLDRQIT--VPGDWAVKRPNLKPGGFAFQ 366
Hs_OSC TLDCGWIVSDCTAEALKAVLLLQEKCP-HVT-EHIPRERLCDAVAVLLNMRNPD----GG 499 At_CAS TADHGWPISDCTAEGLKAALLLSKVPK-AIVGEPIDAKRLYEAVNVIISLQNAD----GG 528 Sc_OSC TKTQGYTVADCTAEAIKAIIMVKNSPVFSEVHHMISSERLFEGIDVLLNLQNIGSFEYGS 506 Aa_SHC FDNVYYPDVDDTAVVVWALNTLR---LPDERRRRDAMTKGFRWIVGMQSSN----GG 416 Hs_OSC FATYETKRGGHLLELLNPSEVFGDIMIDYTYVECTSAVMQALKYFHKRFPEHRAAEIRET 559 At_CAS LATYELTRSYPWLELINPAETFGDIVIDYPYVECTSAAIQALISFRKLYPGHRKKEVDEC 588 Sc_OSC FATYEKIKAPLAMETLNPAEVFGNIMVEYPYVECTDSSVLGLTYFHKYF-DYRKEEIRTR 565 Aa_SHC WGAYDVDNTSDLPNHIPFCDFG--EVTDPPSEDVTAHVLECFG---SFGYDDAWKV 467 Hs_OSC LTQGLEFCRRQQRADGSWEGSWGVCFTYGTWFGLEAFACMGQTYRDGTACAEVSRACDFL 619 At_CAS IEKAVKFIESIQAADGSWYGSWAVCFTYGTWFGVKGLVAVGKTLKN---SPHVAKACEFL 645 Sc_OSC IRIAIEFIKKSQLPDGSWYGSWGICFTYAGMFALEALHTVGETYEN---SSTVRKGCDFL 622 Aa_SHC IRRAVEYLKREQKPDGSWFGRWGVNYLYGTGAVVSALKAVGIDTRE----PYIQKALDWV 523 Hs_OSC LSRQMADGGWGEDFESCEERRY--LQSAQSQIHNTCWAMMGLMAVRHPDIEAQ--ERGVR 675 At_CAS LSKQQPSGGWGESYLSCQDKVYSNLDGNRSHVVNTAWAMLALIGAGQAEVDRKPLHRAAR 705 Sc_OSC VSKQMKDGGWGESMKSSELHSY--VDSEKSLVVQTAWALIALLFAEYPNKEVI--DRGID 678 Aa_SHC EQHQNPDGGWGEDCRSYEDPAY--AGKGASTPSQTAWALMALIAGGRAESEAAR--RGVQ 579 Hs_OSC CLLEKQLPNGDWPQENIAG-VFNKSCAISYTSYRNIFPIWALGRFSQLYPERALAGHP 732 At_CAS YLINAQMENGDFPQQEIMG-VFNRNCMITYAAYRNIFPIWALGEYRCQVLLQQGE--- 759 Sc_OSC LLKNRQEESGEWKFESVEG-VFNHSCAIEYPSYRFLFPIKALGMYSRAYETHTL---- 731 Aa_SHC YLVETQRPDGGWDEPYYTGTGFPGDFYLGYTMYRHVFPTLALGRYKQAIERR--- 631
Figure 1.20 Nucleic acid sequence alignment of OSC, CAS, SHC genes. Abbreviations
Hs, At, Sc, and Aa stand for H. sapoens, A. thaliana, S. cerevisiae, A. acidocaldarius, respectively. Dashed lines indicate the gap introduced for better alignment.
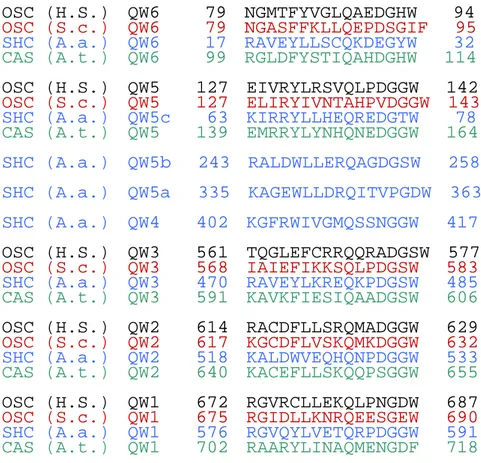
QW motif
Squalene and oxidosqualene cyclase shared a characteristic sequence repeat, [(K/R)(G/A)X2-3(F/Y/W)(L/I/V)3X3QX2-5GXW].[59] This repeat typically occurs five times
in oxidosqualene cyclase and up to eight times in the squalene-hopene cyclase (Fig. 1.21). The high conservation of this repeat throughout the triterpene cyclases has led to a hypothesis that the conserved and unusual rich aromatic amino acids may be involve in the
stabilization of carbocationic intermediates. Such an arrangement has made Poralla to suggest that QW motifs may act to stabilize the transient carbocationic intermediates.[60]
However, the X-ray analysis of the SHC showed that QW motifs are not located in the active-site cavity but rather at the surface of the enzyme. These conserved amino acid residues of the repeats form an complicated network of hydrogen-bonding and hydrophobic interactions that connect the α-barrel helices. Wendt et al. proposed that this arrangement might reinforce the cyclases against an unusually high energy, released during the process of pentacyclic ring formation from acyclic squalene.[31]
Figure 1.21 Aligned QW motifs of squalene and oxidosqualene cyclase. Abbreviations
Hs, Aa, Sc, and At stand for H. sapoens, A. acidocaldarius, S. cerevisiae, A. thaliana, respectively.
