Recently, cardiovascular morbidity and mor-tality have been linked to particulate matter (PM) air pollution (Lanki et al. 2006; Pekkanen et al. 2002; Samet et al. 2000; Zanobetti and Schwartz 2005) and ranked as one of the most costly types of PM-related death (Dockery 2001). The PM-associated activation of the autonomic nervous system, usually expressed as changes in heart rate vari-ability (HRV), has been postulated as one of the major mechanisms linking PM exposures and their cardiovascular effects in the most recent studies (Brook et al. 2004).
Epidemiologic studies (Chuang et al. 2005b; Samet et al. 2000; Schwartz et al. 1999; Seaton et al. 1999), especially those focusing on HRV indices (Chan et al. 2004, 2005; Chuang et al. 2005a; Gong et al. 2004; Tarkiainen et al. 2003; Timonen et al. 2006; Wheeler et al. 2006), have demonstrated that PM-mediated cardiovascular effects are heterogeneous, depending on particle con-tents. Animal models have been used to investigate the effects of different particles (Campen et al. 2001, 2002; Chen et al. 1992; Gordon et al. 1998; Kodavanti et al. 1998, 2002; Ulrich et al. 2002). The cardiovascular toxicities were also demonstrated to be heterogeneous in toxicologic settings focusing
on cytokine release (Hetland et al. 2005; Li et al. 1999), heart rate changes (Gordon et al. 1998; Wellenius et al. 2003), electrocardio-graphic (ECG) changes (Gordon et al. 2000; Hwang et al. 2005; Wellenius et al. 2003), and HRV indices (Chen and Hwang 2005; Godleski et al. 2000). All of these toxicologic studies support the heterogeneity of PM cardiovascular effects. However, the under-lying mechanisms remain to be explored.
Several studies have been devoted to deter-mining the explanation for these observed heterogenic PM effects, including the vulnera-bility and host effects (Bateson and Schwartz 2004; Schwartz et al. 2005). It has also been postulated that the compositional characteris-tics of particles may contribute to their differ-ent health impacts (Ostro et al. 2007). This hypothesis is supported both by epidemiologic observations, and animal toxicologic research. The most appealing observations include the association between HRV indices and trajec-tory (Godleski et al. 2000; Lippmann et al. 2006), and componential groups by statistical modeling (Lippmann et al. 2005). These observations suggested that the cardiovascular effects of PMs varied significantly with their compositional characteristics. Our objectives in this study are to verify that different PM
components can cause different cardioregula-tory effects; that a single PM component exposure can cause different effects at different phases; and that combined exposure to multi-ple PM components can produce interactions modulating the final outcomes.
Materials and Methods
Experimental design. We obtained 60-day-old male spontaneously hypertensive (SH) rats from the National Laboratory Animal Breeding and Research Center (Taipei, Taiwan). They were housed individually on Aspen chip bed-ding and provided with Lab Diet 5001 (PMI Nutrition International, Richmond, IN, USA) and water ad libitum. A 12-hr light/dark cycle, a constant room temperature, and a constant relative humidity were maintained in the ani-mal room during the study.
SH rats were implanted with radio-telemetry transmitters at 11 weeks of age. Experiments were performed over a 2-week period, beginning 10 days after implantation. Phosphate buffered saline (PBS) was given via intratracheal (IT) instillation (under Sevoflurane general anesthesia; Abbott Laboratories Ltd., Queenborough, Kent, UK) at a volume of 0.25 mL per animal in the first week. Radiotelemetric data were subsequently collected for 72 hr, and this served as a base-line template in the analysis process. Materials to be tested were suspended or dissolved in PBS, and were given to the same animals on the same day and time the following week. Data collected for the next 72 hr served as the response data.
As shown in Figure 1, eight groups of experiments were performed. Test materials Address correspondence to T.J. Cheng, Institute of Occupational Medicine and Industrial Hygiene, College of Public Health, National Taiwan University, 17 Xu-Zhou Rd., Rm 720, Taipei, Taiwan 10055. Telephone and Fax: +886-2-3322-8090. E-mail: tcheng@ntu.edu.tw
We thank C.-W. Yeh for her technical assistance. We also thank the National Institute of Environ-mental Analysis, Taiwan EnvironEnviron-mental Protection Administration (EPA) for their assistance on this project.
This study was funded by the Taiwan EPA (grants NSC 94-EPA-Z-002-007; NSC 95-EPA-Z-002-011) and the National Science Council of Taiwan (grant NSC 94-2621-Z-002-019).
The authors declare they have no competing financial interests.
Received 11 October 2006; accepted 27 February 2007.
Interaction Effects of Ultrafine Carbon Black with Iron and Nickel on
Heart Rate Variability in Spontaneously Hypertensive Rats
Chuen-Chau Chang,1,2Jing-Shiang Hwang,3Chang-Chuan Chan,1and Tsun-Jen Cheng1
1Institute of Occupational Medicine and Industrial Hygiene, National Taiwan University, Taipei, Taiwan; 2Department of Anesthesiology,
Taipei Medical University Hospital, Taipei, Taiwan; 3Institute of Statistical Science, Academia Sinica, Taipei, Taiwan
BACKGROUND: Particulate matter (PM) has been reported to be associated with alterations in heart
rate variability (HRV); however, the results are inconsistent. We propose that different components of PM cause the discrepancy.
OBJECTIVE: In this study, our goal was to determine whether different types of exposure would
cause different HRV effects, and to verify the interactions between co-exposing components.
METHODS: Ultrafine carbon black (ufCB; 14 nm; 415 µg and 830 µg), ferric sulfate [Fe2(SO4)3;
105 µg and 210 µg], nickel sulfate (NiSO4; 263 µg and 526 µg), and a combination of high-dose
ufCB and low-dose Fe2(SO4)3or NiSO4were intratracheally instilled into spontaneously
hyper-tensive rats. Radiotelemetry data were collected in rats for 72 hr at baseline and for 72 hr the follow-ing week to determine the response to exposure. Effects of exposure on 5-min average of to-normal intervals (ANN), natural logarithm-transformed standard deviation of the to-normal intervals (LnSDNN), and root mean square of successive differences of adjacent normal-to-normal intervals (LnRMSSD) were analyzed using self-control experimental designs.
