Histopathological and biochemical evidence of hepatopancreatic toxicity
caused by cadmium and zinc in the white shrimp, Litopenaeus vannamei
Jui-Pin Wu
a,*, Hon-Cheng Chen
b, Da-Ji Huang
ca
Division of Environmental Health and Occupational Medicine, National Health Research Institutes, No. 35 Keyan Road, Zhunan Town, Miaoli 350, Taiwan, ROC
bInstitute of Fisheries Sciences, National Taiwan University, Taipei 106, Taiwan, ROC
cDepartment of Environmental Resources Management, Chia Nan University of Pharmacy and Science, Tainan 717, Taiwan, ROC
a r t i c l e
i n f o
Article history:
Received 18 February 2008
Received in revised form 8 August 2008 Accepted 13 August 2008
Available online 21 September 2008 Keywords: Litopenaeus vannamei Cadmium Zinc Hepatopancreas Histopathology
a b s t r a c t
The white shrimp, Litopenaeus vannamei, a globally important cultured prawn species, is an ideal animal for studying the impairment caused by the effects of heavy metals that are often detected in coastal areas. In this study, L. vannamei was exposed to different concentrations of cadmium (Cd) and zinc (Zn) for up to 28 d. Histopathological alterations in the hepatopancreas were observed in L. vannamei after long-term exposure to Cd and Zn. Hepatopancreatic injury was further confirmed by the inductions of two biochem-ical markers, hemolymphatic glutamate-oxalacetate transaminase (GOT) and glutamate-pyruvate trans-aminase (GPT). It was notable that L. vannamei was able to repair its hepatopancreas from the damage caused by Zn, which was evidenced by the results of the histopathological observations, determinations of tissue metal concentrations, and examination of GOT and GPT levels.
Ó 2008 Elsevier Ltd. All rights reserved.
1. Introduction
The hepatopancreas, being analogous to the liver and combin-ing many of the functions of the liver, pancreas, and intestine of vertebrates, plays important roles in several metabolic processes in crustaceans (Caceci et al., 1988; Bhavan and Geraldine, 2000). Previous studies on the hepatopancreas at different biological lev-els such as the structure, development, physiology, metabolism, and biochemistry concluded that this digestive organ possesses several functions, including absorption, digestion, storage, and secretion (Dall and Moriarty, 1983; Caceci et al., 1988). In addition, several specific enzyme systems responsible for biotransformation, e.g., the cytochrome p450 system and glutathione S-transferase, as well as proteins such as metallothioneins appear in the hepatopan-creas, so that this digestive organ also possesses the ability to bio-transform, sequester, and detoxify many kinds of xenobiotics; some biotransformations, however, may in fact actually increase the toxicity of certain xenobiotics (Martinez et al., 1993; Pederson et al., 1997; James and Boyle, 1998; Snyder, 2000; Ahearn et al., 2004). Therefore, the hepatopancreas, or so-called mid-gut gland, is a very important organ for crustaceans, and topics related to it have consistently attracted the attention of many researchers.
Although the hepatopancreas is responsible for the major portion of detoxification activities in crustaceans when exposed to toxicants and pollutants, in aquatic crustaceans, its functions and structure are
likely to be affected by certain xenobiotics, such as pesticides and aflatoxin (Lightner et al., 1982; Bautista et al., 1994; Bhavan and Ger-aldine, 2000). These materials have been reported and demonstrated to have hepatopancreatic toxicities, which resulted in histological alterations to the hepatopancreas of studied organisms. Surpris-ingly, there are relatively few related studies on heavy metals even though they are common pollutants. We previously reported that the heavy metals, cadmium (Cd) and zinc (Zn), caused alterations and the unavailability of biochemical and nutritional materials within the hepatopancreas of the white shrimp, Litopenaeus vanna-mei; these changes might have been the cause of growth retardation we observed after exposure to these metals (Wu and Chen, 2005a). However, the effects of these metals on the histological structure of the hepatopancreas of this species have never been reported.
Therefore, the major objective of the present paper was to study histopathological alterations in the hepatopancreas of L. vannamei, a globally important cultured prawn species, after exposure to Cd and Zn. Furthermore, the activities of two enzymes, glutamate-oxalacetate transaminase (GOT) and glutamate-pyruvate transam-inase (GPT), were also measured in this study to serve as biochem-ical evidence of damage to hepatopancreatic cells and tissues. 2. Materials and methods
2.1. Animal maintenance
Postlarval L. vannamei shrimp were obtained from a commercial shrimp hatchery in Pingtung, southern Taiwan and maintained in
0045-6535/$ - see front matter Ó 2008 Elsevier Ltd. All rights reserved. doi:10.1016/j.chemosphere.2008.08.019
* Corresponding author. Tel.: +886 37 246166x36505; fax: +886 37 587406. E-mail address:rb5_wu@nhri.org.tw(J.-P. Wu).
Contents lists available atScienceDirect
Chemosphere
the laboratory for over 2 months until they reached the juvenile stage (2.15 ± 0.18 g; 9.83 ± 0.59 cm long). Water conditions during shrimp rearing and the experimental period were a temperature of 25 °C, a salinity of 15 p.s.u., dissolved oxygen (DO) of 5.8– 6.5 mg L 1, and a pH of 7.15–7.87, under a 12:12-h light–dark re-gime with continuous aeration and filtration.