OSC (H.S.) QW6 79 NGMTFYVGLQAEDGHW 94
OSC (S.c.) QW6 79 NGASFFKLLQEPDSGIF 95
SHC (A.a.) QW6 17 RAVEYLLSCQKDEGYW 32
CAS (A.t.) QW6 99 RGLDFYSTIQAHDGHW 114
OSC (H.S.) QW5 127 EIVRYLRSVQLPDGGW 142
OSC (S.c.) QW5 127 ELIRYIVNTAHPVDGGW 143
SHC (A.a.) QW5c 63 KIRRYLLHEQREDGTW 78
CAS (A.t.) QW5 139 EMRRYLYNHQNEDGGW 164
SHC (A.a.) QW5b 243 RALDWLLERQAGDGSW 258 SHC (A.a.) QW5a 335 KAGEWLLDRQITVPGDW 363 SHC (A.a.) QW4 402 KGFRWIVGMQSSNGGW 417
OSC (H.S.) QW3 561 TQGLEFCRRQQRADGSW 577
OSC (S.c.) QW3 568 IAIEFIKKSQLPDGSW 583
SHC (A.a.) QW3 470 RAVEYLKREQKPDGSW 485
CAS (A.t.) QW3 591 KAVKFIESIQAADGSW 606
OSC (H.S.) QW2 614 RACDFLLSRQMADGGW 629
OSC (S.c.) QW2 617 KGCDFLVSKQMKDGGW 632
SHC (A.a.) QW2 518 KALDWVEQHQNPDGGW 533
CAS (A.t.) QW2 640 KACEFLLSKQQPSGGW 655
OSC (H.S.) QW1 672 RGVRCLLEKQLPNGDW 687
OSC (S.c.) QW1 675 RGIDLLKNRQEESGEW 690
SHC (A.a.) QW1 576 RGVQYLVETQRPDGGW 591
1.4 Research goal
Oxidosqualene cyclases are key enzymes in sterol biosynthesis. They catalyzed the stereoselective cyclization and precise skeletal rearrangement of (3S)-2,3-oxidosqualene to lanosterol in mammals and fungi and to cycloartenol in algae and higher plant. Complexity, efficiency, and high stereoselectivity of cyclase catalyzed reactions make them prime examples for studying multiple enzyme functions and interesting tools in synthetic chemistry. To provide insight into the catalytic mechanism, functional role of specific residues, and mutation-induced product specificity/diversity profile, many experimental data were reported such as substrate analogues, site-specific mutagenesis coupled with product characterization. Due to the large size and membrane-bound nature of OSCs, three-dimensional structural information is still not available except the SHC from A.
acidocaldarius and human OSC.[31, 33]
The identification for the crystal structure of human OSC provided us a wealthier perception of the enzymatic OS cyclization and attracted us and other researchers to elucidate how the putative active site residues impact cyclization/rearrangement outcome or product specificity and diversity. Nevertheless, the important residues involved in stereo- or rigio-specific control of these product profiles are still far from being fully understood.
The catalytic importance of the Tyr98 residue of the human OSC was inconspicuous until the recent crystal structure of the protein was reported. In view of crystal structures from bacterial SHC and human OSC, it shows that human OSC has an inserted amino acid above and a deleted residue below the molecular plane of 2,3-oxidosqualene, thus preventing the methyl group at C10 to be located above the molecular plane and avoiding the substrate to adopt the energetically favored chair conformation.[7] Further, it was suggested that the Tyr98 of the human OSC positioned spatially to enforce the
energetically unfavorable boat form of oxidosqualene for lanosterol B-ring formation through pushing the methyl group at C-8 (lanosterol numbering) below the molecule plane.[33] However, there is no experimental data to characterize and validate the functional role of Tyr98, as the above-mentioned hypothesis.
The Tyr99 residue of S. cerevisiae oxidosqualene-lanosterol cyclase (ERG7) corresponds to the Tyr98 residue in the human OSC. The multiple sequence alignment revealed that the Tyr99 residue of ERG7 is highly conserved in both the oxidosqualene-lanosterol cyclase and oxidosqualene-cycloartenol synthase, but different substituted amino acids were noticed for β–amyrin synthase and lupeol synthase. Also, a one-residue insertion proximal to the Try99 position of ERG7 was observed specifically for oxidosqualene-lanosterol cyclase from S. cerevisiae, Trypanosoma brucei, and T. cruzi, but not for mammalian oxidosqualene-lanosterol cyclase. According to these observations, we suppose that Tyr99 residue of S. cerevisiae ERG7 may play a different functional role than it of human OSC.