RESULTS: Both high- and low-dose ufCB decreased ANN marginally around hour 30, with
concur-rent increases of LnSDNN. LnRMSSD returned to baseline levels after small initial increases. We observed minor effects after low-dose Fe and Ni instillation, whereas biphasic changes were noted after high-dose instillations. Combined exposures of ufCB and either Fe or Ni resulted in HRV trends different from values estimated from individual-component effects.
CONCLUSIONS: Components in PM may induce different cardioregulatory responses, and a single
component may induce different responses during different phases. Concurrent exposure to ufCB and Fe or Ni might introduce interactions on cardioregulatory effects. Also, the effect of PM may be mediated through complex interaction between different components of PM.
KEY WORDS: ambient particles, heart rate variability, interaction, iron, nickel, spontaneously
hypertensive rats, ultrafine carbon black. Environ Health Perspect 115:1012–1017 (2007). doi:10.1289/ehp.9821 available via http://dx.doi.org/ [Online 27 February 2007]
were dispersed or dissolved in 0.25 mL PBS: 14 nm ultrafine carbon black (ufCB; low-dose: 415 μg/animal, n = 4; and high-dose: 830 μg/animal, n = 4); ferric sulfate [Fe2(SO4)3; low-dose: 105 μg/animal, n = 5;
high-dose: 210 μg/animal, n = 3]; and nickel sulfate (NiSO4; low-dose: 263 μg/animal,
n = 5; high-dose: 526 μg/animal, n = 3). Concomitant exposures of 830 μg ufCB with 105 μg Fe2(SO4)3(n = 4) or 263 μg NiSO4
(n = 4) in 0.25 mL PBS were also performed. All materials to be tested underwent ultra-sonication for 30 min before IT instillation. The exposure dosages of ufCB were previously determined in our laboratory. The low expo-sure dosages of Fe2(SO4)3and NiSO4were
comparable with those used in previous works (Campen et al. 2002); we then doubled those doses to investigate more significant HRV changes. All protocols used in this study were approved by the Committees on Use and Care of Animals of the National Taiwan University. All SH rats used in this study were treated humanely according to institutional guide-lines, with appropriate consideration for the alleviation of suffering and distress.
Heart rate variability measurements. The methodology of HRV measurements with the radiotelemetry system has been described pre-viously (Chang et al. 2004, 2005; Cheng et al. 2003). Briefly, we collected all ECG signals throughout the study on a continuous basis. The sampling rates for the ECG signals were set at 1,000 points per second (1,000 Hz) for better temporal discrimination.
Time intervals between adjacent R waves in the ECG recording (RR) were calculated on a beat-to-beat basis using Dataquest A.R.T. Analysis software, version 2.20 (Data Sciences International, St. Paul, MN, USA). To obtain normal-to-normal (NN) intervals, we used a computer algorithm based on the recommen-dation by Cheung (1981) to eliminate type A and type B errors in NN calculation. Basically, the NN calculation followed a two-step
procedure: the increase or decrease of any RR compared with the previous RR was limited to 33% in a step-1 correction, and data points with distances to the median > 1.5 SDs on Lorenz plots were eliminated in step 2 for every 30 min. The 5-min SD of the normal-to-normal intervals (SDNN) and the root mean square of successive differences of adjacent normal-to-normal intervals (RMSSD) were then calculated from these NN data sets.
Statistical analysis. Owing to individual variation among diseased animals, conven-tional exposure–control experimental designs would necessitate a large sample size to demon-strate minute effects under strong confounding conditions, a scenario commonly seen in the study of PM toxicology. Furthermore, the cir-cadian nature of cardiovascular parameters often complicates the analysis. Thus, we used a self-control experimental designs in this research. Exposures were carried out at the same time (starting from 1200 hours) on Tuesday of two consecutive weeks. Data col-lected in the first week (animals exposed to PBS alone) served as the control group for those collected in the following week (animals exposed to test materials).
We calculated the SDNN and RMSSD as described previously (Chang et al. 2005). Average NN intervals (ANN) and natural log-arithm transformation of SDNN (LnSDNN) and RMSSD (LnRMSSD) were used as out-come measurements to produce approxi-mately symmetrical distributions of response variables for statistical analysis. Time plots of the original data are shown in Figure 2.
We calculated hourly means of the control group data of all three HRV parameters (ANN, LnSDNN, and LnRMSSD); these hourly means served as circadian templates in the ana-lytic procedures. To better illustrate particle effects, we subtracted the hourly means from all 5-min data from each HRV parameter to obtain crude effects, which were then used for the computation of 6-hr average crude effects.
We used the generalized estimation equa-tion (GEE) model to further examine the exposure effect during the 72-hr observation period. We modeled the exposure effects with a set of 13 dummy variables, each standing for average crude effects of the 1-hr preparation and 6-hr succeeding time segments. Yitis the average crude effect for the ith SH rat at time t = 0, 1, 2, … 12, in which t = 0 corresponds to the 1-hr preparation, and the following time points correspond to the 12 6-hr sections dur-ing the 72-hr observation period. For adjustdur-ing rat-to-rat variation and control group effects, dummy variables for the number (n) of ani-mals and the 13 HRV parameter values Bt obtained in the first week are included in the model. Specifically, the GEE model is given by
, [1] where i = 1, …, n, t = 0, 1, 2, … 12, and I (·) is an indicator function. We chose the error term εitto be an autoregressive process with order 1 to model time dependence. The coefficients αm, for m = 0, 1, 2, … 12, were used to describe the 6-hr mean exposure effects during the 72-hr observation period. Because the SH rats were randomly selected from a population, in addition to the overall difference parameter β1i, we added random components amito model the rat-to-rat variation of these effects. All of these random coefficients were assumed to be normally distributed with the mean of 0 and some constant variances. The time plots of the estimation of 6-hr means and 95% confi-dence intervals (CIs) were generated to provide an overall impression of the data.