2.2. Histopathological studies of the hepatopancreas and determination of metal concentrations
L. vannamei shrimp in the intermolt stage were exposed to Cd or Zn and then sacrificed for the histological study of the hepatopan-creas. Animals were divided into seven groups, each of which con-tained 10 shrimp that were exposed to concentrations of either 0.1, 0.2, or 0.4 mg Cd L 1as CdSO
4, or 0.05, 0.2, or 0.6 mg Zn L 1 as ZnSO4, and one control group (with no exposure to either metal). Samples were taken after 7, 14, 21, and 28 d of exposure. The hepa-topancreas samples were very carefully dissected out for both his-topathological studies and determination of metal concentrations. For the histopathological studies, samples were fixed in 4% buf-fered formalin, embedded in paraffin, sectioned at an 8-
l
m thick-ness on a microtone (Microm, HM330, Heidelberg, Germany), stained with hematoxylin and eosin (H&E), and examined with an Olympus microscope (Tokyo, Japan). Metal concentrations in the hepatopancreas were determined by atomic absorption spec-trophotometry as previously described (Wu and Chen, 2005b). 2.3. Determination of GOT and GPT activitiesTo examine the enzymatic activities of GOT and GPT, L. vanna-mei shrimp were exposed to concentrations of either 0.2 mg Cd L 1 or 0.2 mg Zn L 1, in addition to the control set, with each treatment containing at least 15 shrimp in a 20-L tank. Each treatment was repeated three times. Four to six shrimp in each treatment were randomly taken on days 7, 14, and 28. In all cases, hemolymph was extracted by heart puncture using a 0.5-mL syringe pre-rinsed with citrate-EDTA buffer (0.45 M NaCl, 0.1 M glucose, 30 mM triso-dium citrate, 26 mM citric acid, and 10 mM EDTA pH 4.6; pre-cooled to 7 °C), an anticoagulant used with marine invertebrates (Söderhäll and Smith, 1983). Each hemolymph sample was individ-ually and immediately tested to determine the GOT and GPT activities using Merck GOT and GPT diagnostic kits (Cat. Nos. 1.14829.0001 and 1.14820.0001, respectively; Darmstadt, Ger-many) based on the rate of consumption of the reduced form of nicotinamide-adenine dinucleotide (NADH) that could be mea-sured photometrically at 365 nm. If necessary, a sample was diluted with 9 g L 1NaCl before detection as suggested in the kit instructions.
2.4. Statistical analyses
Statistical analysis was performed with t-test to determine the difference between results of treated and control animals, and a p < 0.05 level was considered statistically significant.
3. Results
3.1. Histological studies of the hepatopancreas of L. vannamei The hepatopancreas of control shrimp exhibited the well-orga-nized glandular tubular structure normally seen in prawn species (Bell and Lightner, 1988; Caceci et al., 1988; Lightner et al., 1996; Bhavan and Geraldine, 2000). A longitudinal section of the apical region of a hepatopancreatic tubule showed that the cell surface facing the lumen was covered with a microvillous brush border,
and the tubule apex contained undifferentiated embryonic (embryonalzellen) cells (E-cells;Fig. 1A). Moving away from the apex, the cells began to differentiate into developing absorptive, storage (restzellen) cells (R-cells). A transverse section of the mid-dle-proximal region of the tubules showed that the tubules were empty of food material and appeared in a hexagonal arrangement or ‘‘star shape” in the lumen, and the basal lamina outlined each tubule (Fig. 1B). Also, in this section, different cell types could clearly be observed under a higher magnification (Fig. 1C). Devel-oping R-cells are those in which the cytoplasm characteristically contains numerous vacuoles and lipid droplets, while fibrous (fibrillenzellen) cells (F-cells) are more basophilic, have larger nu-clei than R-cells, and typically contain one prominent nucleoli. This region also contained the large distinctive secretory (blasenzellen) cells (B-cells), each of which contained one large apical secretory vacuole. Immediately surrounding each tubule was a network of myoepithelial cells with prominent nuclei and associated contrac-tile fibers.