In this thesis, the functional role of the Tyr99 position in the ERG7-catalyzed cyclization/rearrangement cascade and product profile of the cyclase was further substantiated, via generation of the site-saturated mutants of ERG7Y99X and characterization of the product profiles.
On the other hand, in the previous studies of oxidosqualene-cycloartenol synthase (A.
thaliana CAS) mutations, a series of amino acid residues sequence,
469AWPFSTADHGWPI481, was found within an upstream region of the putative active site.
Detailed analysis of the mutations indicated that this region is involved in either protein evolution or crucial determination of the cyclization/rearrangement cascade in cyclase product diversity.[61] Therefore, a series of amino acid residues, 441GAWGFSTKTQGYT453 (corresponding to the region Ala469-Ile481 of CAS) within S. cerevisiae ERG7, were subjected to both alanine-scanning mutagenesis and plasmid shuffle selection for
identification of possible residues involved in the complementation of cyclase-deficient yeast strains.[62] The complementation results are showed in Fig. 1.22. Several inactive mutations were identified, including Trp443Ala, Phe445Ala and Lys448Ala mutations, which failed to complement the cyclase deficiency. The functional roles of Phe445 of ERG7 has been characterized and the mutational-induced product profiles has been determined through site-directed mutagenesis.[62]
In order to further understanding the functional role of the Trp443, the effects of substitutions of Trp443 with other 19 essential amino acid residues in the catalytic activity and the product profiles were investigated. A series of saturated mutagenesis were constructed and the product profiles characterizations were also carried out in this thesis. My experimental flowchart is illustrated in Figure 1.23.
Complementary results:
Mutation CBY57 (ERG7△::LEU2)
Wild-Type + Negative control - Gly441Ala (pFHCOSCRS-13) + Trp443Ala (pFHCOSCRS-12) - Gly444Ala (pFHCOSCRS-11) + Phe445Ala (pFHCOSCRS-10) - Ser446Ala (pFHCOSCRS-9) + Thr447Ala (pFHCOSCRS-7) + Lys448Ala (pFHCOSCRS-6) - Thr449Ala (pFHCOSCRS-5) + Gln450Ala (pFHCOSCRS-4) + Gly451Ala (pFHCOSCRS-3) + Tyr452Ala (pFHCOSCRS-2) + Thr453Ala (pFHCOSCRS-1) +
Figure 1.22 The complementary results of 12 Alanine mutants (amino acid sequence
Experimental flowchart
Figure 1.23 Overall experimental flowchart of this study.
Gene cloning:
the construction of plasmid of OSC gene mutant by site-directed mutation
--- Quick change site-directed mutagenesis --- Mapping and sequencing confirmation
Sequence alignment of human and yeast oxidosqualene-lanosterol cyclase
Product profiles characterization --- TLC
--- silica column --- GC-mass, NMR
Analysis of the product profile of saturated mutants through the homology modeling OSC homology modeling based on human
OSC X-ray structure
Functional expression:
---Ergosterol complementary selection ---Plasmid shuffle/ counter selection
Chapter2 Materials and Methods
2.1 Materials
Chemicals and reagents:
Acetic acid (Merck) Acetic anhydride (Sigma) Acetone (Merck)
Adenine (Sigma) Agarose-LE (USB) 95% Alcohol (Merck) Ampicillin sulfate (Sigma) Anisaldehyde (Mecrk) BactoTMAgar (DIFCO) Bromophenol blue (USB) Dichloromethane (Merck)
Dimethyl sulfoxide (MP Biomedicals) DNA 10Kb Ladder (Bio Basic Inc., Tanwan) D-Sorbitol (Sigma)
Ergosterol (Sigma) Ethyl acetate (Merck)
Ethylenediamine-tetraacetic acid (Merck) Ether (Merck)
G418 (Gibco) Glycerol (Merck) Glucose (Sigma)
Hisidine (Sigma)
LB Broth, Miller (DIFCO) Lysine (Sigma)
Methanol (Merk) Methioine (Sigma)
dNTP Set, 100mM Solutions (GE Healthcare) Primers (Bio Basic Inc., Tanwan)
Potassium hydroxide (Merck) Pyridine (Sigma)
Pyrogallol (Merck)
Restriction enzyme (New England BioLabs Inc.) Sea sand (Merck)
Silica gel (Merck) Silver nitrate (Merck) Sodium sulfate (Merck) Sulfonic Acid (Merck) SYBR®
Green I (Roche) TLC plate (Mecrk) Tris base (USB) Trptophan (Sigma) Tween 80 (Merck) Trypton (DIFCO) Yeast Extra (DIFCO)
Yeast Nitrogen Base w/o amino acid (DIFCO) Uracil (Sigma)