The exposure effects of high-dose ufCB and low-dose Fe2(SO4)3or NiSO4were used
to generate two virtual series by temporal sum-mation of means and variances of the data from every 6 hr. These two virtual series were defined as the expected combined effects. Time plots of real combined effects of high dose ufCB and low dose Fe2(SO4)3or NiSO4
were used to analyze the interactions between ufCB and Fe2(SO4)3or NiSO4.
We used SAS 8.2 statistical software pack-age (SAS Institute, Cary, NC, USA) to manpack-age data and estimate the parameters and standard errors in the models.
Results
Exposure effects. The GEE model–estimated exposure effects of ufCB, Fe2(SO4)3, and
NiSO4are shown in Figure 3. As shown in
Figure 3, for both low-dose (415 μg) and high-dose (830 μg) ufCB exposures, the ANN basi-cally exhibited a borderline depressed level centering around 30 hr after exposure (Figure 3A). Increased LnSDNN was followed by nonsignificant changes (Figure 3D). Initially
Y I i j B a it nj i t t mi m m = +∑ = + + + = = β β β α 0 1 1 ( ) 2 [( ) 0 0 12 ∑ I t( =m)]+εit
Figure 1. Summary of IT instillation protocol. See “Materials and Methods” for details of experiments. Data
collected from the first week (PBS alone) served as controls for data from the second week (test materials). Postsurgery recovery,
10 days
Week 1: control data collected for 72 hr
Week 2: response data collected for 72 hr Implantation of radiotelemeter IT at 1200 hours on Tuesday (0.25 mL/PBS) IT at 1200 hours on Tuesday ufCB Fe2(SO4)3 NiSO4 Low High Low High Low High 415 μg 830 μg 105 μg 210 μg 263 μg 526 μg 830 μg ufCB + 105 μg Fe2(SO4)3 830 μg ufCB + 263 μg NiSO4
elevated LnRMSSD followed a back-to-baseline trend 6 hr after exposure (Figure 3G).
Exposure to low-dose (105 μg) Fe2(SO4)3
resulted in increased LnSDNN at the end of the 72-hr observation (Figure 3E). The increase in LnRMSSD was small and persistent, and reached significance in the latter half of the observation period (Figure 3H). Exposure to high-dose (210 μg) Fe2(SO4)3resulted in
signif-icantly biphasic responses in ANN (Figure 3B) and LnRMSSD (Figure 3H), which increased in the first 24 hr and decreased in the last 24 hr. Increased LnSDNN in the first and last 24 hr, however, rendered the responses multimodal (Figure 3E).
The exposure effects of NiSO4are shown
in Figure 3C, 3F, and 3I. Exposure to low-dose (263 μg) NiSO4did not generate prominent
responses in HRV measurements. In contrast, exposure to high-dose (516 μg) NiSO4resulted
in biphasic responses in all three measurements, which increased in the first 24 hr and decreased in the last 24 hr.
Expected and real combined effects. The expected and real combined effects are illus-trated in Figure 4. The expected combined effects in both Fe2(SO4)3and NiSO4basically
followed similar trends. Compared with the expected combined effects, the real combined effects of ufCB and Fe2(SO4)3 tend to
demonstrate milder changes in all three para-meters during the 36 hr after exposure. In the combined exposure of ufCB and NiSO4, the
real combined effects had a tendency to show more prominent changes in all three parame-ters during the same period. For the last 24 hr, the real combined effects for both groups tracked comparable trends, and were frequently separated from the expected com-bined effects.
Discussion
Dose responses to ufCB. Effects of high-dose ufCB were not obviously different from those of low-dose ufCB. According to Harder et al. (2005), the decrease in ANN around 30 hr after ufCB instillation might reflect low-grade but significant pulmonary inflammation. Failure of high-dose ufCB to induce more prominent responses might be due to aggrega-tion effects. Although ultrasonicaaggrega-tion was applied to all materials before instillation, the aggregation of ufCB still should be considered, particularly when the concentrations are high (Gilmour et al. 2004).
Dose and phased responses to Fe2(SO4)3or NiSO4. Whereas reactions to low-dose Fe2(SO4)3 and NiSO4were modest, the
responses to high doses were noticeably bipha-sic in the present study. Campen et al. (2002) found that low-dose Fe2(SO4)3produced no
obvious changes in heart rate and core body temperature on monocrotaline-induced pul-monary hypertensive Sprague-Dawley rats,
whereas NiSO4demonstrated acute
bradycar-dia. In the present study, we found increased LnSDNN and LnRMSSD toward the end of the 72-hr observation period in response to the same level of Fe2(SO4)3. Exposure to low-dose
NiSO4did not result in significant HRV
changes. We thus tested the HRV responses to “double-dosed” exposures, and demonstrated biphasic effects. The difference of the effects between these studies might have derived from model dissimilarity (Chang et al. 2004; Cheng et al. 2003).
Decreased ANN and LnSDNN 48 hr after IT instillation of Ni are comparable with the most recent study found on ApoE–/–
(apolipoprotein deficient) mice by inhalation of Ni-rich concentrated ambient particles (Lippmann et al. 2006). Biological plausibility of Ni-induced cardiovascular effects was well reviewed in their work and is applicable to our results. The discrepancy between response phases may be caused by differences in the model and the experimental design.