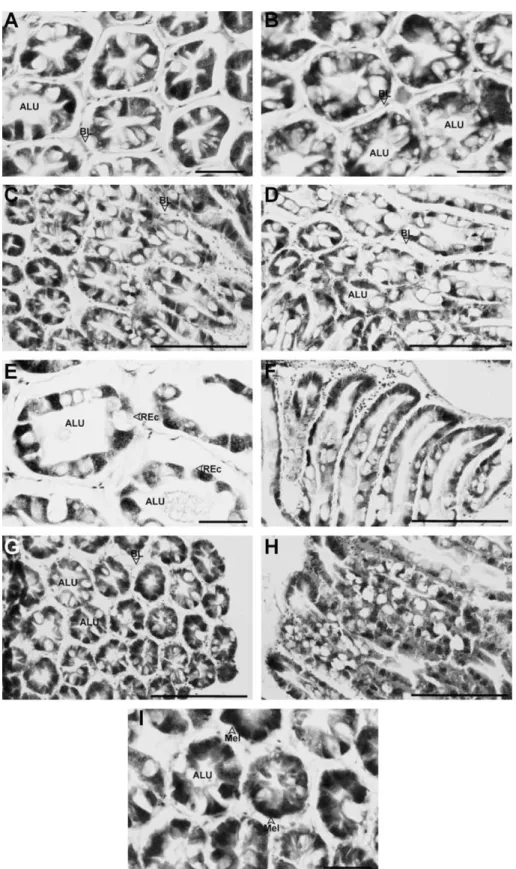
After exposure to Cd, alterations to the hepatopancreatic tissue of treated L. vannamei were observed. In shrimp exposed to lower concentrations of 0.1 and 0.2 mg Cd L 1, large numbers of vacu-oles appeared in the tubular epithelial cells of the hepatopancreas of animals exposed to 0.2 mg Cd L 1for 28 d, as well as one dee-ply affected individual exposed to 0.1 mg Cd L 1for 21 d (Fig. 2A and B). In addition, the star shape of the lumen was partially lost due to morphological changes of the tubular epithelial cells, be-cause some cells decreased in height from a normal columnar height to a low cuboidal form. Also, a slight thickening of the ba-sal lamina increased the distance between adjacent tubules in shrimp exposed to lower concentrations of Cd. When L. vannamei was exposed to a higher concentration of Cd (0.4 mg L 1), severe lesions in the hepatopancreas were very obvious. In shrimp ex-posed to 0.4 mg Cd L 1for 14 d, tubular epithelial cells were heav-ily vacuolated and even ruptured (Figs. 2C and D). Thickening of the basal lamina and a decrease in cell height were also conspic-uous with this treatment. Cellular hypertrophy and vacuolization resulting in a scraggy lumen surface were obvious, when the sec-tion was examined longitudinally in comparison to the hepato-pancreas of normal animals (Fig. 2E). Furthermore, after exposure for 21 d, the arrangement of the tubules and shape of the lumens had substantially been altered (Fig. 2F). Infiltration of hemocytes began in the intertubular hemocoel between the middle-proximal portions of the hepatopancreas tubules. Gener-ally, the distance between adjacent tubules was longer than that of normal shrimp. Atrophy and necrosis of tubules were conspic-uous. Necrotic tubules of the hepatopancreas were rounded off and began to slough off their basement membrane (Fig. 2G). In this study, no shrimp survived after exposure to 0.4 mg Cd L 1 for 28 d, and thus no histological results from this treatment are available.
Similarly, exposure to Zn caused some histological effects on the hepatopancreas of L. vannamei, especially those exposed to 0.2 and 0.6 mg Zn L 1. Histological differences between the hepa-topancreas of 0.2 mg Zn L 1-treated shrimp and normal ones in-cluded vacuolization resulting in hypertrophy in a large number of tubular epithelial cells and an increase in the distance be-tween adjacent tubules, probably due to detachment of tubules from their basement membrane ( Fig. 3A and B). It is very interesting that symptoms of treated shrimps exposed to 0.2 mg Zn L 1 for both 14 and 28 d were almost the same, and showed no time-dependence with these two treatments. In other words, the hepatopancreas of shrimp exposed to 0.2 mg Zn L 1 for 28 d showed no more-serious or -substantial alterations than those individuals exposed to the same concentration of Zn for 14 d. However, when shrimp were exposed to a higher concen-tration of Zn (0.6 mg Zn L 1), different alterations were apparent
in shrimp at different exposure times. In the hepatopancreatic tubular systems of shrimp exposed to 0.6 mg Zn L 1 for 14 and 21 d, slight to moderate vacuolization was apparent in tubular epithelial cells (Fig. 3C and D). In some regions of the hepatopan-creatic tubules from shrimp exposed to 0.6 mg Zn L 1 for 21 d, a decrease in the epithelial cell height, cell rupture, and formation of an abnormal lumen shape were observed (Fig. 3E and F). The most impressive symptoms appeared in the section from shrimp exposed to 0.6 mg Zn L 1for 28 d (Fig. 3G and I). Tubular epithe-lial cells had detached from the basal lamina. The tubules were seriously atrophied, and some epithelial cells were hypertro-phied and vacuolated, so that the tubules looked like they were compressed and the lumen-facing surface was oppilated and irregular (Fig. 3H). Furthermore, melanization was observed in the epithelial cells, especially in the periphery of the tubules (Fig. 3I).
No obvious alterations were observed by light microscopy of the hepatopancreatic tissue of treated L. vannamei after exposure to 0.1 mg Cd L 1 for 14 or 21 d, 0.2 mg Cd L 1 for 14 or 21 d, or 0.05 mg Zn L 1for 14, 21, or 28 d in this study, except for one dee-ply affected individual exposed to 0.1 mg Cd L 1for 21 d, which was moribund when sampled.
3.2. Metal concentrations in the hepatopancreas of L. vannamei Metal concentrations accumulating in the hepatopancreas of L. vannamei were determined (Tables 1 and 2). The hepatopancreatic Cd concentrations were undetectable in control animals at each sampling time (Table 1). After exposure to 0.1 mg Cd L 1, however, the hepatopancreatic Cd concentration was 0.07 ± 0.03 mg g 1 after 7 d of exposure and significantly increased to 0.19 ± 0.0.08, 0.24 ± 0.06 and 0.33 ± 0.08 mg g 1 after 14, 21 and 28 d, respec-tively. A similar increasing trend was also observed in 0.2 mg Cd L 1-treated animals, and the hepatopancreatic Cd concentra-tions were 0.18 ± 0.09, 0.37 ± 0.07, 0.38 ± 0.05, and 0.52 ± 0.18 mg g 1, respectively, after 7, 14, 21, and 28 d of exposure.
The hepatopancreatic zinc concentration in control shrimp was 0.19 ± 0.04 mg g 1(Table 2). No significant difference was appar-ent in hepatopancreatic zinc concappar-entrations of 0.05 mg Zn L 1 -treated animals compared to the control. In the 0.2 mg Zn L 1 treatment, the hepatopancreatic zinc concentration significantly increased to 0.31 ± 0.04 and 0.34 ± 0.06 mg g 1 (p < 0.05) after exposure for 7 and 14 d, but then declined to levels that showed no significant difference compared to those of the control after exposure for 21 and 28 d.
3.3. Effects of Cd and Zn on hemolymphatic GOT and GPT activities of L. vannamei
According to the results of the enzyme activity determinations, hemolymphatic GOT and GPT activities of control L. vannamei were 19.8 ± 4.4 and 14.7 ± 4.9 U L 1, respectively. Exposure of L. vanna-mei to Cd for 28 d resulted in increased hemolymphatic GOT and GPT activities, according to our observations (Table 1). After expo-sure for 7, 14, and 28 d, the average levels of GOT activities of Cd-treated L. vannamei were 180.3%, 264.4%, and 218.7% higher com-pared to the average level of the control animals. Similarly, the average levels of GPT activities of individuals exposed to Cd for 7, 14, and 28 d were 352.4%, 337.1%, and 381.0% higher, respectively. Although both hemolymphatic GOT and GPT activities also in-creased after shrimp were exposed to Zn for 7 d and were 361.4% and 521.4% higher than those of control individuals, declines in both enzyme activities were observed and no significant differ-ences were evident from those of the control group after exposure for 14 and 28 d (Table 2).
4. Discussion
The liver and hepatopancreas are known to be very sensitive to different diets and water-borne pollutants; thus these organs are often used to monitor the effects of various toxicants ( Bau-tista et al., 1994). The hepatopancreas is essentially composed
Fig. 1. Hepatopancreas from a control Litopenaeus vannamei. (A) Longitudinal section of the hepatopancreas showing that the inner surface of the tubular structure is covered by a microvillous brush border. The tubule apex contains undifferentiated embryonic (embryonalzellen) cells (E-cells). Bar = 50lm. (B) Transverse section of the middle-proximal region of tubules showing that tubules are well arranged and appear as a star shape in the lumen. Bar = 200lm. (C) In transverse section, different cell types can be observed, including secretory (blasenzellen) cells (B-cells), fibrous (fibrillenzellen) cells (F-cells), and absorptive, storage (restzellen) cells (R-cells). A network of myoepithelial cells with prominent nuclei and associated contractile fibers surrounds each tubule. Bar = 50lm. B, B-cell; BL, basal lamina; Brb, microvillous brush border; E, E-cell; F, F-cell; Mef, myoepithelial fibers; Mfn, myoepithelial cells with prominent nuclei; R, R-cell; *, star shape of the lumen.
of branched tubules and different types of epithelial cells lining the tubules. Therefore, it is likely that exposure to noxious chemicals or xenobiotics would be reflected in alterations to the structures of tubules and epithelial cells (Bhavan and Geral-dine, 2000). The effects of exposure to various toxicants on his-tological and cellular changes to the liver and hepatopancreas of several aquatic organisms have been investigated (Lightner et al., 1982; Doughtie and Rao, 1984; Förlin et al., 1986; Khangarot, 1992; Bautista, 1994; Lightner et al., 1996; Bhavan and Geral-dine, 2000). The effects on the hepatopancreas of several prawn
species of aflatoxin, naturally produced by the fungi, Aspergillus flavus and Aspergillus parasiticus, have been studied (Lightner et al., 1982; Bautista, 1994), and it is thought to possess toxicity to the liver of higher organisms. Aflatoxin-induced structural changes in the hepatopancreas of prawns included a decrease in the cellular height of the tubular epithelium, a reduction in the abundance of secretory and lipid vacuoles, infiltration of hemocytes, atrophy of epithelial cells, development of pyknotic nuclei, cytolysis, the formation of fibrosis, and the melanized encapsulation of necrotic tissues. Likewise, similar studies have
Fig. 2. Hepatopancreas from cadmium (Cd)-treated Litopenaeus vannamei. (A) The hepatopancreas from a shrimp exposed to 0.2 mg Cd L 1for 28 d. Bar = 50lm. (B) The
hepatopancreas from one deeply affected and moribund individual exposed to 0.1 mg Cd L 1
for 21 d. Bar = 50lm. Both (A) and (B) show that large numbers of vacuoles appeared in the tubular epithelial cells, and the lumina are abnormal. The slight thickening of the basal lamina is obvious. (C) The hepatopancreas from a shrimp exposed to 0.4 mg Cd L 1
for 14 d showing that the tubular epithelial cells are heavily vacuolated; and some are ruptured. Bar = 200lm. (D) The same section shown in (C), but at a higher magnification. Bar = 50lm. (E) Longitudinal section of the hepatopancreas from a shrimp exposed to 0.4 mg Cd L1
for 14 d. In this section, the lumen appears scraggly due to cellular hypertrophy as indicated by the arrow. Bar = 50lm. (F) The hepatopancreas from a shrimp exposed to 0.4 mg Cd L1
for 21 d. Arrangement of the tubules and the shape of the lumina have substantially been altered. Infiltration of hemocytes and necrosis of tubules appeared. Bar = 200lm. (G) Further examination at a higher magnification showing that necrotic tubules and cells appeared in the hepatopancreas of a shrimp exposed to 0.4 mg Cd L1. Necrotic tubules are rounded off and have
sloughed off the basement membrane. Bar = 50lm. ALU, abnormal lumen; BL, basal lamina; IHc, infiltration of hemocytes; NC, necrosis of epithelial cells; NT, necrosis of tubules; REc, ruptured epithelial cells.