Significance and possible mechanisms of phased responses. Single-phased and dose-dependent decreases in heart rate have been demonstrated in SH rats after IT instillation of an oil combustion–derived PM rich in transi-tion metals (Wichers et al. 2004). Although biphasic heart rate and thermoregulatory effects
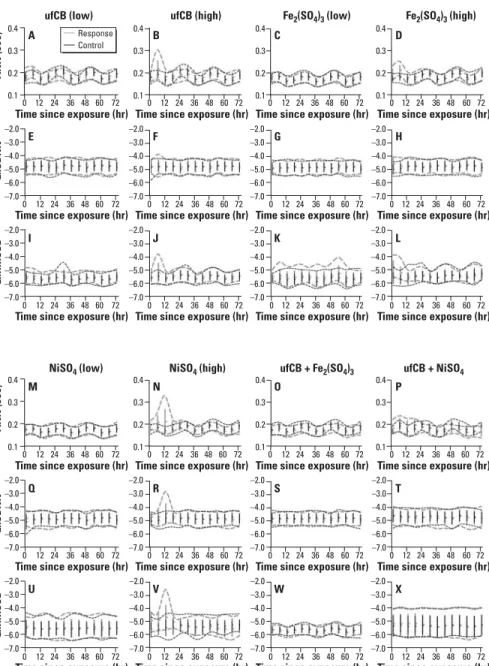
Figure 2. Original data distribution of response and baseline (control) for ANN, LnSDNN, and LnRMSSD for
low-dose ufCB (A, E, and I), high-dose ufCB (B, F, and J), low-dose Fe2(SO4)3(C, G, and K), high-dose (D, G,
and L), low-dose NiSO4(M, Q, and U), high-dose NiSO4(N, R, andV), ufCB + Fe2(SO4)3(O, S, and W), and
ufCB + NiSO4(P, T, and X). Values shown are mean ± SE; dashed lines represent the 95% distribution
envelopes (the region of distribution for 2.5 percentile up to 97.5 percentile data points).
0.4 0.3 0.2 0.1 0 12 24 36 48 60 72 0.4 0.3 0.2 0.1 0.4 0.3 0.2 0.1 0.4 0.3 0.2 0.1 –2.0 –3.0 –4.0 –5.0 –6.0 –7.0 0 12 24 36 48 60 72 0 12 24 36 48 60 72 0 12 24 36 48 60 72 0 12 24 36 48 60 72 0 12 24 36 48 60 72 0 12 24 36 48 60 72 0 12 24 36 48 60 72 0 12 24 36 48 60 72 0 12 24 36 48 60 72 0 12 24 36 48 60 72 0 12 24 36 48 60 72 0 12 24 36 48 60 72 0 12 24 36 48 60 72 0 12 24 36 48 60 72 0 12 24 36 48 60 72 0.4 0.3 0.2 0.1 0.4 0.3 0.2 0.1 0.4 0.3 0.2 0.1 0.4 0.3 0.2 0.1 0 12 24 36 48 60 72 0 12 24 36 48 60 72 0 12 24 36 48 60 72 0 12 24 36 48 60 72 0 12 24 36 48 60 72 0 12 24 36 48 60 72 0 12 24 36 48 60 72 0 12 24 36 48 60 72 ANN (sec) LnSDNN LnRMSSD ANN (sec) LnSDNN LnRMSSD A B C D E F G H I J K L M N O P Q R S T U V W X
Time since exposure (hr) Time since exposure (hr) Time since exposure (hr) Time since exposure (hr)
Time since exposure (hr) Time since exposure (hr) Time since exposure (hr) Time since exposure (hr)
Time since exposure (hr) Time since exposure (hr) Time since exposure (hr) Time since exposure (hr)
Time since exposure (hr) Time since exposure (hr) Time since exposure (hr) Time since exposure (hr) Time since exposure (hr) Time since exposure (hr) Time since exposure (hr) Time since exposure (hr) Time since exposure (hr) Time since exposure (hr) Time since exposure (hr) Time since exposure (hr)
ufCB (low) ufCB (high) Fe2(SO4)3 (low) Fe2(SO4)3 (high)
NiSO4 (low) NiSO4 (high) ufCB + Fe2(SO4)3 ufCB + NiSO4
–2.0 –3.0 –4.0 –5.0 –6.0 –7.0 –2.0 –3.0 –4.0 –5.0 –6.0 –7.0 –2.0 –3.0 –4.0 –5.0 –6.0 –7.0 –2.0 –3.0 –4.0 –5.0 –6.0 –7.0 –2.0 –3.0 –4.0 –5.0 –6.0 –7.0 –2.0 –3.0 –4.0 –5.0 –6.0 –7.0 –2.0 –3.0 –4.0 –5.0 –6.0 –7.0 –2.0 –3.0 –4.0 –5.0 –6.0 –7.0 –2.0 –3.0 –4.0 –5.0 –6.0 –7.0 –2.0 –3.0 –4.0 –5.0 –6.0 –7.0 –2.0 –3.0 –4.0 –5.0 –6.0 –7.0 –2.0 –3.0 –4.0 –5.0 –6.0 –7.0 –2.0 –3.0 –4.0 –5.0 –6.0 –7.0 –2.0 –3.0 –4.0 –5.0 –6.0 –7.0 –2.0 –3.0 –4.0 –5.0 –6.0 –7.0 Response Control
have been demonstrated in cardiopulmonary-compromised rats exposed to residual oil fly ash (Campen et al. 2000; Watkinson et al. 1998), this is not the case in single component expo-sures. Conversely, exposure to high-dose Fe2(SO4)3and NiSO4generated biphasic
changes in all three parameters in the present study. To the best of our knowledge, this is the first study to demonstrate biphasic HRV responses to single component exposures.
We have speculated that the time lag and complex interplay among incoming C-fiber stimulation, reactive oxygen species (ROS) production (Adler et al. 1999; Avshalumov et al. 2000; Girouard and de Champlain 2005; Zanzinger and Czachurski 2000), and inflammation with proinflammatory cytokines release (Elder et al. 2004; Hirano et al. 1994; Kang et al. 2002; Lei et al. 2004a, 2004b, 2005; Shwe et al. 2005; Tracey 2002; Yang et al. 1997) might all contribute to the synthe-sis of the observed biphasic responses. However, this speculation warrants further testing and verification.