Fig. 3. Hepatopancreas from zinc (Zn)-treated Litopenaeus vannamei. (A) The hepatopancreas from a shrimp exposed to 0.2 mg Zn L1for 14 d. Bar = 50lm. (B) The
hepatopancreas from a shrimp exposed to 0.2 mg Zn L1
for 28 d. Bar = 50lm. Both (A) and (B) show that large numbers of vacuoles appeared in the tubular epithelial cells, and the lumens are abnormal. (C) The hepatopancreas from a shrimp exposed to 0.6 mg Zn L1
for 14 d. Bar = 200lm. (D) The hepatopancreas from a shrimp exposed to 0.6 mg Zn L1
for 21 d. Bar = 200lm. In sections shown in (C) and (D), slight to moderate vacuolization of the tubular epithelial cells is apparent. (E) The hepatopancreas from a shrimp exposed to 0.6 mg Zn L 1
for 21 d. In this section, a decrease in epithelial cell height as well as cell rupture resulting in the formation of an abnormal lumen are clearly evident. Bar = 50lm. (F) A longitudinal section of the hepatopancreas from a shrimp exposed to 0.6 mg Zn L1
for 21 d. Note that the lumen of the tubules is abnormal due to vacuolization and hypertrophy of the epithelial cells. Bar = 200lm. (G) The hepatopancreas from a shrimp exposed to 0.6 mg Zn L1for 28 d. Tubular epithelial cells
have detached from the basal lamina with this treatment, and the tubules have atrophied. Bar = 200lm. (H) A longitudinal section of the hepatopancreas from a shrimp exposed to 0.6 mg Zn L 1
for 28 d. Note that the lumen-facing surface is almost completely oppilated and irregular. Bar = 200lm. (I) The hepatopancreas from a shrimp exposed to 0.6 mg Zn L1
for 28 d. Melanization of the epithelial cells has appeared. Bar = 50lm. ALU, abnormal lumen; BL, basal lamina; Mel, melanization of cells; REc, ruptured epithelial cells.
also examined the effects of pesticides and fungicides on other decapods (Lightner et al., 1996; Bhavan and Geraldine, 2000).
Förlin et al. (1986) and Khangarot (1992)studied the effects of the heavy metals, copper (Cu) and Cd, on ultrastructural changes in the liver of a teleost. Major alterations of liver cells after fish were exposed to Cu or Cd included proliferation of smooth endoplasmic reticula (SER), fragmentation and dilation of cisternae of rough endoplasmic reticula (RER), detachment of ribosomes from endo-plasmic reticula (ER), hypertrophy of Golgi complexes, swelling of mitochondria with an amorphous, dense and intramaterial deposition, the appearance of a large number of lysosome-like inclusion bodies and autophagic vacuoles, and a tendency towards fibrosis. A related study also examined the effects of hexavalent chromium on the grass shrimp, Palaemonetes pugio (Doughtie and Rao, 1984). Various degrees of nuclear hypertrophy and nucle-ar inclusions, a relative decrease in mitotic activities within the tubule apices, an obvious loss of intracellular organization, degen-erative cytoplasmic changes, and thickening of the basal lamina were observed in hepatopancreatic cells of P. pugio treated with hexavalent chromium.
Previous studies reported that, in unpolluted water, the average concentration of Cd is about 0.05
l
g L 1, while in coastal waters it tends to increase to over 0.1l
g L 1 and even over 10l
g L 1 insome areas due to anthropogenic input, local geological conditions, and human activities (Soegianto et al., 1999). Likewise, Zn concen-trations in coast water had also been detected as 4–800
l
g L 1 (Bryan, 1976). Hence, the existence of heavy metals in coastal water should be considered when directly using coastal waters for shrimp farming. We had previously reported the acute toxicities of Zn and Cd for L. vannamei (Wu and Chen, 2004). The 24-h LC50 values for L. vannamei were 3.98 mg Zn L 1 and 2.58 mg Cd L 1. In this study, we investigated the structural and biochemical alterations of hepatopancreas of L. vannamei after ex-posed to sub-lethal concentrations of these two metals. From our studies, structural changes in the hepatopancreas after metal expo-sure were obvious. Infiltration of hemocytes, separation of necrotic cells from the basal lamina, and the formation of necrotic tubules were observed after L. vannamei was exposed to heavy metals in this study. These symptoms are likely common and typical re-sponses when prawn are exposed to toxicants causing lesions in the hepatopancreas, since the same symptoms were also reported in other prawn species exposed to higher concentrations of afla-toxin, endosulfan, or a fungicide (Lightner et al., 1982; Bautista, 1994; Lightner et al., 1996; Bhavan and Geraldine, 2000). A thick-ening of the basal lamina was also observed in Macrobrachium mal-colmsonii after exposure to endosulfan, and the authors consideredTable 1
Hepatopancreatic cadmium (Cd) concentrations and hemolymphatic glutamate-oxalacetate transaminase (GOT) and glutamate-pyruvate transaminase (GPT) activities of Litopenaeus vannamei exposed to Cd
Exposure time (d) Parameter Treatment
Control Cd 0.1 mg L 1
Cd 0.2 mg L 1
7 Cd concentration (mg g1
dry weight) N.D. 0.07 (0.03)*
0.18 (0.09)*
GOT activity (U L1) 23.73 (7.00) N/A 42.79 (27.30)
GPT activity (U L 1) 15.00 (4.00) N/A 52.85 (31.52)* 14 Cd concentration (mg g1 dry weight) N.D. 0.19 (0.08)* 0.37 (0.07)* GOT activity (U L1 ) 19.42 (3.05) N/A 51.34 (24.62)* GPT activity (U L 1 ) 15.10 (3.05) N/A 50.91 (35.07)* 21 Cd concentration (mg g1 dry weight) N.D. 0.24 (0.06)* 0.38 (0.05)* GOT activity (U L1
) N/A N/A N/A
GPT activity (U L 1) N/A N/A N/A
28 Cd concentration (mg g1dry weight) N.D. 0.33 (0.08)* 0.52 (0.18)*
GOT activity (U L1
) 16.18 (3.00) N/A 35.38 (11.68)*
GPT activity (U L 1
) 14.38 (6.59) N/A 54.80 (32.42)*
N.D., the result of measurement was under detection limit. N/A, the data was not available.
Values are expressed as mean (SD) of all measurements.
*Mean values of treated groups significantly differ from those of the corresponding control group (p < 0.05).
Table 2
Hepatopancreatic zinc (Zn) concentrations and hemolymphatic glutamate-oxalacetate transaminase (GOT) and glutamate-pyruvate transaminase (GPT) activities of Litopenaeus vannamei exposed to Zn
Exposure time (d) Parameter Treatment
Control Zn 0.05 mg L 1 Zn 0.2 mg L 1
7 Zn concentration (mg g 1dry weight) 0.16 (0.01) 0.21 (0.02) 0.31 (0.04)*
GOT activity (U L 1 ) 23.73 (7.00) N/A 85.75 (27.65)* GPT activity (U L 1 ) 15.00 (4.00) N/A 78.20 (41.85)* 14 Zn concentration (mg g 1 dry weight) 0.17 (0.01) 0.20 (0.02) 0.34 (0.05)* GOT activity (U L 1 ) 19.42 (3.05) N/A 24.16 (12.43) GPT activity (U L 1 ) 15.10 (3.05) N/A 19.85 (9.93)
21 Zn concentration (mg g1dry weight) 0.21 (0.02) 0.19 (0.02) 0.21 (0.01)
GOT activity (U L 1) N/A N/A N/A
GPT activity (U L 1
) N/A N/A N/A
28 Zn concentration (mg g 1 dry weight) 0.19 (0.04) 0.18 (0.01) 0.22 (0.04) GOT activity (U L 1 ) 16.18 (3.00) N/A 22.65 (4.58) GPT activity (U L 1 ) 14.38 (6.59) N/A 29.12 (13.73)
N.D., the result of measurement was under detection limit. N/A, the data was not available.
Values are expressed as mean (SD) of all measurements.
*Mean values of treated groups significantly differ from those of the corresponding control group (p < 0.05).
that it might have been due to production of collagen and walling off by hemocytes and also represented a defensive reaction against the toxicity of xenobiotics (Bhavan and Geraldine, 2000). Among the different types of cells in the tubular epithelia of the hepato-pancreas, R-cells have been found to be the most readily and severely affected (Vogt, 1990; Bautista, 1994). R-cells are charac-terized by numerous vacuoles and lipid droplets appearing within the cytoplasm, and they are considered to be the major lipid re-serve in the hepatopancreas. We found that a common feature of the hepatopancreas of Cd- and Zn-exposed L. vannamei was the appearance of moderate to heavy vacuolization in tubular epithe-lial cells. Since xylene was used when we stained the tissue paraf-fin sections with hematoxylin and eosin, it is possible that lipids within the lipid droplets or lipid-containing vacuoles may have been dissolved away, and the resulting tissue section showed only the existence of hollow vacuoles of cells. It was reported in M. mal-colmsonii that the number of R-cells in the tubular epithelium of the hepatopancreas of test prawns exposed to 10.6 ng L 1 endosul-fan was higher than that of control prawns (Bhavan and Geraldine, 2000). Therefore, the appearance of heavy vacuolization in R-cells might have resulted from an increase in the lipid content of cells and/or an increase in the number of R-cells.
Zn is a ubiquitous and nutritionally essential element that is re-quired for several normal physiological functions, while Cd is a non-essential trace metal that exerts its toxic effects on animals. Hence, it is not surprising that Zn was detected in the hepatopan-creas of control L. vannamei, while Cd was not. In our study, both exogenous Cd and Zn accumulated in the hepatopancreas of L. van-namei after exposure for 14 d. However, these two metals behaved differently after exposure for 21 and 28 d. It had been reported that marine prawns possess the ability to regulate tissue Zn concentra-tions for normal physiology (White and Rainbow, 1985; Vijayram and Geraldine, 1996). It was believed that metallothioneins in-volved in the regulation of essential trace elements (Roesijadi, 1992). Also, we had previously demonstrated that the pattern of matellothionein expressed deeply associated with the distribution of Zn in the tissues of L. vannamei (Wu and Chen, 2005b). Thus, our histopathological observations from 0.2 mg Zn L 1-treated shrimp exposed for 14 and 28 d showed no obvious differences from con-trol shrimp, which can possibly be explained by the declining accu-mulation of Zn, which might have decreased its hepatopancreatic toxicity.