Interactions between ufCB and/or Fe2(SO4)3or NiSO4. The expected combined effects are the virtual series generated by tem-poral summation of means and variances of the real exposure effects of high-dose ufCB and
low-dose Fe2(SO4)3or NiSO4. Because the
aim of the present study was to examine inter-actions between ufCB and transition metals, we selected transition metals at doses that produced minimal HRV effects: low-dose Fe2(SO4)3and NiSO4. Because neither low
nor high doses of ufCB generate significantly different HRV effects, we chose high-dose ufCB for more complete “absorption” of tran-sition metals on the carbonaceous surfaces. In the present study, combined exposures of ufCB and Fe2(SO4)3or NiSO4demonstrated
real combined effects that were significantly different from the expected combined effects. These trends verified significant interactions between the exposure components.
Transition metals have demonstrated interactions on cardioregulatory and thermo-regulatory effects (Campen et al. 2002). Interactions between ufCB and Fe were also verified on pulmonary inflammation and ROS production (Wilson et al. 2002). We speculate that these interactions might involve a complex interplay among chelating/leaching kinetics, inflammatory processes, and ROS reactions (Arimoto et al. 2005). Ambient ufCB and tran-sition metals provoke different cardioregulatory effects when administered jointly, and these effects might be augmented in compromised
vulnerable subjects. This speculation deserves further research and verification.
Experimental niches and limitations. IT
under general anesthesia. To precisely control
the dosage, we used IT instillation as the expo-sure route. However, this procedure is consid-ered invasive and less physiologic (Driscoll et al. 2000) and requires general anesthesia. We chose the ultra-short inhalation anesthetic Sevoflurane to shorten the postanesthetic recovery to within 2 min, and we discarded data acquired within the first hour. This new and improved technology has minimized the anesthesia-associated variations to a negligible level. IT instillation disperses the particles evenly throughout most airways independent of particle size (Leong et al. 1998). We believe that, within the lung, the pattern of distribu-tion of instilled ufCB, Ni, and Fe compounds is similar, and that the response discrepancy might not have originated from distribution pattern differences.
SH rats. Kodavanti et al. (2000) and
Watkinson et al. (2001) observed exacerbated cardiopulmonary injury and oxidative stress in SH rats exposed to PM and concluded that the SH rat is a potentially useful model to study the susceptibility to PM effects on the cardio-vascular system. In the present study we used
Figure 3. Dosage effects and dynamic responses for low-dose and high-dose ufCB (A, D, G), Fe2(SO4)3(B, E, H), and NiSO4(C, F, I). (A–C) ΔANN. (D–F) ΔLnSDNN.
(G–I) ΔLnRMSSD. Values shown are mean ± SE; dashed lines indicate 95% CI.
0.06 0.04 0.02 0.00 –0.02 –0.04 12 24 36 48 60 72 Δ ANN (sec) A
Time since exposure (hr)
12 24 36 48 60 72 0.06 0.04 0.02 0.00 –0.02 –0.04 72 60 48 36 24 12
Time since exposure (hr) Time since exposure (hr)
0.06 0.04 0.02 0.00 –0.02 –0.04 12 24 36 48 60 72 12 24 36 48 60 72 1.0 0.5 0.0 –0.5 Δ LnSDNN 1.0 0.5 0.0 –0.5 12 24 36 48 60 72
Time since exposure (hr) Time since exposure (hr) Time since exposure (hr)
1.6 1.2 0.8 0.4 0.0 –0.4 12 24 36 48 60 72 12 24 36 48 60 72 12 24 36 48 60 72 1.6 1.2 0.8 0.4 0.0 –0.4 1.0 0.5 0.0 –0.5 1.6 1.2 0.8 0.4 0.0 –0.4
Time since exposure (hr) Time since exposure (hr) Time since exposure (hr)
B C
D E F
G H I
Δ
LnRMSSD
ufCB Fe2(SO4)3 NiSO4
Low dose High dose
SH rats as an oxidation-deficient animal model, and we suggest that this model might be useful in assessing the potential biological plausibility linking PM exposures and the cardioregulatory effects in subpopulations with increased oxida-tive stress (Schwartz et al. 2005). Although SH rats have been suggested to be suitable for mim-icking human essential hypertensive subgroups (Sun and Zhang 2005), their pathophysiology may not completely match that of humans (Watkinson et al. 2003). Extrapolation of these PM-associated cardiovascular effects to human beings deserves further studies on healthy con-trols, including Wistar-Kyoto rats.
Time domain HRV. In the present study,
only time domain HRV parameters were used to measure the cardioregulatory effects, spar-ing the more sophisticated frequency domain parameters. However, we have previously
demonstrated the applicability of these para-meters (Chang et al. 2005) and the correlation with other hemodynamic indices (Chang et al. 2004). Owing to the close correlations among these parameters and those of frequency domain (Kleiger et al. 1991), this limitation does not seriously restrict the interpretation of results. Besides, the index ANN is equivalent to the inverse of heart rate in beats per minute. The use of ANN may cause some inconve-nience in biologic interpretation, but it com-plies with HRV analysis for better symmetry of data distribution.
Statistical strategy and experimental design. Owing to technical demands,
large-scaled experiments were impractical in our study. We used self-control experimental designs and GEE models to remedy the inter-ference introduced by the relatively small
sample size. In contrast, only three PM com-ponents were used in the present study, spar-ing many others. These factors have limited the scope of the study to some extent. However, to the best of our knowledge, this is the first study investigating the interactions between ufCB and transition metals on HRV and might indicate further investigations on many other major PM components for their dynamic effects and potential interactions.