GOT, also called aspartate aminotransferase (ASAT), catalyzes an important reaction of the molecular rearrangement involving amino acids linked to the citric acid cycle at two points (oxaloace-tic and ketoglutaric acids), being the most important mechanism for introducing reduction equivalents into mitochondria (Urich, 1994). GPT, also called alanine aminotransferase (ALAT), predomi-nates in organs with intensive glycogenesis, such as the liver ( Ur-ich, 1994; Torre et al., 2000). Serum GOT and GPT are important diagnostic tools in medicine and clinics, and are used to detect the toxic effects of various pollutants (Nelson and Cox, 2000). The effects of heavy metals on serum or liver enzyme activities including GOT and GPT activities of several teleost species have re-cently been studied (Vaglio and Landriscina, 1999; Torre et al., 2000; Kim and Kang, 2004). After sub-chronic dietary Cu exposure for 40 d, increased serum GOT and GPT concentrations with increasing time and dose were observed in the rockfish, Sebastes schlegeli (Kim and Kang, 2004). In addition, it was also reported in another teleost (Sparus aurata) that Cd exposure may decrease GOT and GPT activities in liver cells (Vaglio and Landriscina, 1999). The authors of those two articles suggested that the liver is rich in GOT and GPT, and damage to it can result in the liberation of large quantities of these enzymes into the blood. Therefore, in-creases in GOT and GPT activities in the serum of heavy metal-trea-ted fish are assumed to be a result of liver damage by the heavy
metals. In fact, related studies on crustacean and prawn species are fairly limited.Galindo-Reyes et al. (2000)studied some enzy-matic alterations in L. vannamei exposed to pesticides, including hemolymphatic GOT and GPT activities. However, they found no statistically significant differences in either GOT or GPT activities among treatments and control sets, and concluded that the pesti-cides assayed had no impact on GOT or GPT activities in shrimp hemolymph. In this study, we found that both hemolymphatic GOT and GPT activities of L. vannamei were influenced after expo-sure to Cd and Zn. It is very interesting that Cd and Zn showed dif-ferent effects on the hemolymphatic GOT and GPT activities. Unlike Cd, a non-essential and xenobiotic element, Zn is an essential ele-ment for maintaining normal functions of most organisms. Many organisms, such as the prawn species, M. malcolmsonii, have been reported to possess the ability to regulate the levels of Zn entering into and being excreted from the body in order to maintain a stable level within the body when they are exposed to ambient Zn ( Vijay-ram and Geraldine, 1996). Therefore, the recovery of hemolym-phatic GOT and GPT activities was considered to be related to the intrinsic regulation of Zn by L. vannamei to decrease its toxicity, and shows that the damage to the hepatopancreas caused by expo-sure to Zn can be repaired. This was also supported by our histolog-ical results from the hepatopancreas of shrimp exposed to 0.2 mg Zn L 1for 14 and 28 d, in that the lesions of the hepatopan-creas did not worsen and probably began to recover from the dam-age caused by exposure to a lower concentration of Zn. A concentration of 0.6 Zn mg L 1 might be so toxic for L. vannamei that its recovery mechanism failed, since hepatopancreatic tubules of individuals exposed for 28 d were seriously atrophied, and the lumina were almost completely oppilated in this study.
Hepatopancreas is not only a digestive organ possesses abilities of absorption, digestion, storage, and secretion, but also a major site where biotransformation and detoxification undergo in crusta-ceans. In this study, we demonstrated that histological alterations appeared in the hepatopancreas of L. vannamei after long-term exposure to Cd and Zn, and included vacuolization of epithelial cells, loss of the star shape of the lumen, a thickening of the basal lamina, an increased distance between adjacent tubules, the appearance of a scraggy lumen surface, and infiltration of hemo-cytes. In addition, atrophy and necrosis of tubules as well as mel-anization were observed in animals treated at higher doses. GOT and GPT levels were also determined in this study as biochemical evidence to confirm that the hepatopancreas of L. vannamei was damaged after exposure to Cd and Zn. Therefore, results of the present study provided a reasonable explanation for our previous observations (Wu and Chen, 2005a), that the structure and func-tions of hepatopancreas of L. vannamei were damaged by Cd and Zn, resulting in the alterations and unavailability of biochemical and nutritional materials within the hepatopancreas and conse-quently growth retardation. Also, the influence of heavy metals on the biotransformation ability and efficiency of hepatopancreas to other environmental pollutants should also be considered and will be investigated in the future.
References
Ahearn, G.A., Mandal, P.K., Mandal, A., 2004. Mechanisms of heavy-metal sequestration and detoxification in crustaceans: a review. J. Comp. Physiol. B 174, 439–452.
Bautista, M.N., Lavilla-Pitogo, C.R., Subosa, P.F., 1994. Aflatoxin B1contamination of
shrimp feeds and its effect on growth and hepatopancreas of pre-adult Penaeus monodon. J. Sci. Food Agric. 65, 5–11.
Bell, T.A., Lightner, D.V., 1988. A Handbook of Normal Penaeid Shrimp Histology. World Aquaculture Society, Baton Rouge, LA.
Bhavan, P.S., Geraldine, P., 2000. Histopathology of the hepatopancreas and gills of the prawn Macrobrachium malcolmsonii exposed to endosulfan. Aquat. Toxicol. 50, 331–339.
Bryan, G.W., 1976. Heavy metal contamination in the sea. In: Johnston, R. (Ed.), Marine Pollution. Academic Press, New York, USA, pp. 185–302.
Caceci, T., Neck, K.F., Lewis, D.H., Sis, R.F., 1988. Ultrastructure of the hepatopancreas of the pacific white shrimp, Penaeus vannamei (Crustacea: Decapoda). J. Mar. Biol. Assoc. UK 68, 323–337.
Dall, W., Moriarty, D.J.W., 1983. Functional aspects of nutrition and digestion. In: Mantel, L.H. (Ed.), The Biology of Crustacea. Internal Anatomy and Physiological Regulation, vol. 5. Academic Press, New York, pp. 215–261.
Doughtie, D.G., Rao, K.R., 1984. Histopathological and ultrastructural changes in the antennal gland, midgut, hepatopancreas, and gill of grass shrimp following exposure to hexavalent chromium. J. Inver. Pathol. 43, 89–108.
Förlin, L., Haux, C., Karlsson-Norrgren, L., Runn, P., Larsson, Á., 1986. Biotransformation enzyme activities and histopathology in rainbow trout, Salmo gairdneri, treated with cadmium. Aquat. Toxicol. 8, 51–64.
Galindo-Reyes, J.G., Venezia, L.D., Lazcano-Alvarez, G., Rivas-Mendoza, H., 2000. Enzymatic and osmoregulative alterations in white shrimp Litopenaeus vannamei exposed to pesticides. Chemosphere 40, 233–237.
James, M.O., Boyle, S.M., 1998. Cytochrome P450 in Crustacea. Comp. Biochem. Phys. C 121, 157–172.
Khangarot, B.S., 1992. Copper-induced hepatic ultrastructural alterations in the snake-headed fish, Channa punctatus. Ecotoxicol. Environ. Saf. 23, 82–293. Kim, S.-G., Kang, J.-C., 2004. Effect of dietary copper exposure on accumulation,
growth and hematological parameters of the juvenile rockfish, Sebastes schlegeli. Mar. Environ. Res. 58, 65–82.
Lightner, D.V., Redman, R.M., Price, R.L., Wiseman, M.O., 1982. Histopathology of aflatoxicosis in the marine shrimp Penaeus stylirostris and P. vannamei. J. Inver. Pathol. 40, 279–291.
Lightner, D.V., Hasson, K.W., White, B.L., Redman, R.M., 1996. Chronic toxicity and histopathological studies with Benlate, a commercial grade of benomyl, in Penaeus vannamei (Crustacea: Decapoda). Aquat. Toxicol. 34, 105–118.
Martinez, M., Torreblanca, A., Ramo, J.D., Pastor, A., Diaz-Mayans, J., 1993. Cadmium induced metallothionein in hepatopancreas of Procambarus clarkii: quantification by a silver-saturation method. Comp. Biochem. Phys. C 105, 263–267.
Nelson, D.L., Cox, M.M., 2000. Lehninger Principles of Biochemistry. Worth Publishers, New York. pp. 623–658.
Pederson, S.N., Lundebye, A.-K., Depledge, M.H., 1997. Field application of metallothionein and stress protein biomarkers in the shore crab Carcinus maenas exposed to trace metals. Aquat. Toxicol. 37, 183–200.
Roesijadi, G., 1992. Metallothioneins in metal regulation and toxicity in aquatic animals. Aquat. Toxicol. 22, 81–114.
Snyder, M.J., 2000. Cytochrome P450 enzymes in aquatic invertebrates: recent advances and future directions. Aquat. Toxicol. 48, 529–547.
Söderhäll, K., Smith, V.J., 1983. Separation of the haemocyte populations of Carcinus maenas and other marine decapods, and prophenoloxidase distribution. Dev. Comp. Immunol. 7, 229–239.
Soegianto, A., Charmantier-Daures, M., Trilles, J.-P., Charmantier, G., 1999. Impact of cadmium on the structure of gills and epipodites of the shrimp Penaeus japonicus. Aquat. Living Resour. 12 (1), 57–70.
Torre, F.R., Salibián, A., Ferrari, L., 2000. Biomarkers assessment in juvenile Cyprinus carpio exposed to waterborne cadmium. Environ. Pollut. 109, 277–282. Urich, K., 1994. Comparative Animal Biochemistry. Springer, Berlin.
Vaglio, A., Landriscina, C., 1999. Change in liver enzyme activity in the teleost Sparus aurata in response to cadmium intoxication. Ecotoxicol. Environ. Saf. 43, 111– 116.
Vijayram, K., Geraldine, P., 1996. Regulation of essential heavy metals (Cu, Cr, and Zn) by the freshwater prawn Macrobrachium malcolmsonii. Bull. Environ. Contam. Toxicol. 56, 335–342.
Vogt, G., 1990. Pathology of midgut gland-cells of Penaeus monodon post-larvae after Leucaena leucocephala feeding. Dis. Aquat. Organ. 9, 45–61.
White, S.L., Rainbow, P.S., 1985. On the metabolic requirements for copper and zinc in mollusks and crustaceans. Mar. Environ. Res. 16, 215–229.
Wu, J.P., Chen, H.-C., 2004. Effects of cadmium and zinc on oxygen consumption, ammonium excretion, and osmoregulation of white shrimp (Litopenaeus vannamei). Chemosphere 57, 1591–1598.
Wu, J.-P., Chen, H.-C., 2005a. Effects of cadmium and zinc on the growth, food consumption, and nutritional conditions of the white shrimp, Litopenaeus vannamei (Boone). Bull. Environ. Contam. Toxicol. 74, 234–241.
Wu, J.-P., Chen, H.-C., 2005b. Metallothionein induction and heavy metal accumulation in white shrimp Litopenaeus vannamei exposed to cadmium and zinc. Comp. Biochem. Phys. C 140, 383–394.