Concomitant exposure to ufCB and tran-sition metals. In the present study, we presume
that administering Ni or Fe individually is comparable to having these two substance leach from ufCB once instilled. A recent study investigating the interaction between 14 nm ufCB and transition metals on pulmonary inflammation and ROS formation also used a similar approach (Wilson et al. 2002). However, the kinetics of Ni or Fe leaching from ufCB has not been completely studied. We suggest that caution is required in interpre-tation before more detailed binding/leaching kinetics are available.
Conclusion
In the present study, we demonstrated that ufCB has different cardioregulatory effects at different phases. The HRV responses to high-dose Fe2(SO4)3and NiSO4were noticeably
biphasic, although the reactions to low-dose exposures were modest. Whereas the dose effects of Fe2(SO4)3and NiSO4were obvious,
those for ufCB were obscure. Concurrent exposure to ufCB and Fe2(SO4)3or NiSO4
introduced cardioregulatory responses that were more significant than those to single-component exposures.
We concluded that different components in PM might induce different cardioregulatory effects. A single-component exposure might also induce different effects at different phases, resulting in biphasic or even more complex cardioregulatory responses. Combined expo-sure to multiple components could introduce interactions among copollutants, and tem-poral summation of componential toxic responses might not be appropriate in the esti-mation of cardiovascular effects in real-life exposures.
REFERENCES
Adler V, Yin Z, Tew KD, Ronai Z. 1999. Role of redox potential and reactive oxygen species in stress signaling. Oncogene 18:6104–6111.
Arimoto T, Kadiiska MB, Sato K, Corbett J, Mason RP. 2005. Synergistic production of lung free radicals by diesel exhaust particles and endotoxin. Am J Respir Crit Care Med 171:379–387.
Avshalumov MV, Chen BT, Rice ME. 2000. Mechanisms under-lying H2O2-mediated inhibition of synaptic transmission in
rat hippocampal slices. Brain Res 882:86–94.
Bateson TF, Schwartz J. 2004. Who is sensitive to the effects of particulate air pollution on mortality? A case-crossover analysis of effect modifiers. Epidemiology 15:143–149. Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M,
Figure 4. Interactions between ufCB and transition metals shown as expected and real combined effects.
ΔANN (A, B), ΔLnSDNN (C, D), and ΔLnRMSSD (E–F) for ufCB + Fe2(SO4)3(A, C, E), and ufCB + NiSO4
(B, D,F). Values shown are mean ± SE; dashed lines represent 95% CIs.
0.06 0.04 0.02 0.00 –0.02 –0.04 12 24 36 48 60 72 Δ ANN (sec) A
Time since exposure (hr) Expected Real 0.06 0.04 0.02 0.00 –0.02 –0.04
Time since exposure (hr)
12 24 36 48 60 72 Δ LnSDNN 1.0 0.5 0.0 –0.5 12 24 36 48 60 72
Time since exposure (hr) Time since exposure (hr)
12 24 36 48 60 72 1.0 0.5 0.0 –0.5 1.6 1.2 0.8 0.4 0.0 –0.4 Δ LnRMSSD 12 24 36 48 60 72
Time since exposure (hr) Time since exposure (hr)
12 24 36 48 60 72 1.6 1.2 0.8 0.4 0.0 –0.4 B C D E F
et al. 2004. Air pollution and cardiovascular disease: a state-ment for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 109:2655–2671.
Campen MJ, Costa DL, Watkinson WP. 2000. Cardiovascular and thermoregulatory toxicity of residual oil fly ash in cardio-pulmonary-compromised rats. Inhal Toxicol 12(suppl 2):7–22. Campen MJ, Nolan JP, Schladweiler MC, Kodavanti UP, Costa DL, Watkinson WP. 2002. Cardiac and thermoregula-tory effects of instilled particulate matter associated tran-sition metals in healthy and cardiopulmonary-compromised rats. J Toxicol Environ Health A 65:1615–1631.
Campen MJ, Nolan JP, Schladweiler MC, Kodavanti UP, Evansky PA, Costa DL, et al. 2001. Cardiovascular and thermoregulatory effects of inhaled PM-associated transi-tion metals: a potential interactransi-tion between nickel and vanadium sulfate. Toxicol Sci 64:243–252.
Chan CC, Chuang KJ, Shiao GM, Lin LY. 2004. Personal exposure to submicrometer particles and heart rate variability in human subjects. Environ Health Perspect 112:1063–1067. Chan CC, Chuang KJ, Su TC, Lin LY. 2005. Association between
nitrogen dioxide and heart rate variability in a susceptible population. Eur J Cardiovasc Prev Rehabil 12:580–586. Chang CC, Hwang JS, Chan CC, Wang PY, Hu TH, Cheng TJ.
2004. Effects of concentrated ambient particles on heart rate, blood pressure, and cardiac contractility in sponta-neously hypertensive rats. Inhal Toxicol 16:421–429. Chang CC, Hwang JS, Chan CC, Wang PY, Hu YW, Cheng TJ.
2005. Effects of concentrated ambient particles on heart rate variability in spontaneously hypertensive rats. J Occup Health 47:471–480.
Chen LC, Hwang JS. 2005. Effects of subchronic exposures to concentrated ambient particles in mice: IV. Characterization of acute and chronic effects of ambient air fine particulate matter exposures on heart rate variability. Inhal Toxicol 17:209–216.
Chen LC, Miller PD, Amdur MO, Gordon T. 1992. Airway hyper-responsiveness in guinea pigs exposed to acid-coated ultrafine particles. J Toxicol Environ Health 35:165–174. Cheng TJ, Hwang JS, Wang PY, Tsai CF, Chen CY, Lin SH, et al.
2003. Effects of concentrated ambient particles on heart rate and blood pressure in pulmonary hypertensive rats. Environ Health Perspect 111:147–150.
Cheung MN. 1981. Detection of and recovery from errors in cardiac interbeat intervals. Psychophysiology 18:341–346. Chuang KJ, Chan CC, Chen NT, Su TC, Lin LY. 2005a. Effects of
particle size fractions on reducing heart rate variability in cardiac and hypertensive patients. Environ Health Perspect 113:1693–1697.
Chuang KJ, Chan CC, Shiao GM, Su TC. 2005b. Associations between submicrometer particles exposures and blood pressure and heart rate in patients with lung function impairments. J Occup Environ Med 47:1093–1098. Dockery DW. 2001. Epidemiologic evidence of cardiovascular
effects of particulate air pollution. Environ Health Perspect 109(suppl 4):483–486.
Driscoll KE, Costa DL, Hatch G, Henderson R, Oberdorster G, Salem H, et al. 2000. Intratracheal instillation as an exposure technique for the evaluation of respiratory tract toxicity: uses and limitations. Toxicol Sci 55:24–35.
Elder AC, Gelein R, Azadniv M, Frampton M, Finkelstein J, Oberdorster G. 2004. Systemic effects of inhaled ultrafine particles in two compromised, aged rat strains. Inhal Toxicol 16:461–471.
Gilmour PS, Ziesenis A, Morrison ER, Vickers MA, Drost EM, Ford I, et al. 2004. Pulmonary and systemic effects of short-term inhalation exposure to ultrafine carbon black particles. Toxicol Appl Pharmacol 195:35–44.
Girouard H, de Champlain J. 2005. Acute and chronic effects of free radicals on alpha1-adrenergic-induced vasoconstric-tion in mesenteric beds of spontaneously hypertensive rats. J Hypertens 23:807–814.
Godleski JJ, Verrier RL, Koutrakis P, Catalano P, Coull B, Reinisch U, et al. 2000. Mechanisms of morbidity and mor-tality from exposure to ambient air particles. Res Rep Health Eff Inst (91):5–88.
Gong H Jr, Linn WS, Terrell SL, Clark KW, Geller MD, Anderson KR, et al. 2004. Altered heart-rate variability in asthmatic and healthy volunteers exposed to concentrated ambient coarse particles. Inhal Toxicol 16:335–343. Gordon T, Nadziejko C, Chen LC, Schlesinger R. 2000. Effects of
concentrated ambient particles in rats and hamsters: an exploratory study. Res Rep Health Eff Inst (93):5–34.
Gordon T, Nadziejko C, Schlesinger R, Chen LC. 1998. Pulmonary and cardiovascular effects of acute exposure to concen-trated ambient particulate matter in rats. Toxicol Lett 96–97:285–288.
Harder V, Gilmour P, Lentner B, Karg E, Takenaka S, Ziesenis A, et al. 2005. Cardiovascular responses in unrestrained WKY rats to inhaled ultrafine carbon particles. Inhal Toxicol 17:29–42.
Hetland RB, Cassee FR, Lag M, Refsnes M, Dybing E, Schwarze PE. 2005. Cytokine release from alveolar macrophages exposed to ambient particulate matter: hetero-geneity in relation to size, city and season. Part Fibre Toxicol 2:4; doi:10.1186/1743-8977-2-4 [Online 17 August 2005]. Hirano S, Shimada T, Osugi J, Kodama N, Suzuki KT. 1994.
Pulmonary clearance and inflammatory potency of intra-tracheally instilled or acutely inhaled nickel sulfate in rats. Arch Toxicol 68:548–554.
Hwang JS, Nadziejko C, Chen LC. 2005. Effects of subchronic exposures to concentrated ambient particles in mice: III. Acute and chronic effects of CAPs on heart rate, heart-rate fluctuation, and body temperature. Inhal Toxicol 17:199–207. Kang YJ, Li Y, Zhou Z, Roberts AM, Cai L, Myers SR, et al. 2002. Elevation of serum endothelins and cardiotoxicity induced by particulate matter (PM2.5) in rats with acute myocardial
infarction. Cardiovasc Toxicol 24:253–261.
Kleiger RE, Bigger JT, Bosner MS, Chung MK, Cook JR, Rolnitzky LM, et al. 1991. Stability over time of variables measuring heart rate variability in normal subjects. Am J Cardiol 68:626–630.
Kodavanti UP, Coata DL, Bromberg PA. 1998. Rodent model of cardiopulmonary disease: their potential applicability in studies of air pollutant susceptibility. Environ Health Perspect 106(suppl 1):111–130.
Kodavanti UP, Schladweiler MC, Ledbetter AD, Hauser R, Christiani DC, McGee J, et al. 2002. Temporal association between pulmonary and systemic effects of particulate matter in healthy and cardiovascular compromised rats. J Toxicol Environ Health A 65:1545–1569.
Kodavanti UP, Schladweiler MC, Ledbetter AD, Watkinson WP, Campen MJ, Winsett DW, et al. 2000. The spontaneously hypertensive rat as a model of human cardiovascular dis-ease: evidence of exacerbated cardiopulmonary injury and oxidative stress from inhaled emission particulate matter. Toxicol Appl Pharmacol 164:250–263.
Lanki T, de Hartog JJ, Heinrich J, Hoek G, Janssen NA, Peters A, et al. 2006. Can we identify sources of fine parti-cles responsible for exercise-induced ischemia on days with elevated air pollution? The ULTRA study. Environ Health Perspect 114:655–660.
Lei YC, Chan CC, Wang PY, Lee CT, Cheng TJ. 2004a. Effects of Asian dust event particles on inflammation markers in peripheral blood and bronchoalveolar lavage in pulmonary hypertensive rats. Environ Res 95:71–76.
Lei YC, Chen MC, Chan CC, Wang PY, Lee CT, Cheng TJ. 2004b. Effects of concentrated ambient particles on airway responsiveness and pulmonary inflammation in pulmonary hypertensive rats. Inhal Toxicol 16:785–792.
Lei YC, Hwang JS, Chan CC, Lee CT, Cheng TJ. 2005. Enhanced oxidative stress and endothelial dysfunction in strepto-zotocin-diabetic rats exposed to fine particles. Environ Res 99:335–343.
Leong BK, Coombs JK, Sabaitis CP, Rop DA, Aaron CS. 1998. Quantitative morphometric analysis of pulmonary deposi-tion of aerosol particles inhaled via intratracheal nebuliza-tion, intratracheal instillation or nose-only inhalation in rats. J Appl Toxicol 18:149–160.
Li XY, Brown D, Smith S, MacNee W, Donaldson K. 1999. Short-term inflammatory responses following intratracheal instil-lation of fine and ultrafine carbon black in rats. Inhal Toxicol 11:709–731.
Lippmann M, Hwang JS, Maciejczyk P, Chen LC. 2005. PM source apportionment for short-term cardiac function changes in ApoE– / –mice. Environ Health Perspect
113:1575–1579.
Lippmann M, Ito K, Hwang JS, Maciejczyk P, Chen LC. 2006. Cardiovascular effects of nickel in ambient air. Environ Health Perspect 114:1662–1669.
Ostro B, Feng WY, Broadwin R, Green S, Lipsett M. 2007. The effects of components of fine particulate air pollution on mortality in California: results from CALFINE. Environ Health Perspect 115:13–19.
Pekkanen J, Peters A, Hoek G, Tiittanen P, Brunekreef B, de Hartog J, et al. 2002. Particulate air pollution and risk of
ST-segment depression during repeated submaximal exer-cise tests among subjects with coronary heart disease: the Exposure and Risk Assessment for Fine and Ultrafine Particles in Ambient Air (ULTRA) study. Circulation 106:933–938.
Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. 2000. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med 343:1742–1749.
Schwartz J, Norris G, Larson T, Sheppard L, Claiborne C, Koenig J. 1999. Episodes of high coarse particle concen-trations are not associated with increased mortality. Environ Health Perspect 107:339–342.
Schwartz J, Park SK, O’Neill MS, Vokonas PS, Sparrow D, Weiss ST, et al. 2005. Glutathione-S-transferase M1, obesity, statins, and autonomic effects of particles. Am J Respir Crit Care Med 172:1529–1533.
Seaton A, Soutar A, Crawford V, Elton R, McNerlan S, Cherrie J, et al. 1999. Particulate air pollution and the blood. Thorax 54:1027–1032.
Shwe TT, Yamamoto S, Kakeyama M, Kobayashi T, Fujimaki H. 2005. Effect of intratracheal instillation of ultrafine carbon black on proinflammatory cytokine and chemokine release and mRNA expression in lung and lymph nodes of mice. Toxicol Appl Pharmacol 209:51–61.
Sun ZJ, Zhang ZE. 2005. Historic perspectives and recent advances in major animal models of hypertension. Acta Pharmacol Sin 26:295–301.
Tarkiainen TH, Timonen KL, Vanninen EJ, Alm S, Hartikainen JE, Pekkanen J. 2003. Effect of acute carbon monoxide expo-sure on heart rate variability in patients with coronary artery disease. Clin Physiol Funct Imaging 23:98–102. Timonen KL, Vanninen E, de Hartog J, Ibald-Mulli A, Brunekreef
B, Gold DR, et al. 2006. Effects of ultrafine and fine particu-late and gaseous air pollution on cardiac autonomic control in subjects with coronary artery disease: the ULTRA study. J Expo Sci Environ Epidemiol 16:332–341.
Tracey KJ. 2002. The inflammatory reflex. Nature 420(6917): 853–859.
Ulrich MM, Alink GM, Kumarathasan P, Vincent R, Boere AJ, Cassee FR. 2002. Health effects and time course of particu-late matter on the cardiopulmonary system in rats with lung inflammation. J Toxicol Environ Health A 65:1571–1595. Watkinson WP, Campen MJ, Costa DL. 1998. Cardiac arrhythmia induction after exposure to residual oil fly ash particles in a rodent model of pulmonary hypertension. Toxicol Sci 41:209–216.
Watkinson WP, Campen MJ, Nolan JP, Costa DL. 2001. Cardiovascular and systemic responses to inhaled pollu-tants in rodents: effects of ozone and particulate matter. Environ Health Perspect 109(suppl 4):539–546. Watkinson WP, Campen MJ, Wichers LB, Nolan JP, Costa DL.
2003. Cardiac and thermoregulatory responses to inhaled pollutants in healthy and compromised rodents: modulation via interaction with environmental factors. Environ Res 92:35–47.
Wellenius GA, Coull BA, Godleski JJ, Koutrakis P, Okabe K, Savage ST, et al. 2003. Inhalation of concentrated ambient air particles exacerbates myocardial ischemia in con-scious dogs. Environ Health Perspect 111:402–408. Wheeler A, Zanobetti A, Gold DR, Schwartz J, Stone P, Suh HH.
2006. The relationship between ambient air pollution and heart rate variability differs for individuals with heart and pulmonary disease. Environ Health Perspect 114:560–566. Wichers LB, Nolan JP, Winsett DW, Ledbetter AD, Kodavanti UP,
Schladweiler MC, et al. 2004. Effects of instilled combus-tion-derived particles in spontaneously hypertensive rats. Part I: Cardiovascular responses. Inhal Toxicol 16:391–405. Wilson MR, Lightbody JH, Donaldson K, Sales J, Stone V. 2002.
Interactions between ultrafine particles and transition met-als in vivo and in vitro. Toxicol Appl Pharmacol 184:172–179. Yang HM, Ma JY, Castranova V, Ma JK. 1997. Effects of diesel
exhaust particles on the release of interleukin-1 and tumor necrosis factor-alpha from rat alveolar macrophages. Exp Lung Res 23:269–284.
Zanobetti A, Schwartz J. 2005. The effect of particulate air pollu-tion on emergency admissions for myocardial infarcpollu-tion: a multicity case–crossover analysis. Environ Health Perspect 113:978–982.
Zanzinger J, Czachurski J. 2000. Chronic oxidative stress in the RVLM modulates sympathetic control of circulation in pigs. Pflugers Arch 439:489–494.