975
Major Factors Controlling Arsenic Occurrence in the Groundwater
and Sediments of the Chianan Coastal Plain, SW Taiwan
Kuan-Yu Chen1 and Tsung-Kwei Liu1,
*
(Manuscript received 16 May 2007, in final form 8 June 2007)
ABSTRACT
1
Department of Geosciences, National Taiwan University, Taipei, Taiwan, ROC
*
Corresponding author address: Prof. Tsung-Kwei Liu, Department of Geosciences, National Taiwan University, Taipei, Taiwan, ROC; E-mail: liutk@ntu.edu.twdoi: 10.3319/TAO.2007.18.5.975(TT)
The Chianan coastal plain in southwestern Taiwan has long been well known for the prevalence of black-foot disease during the period 1961 to 1985. This disease resulted from drinking groundwater rich in arsenic and dissolved humic substances. However, the occurrence of arsenic and its controlling factors have not yet been studied in detail. Water samples from a total of 86 newly established monitoring wells and sediment samples from 28 fully cored boreholes were analyzed in this study to investigate major chemical [such as SO42-, Cl-, redox potential (Eh), pH, and 14CDIC ages] and
geological (such as depositional environment, grain size and arsenic content of sediments) factors which may control the distribution of arsenic in ground-water of the upper ~300-m thick strata. This study shows that arsenic
con-centrations of almost all groundwater samples exceed the 0.01 mg L-1 limit
of the WHO guidelines, with the highest values up to ~1.2 mg L-1. Only the
deep aquifers (> 50 m) deposited before Holocene transgression contain groundwaters with relatively high arsenic contents (> 0.1 mg L-1). All the
arsenic-rich groundwaters are under strongly reducing conditions with low Eh (< 110 mV) and low SO42- (nearly all < 2 mg L-1). The different
combina-tion of As, Fe, and SO42- concentrations of groundwater reflect various redox
potentials. The reductive dissolution of As-rich Fe-(hydr)oxides is believed to be the major source of As in the groundwater, but the contents of SO42-,
humic substances and residence time of water are also responsible for the variation of dissolved As in the Chianan coastal plain.
The occurrence and factors controlling the arsenic contents in groundwater have drawn much attention in recent years (e.g., Hering and Kneebone 2001; Inskeep et al. 2002; Mandal and Suzuki 2002; Smedley and Kinniburg 2002; Anawar et al. 2003; Ahmed et al. 2004; Huntsman-Mapila et al. 2006; Pedersen et al. 2006; Varsanyi and Kovács 2006). The Chianan coastal plain in southwestern Taiwan (Fig. 1) has been well known for the occurrence of a peculiar peripheral vascular disorder in humans called “black-foot disease”. This serious chronic disease was first reported between 1910 and 1920 (Kao and Kao 1954), but awareness of the problem did not begin until 1960s (e.g., Tseng et al. 1961) when the disease became endemic in coastal areas. The cause of the disease was later attributed to high arsenic (Chen and Wu 1962) or fluorescent humic substances (Lu et al. 1975; Lu 1990a, b) in groundwater used for drinking. It was reported that both black-foot disease and arsenism was limited to people drinking deep well water that contained a variable but high concentration of arsenic (0.10 -1.81 mg L-1; Tseng et al. 1961). Since then, intensive medical studies on the disease have been carried out (e.g., Chen et al. 1988). However, the occurrence and the controlling factor(s) of
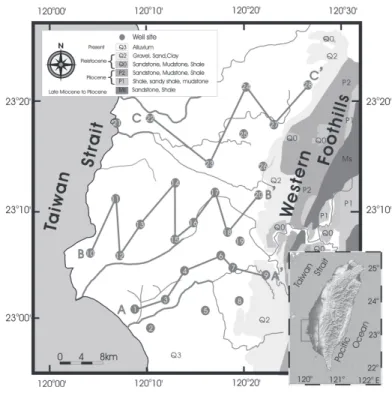
Fig. 1. Groundwater well locations and the simplified geologic map around the
Chianan coastal plain in southwestern Taiwan. Profiles AA’, BB’, and CC ’ shown in Fig. 2 are also shown.
arsenic enrichment in the groundwater were not examined in detail. The water samples ana-lyzed by early studies were mainly collected from private wells, and the depths of each well screen (i.e., tapped portions of well casings) were not known exactly and/or the potential existed for water to be a mixture from different aquifers.
Over the past few decades, excess pumping of groundwater in the plain has resulted in environmental problems such as seawater intrusion and land subsidence. To enable better development and management of the groundwater resource and better understanding and pro-tection of water quality, a Groundwater Monitoring Network (GMN) program, involving in-tensive drilling for sediment cores and construction of monitoring wells, has been undertaken in recent years. This study uses data provided by 28 boreholes, each about 250 ~ 300 m deep, and 86 wells which were drilled during the GMN program. The main results obtained from analyses of the core sediments and water samples are presented herein, with a focus on major hydrochemical and hydrogeological factors controlling the spatial distribution of arsenic in the groundwater.
Taiwan is a young and active mountainous island formed due to the collision between the Luzon volcanic arc of the Philippine Sea plate and the continental margin of the Eurasian plate (Ho 1986; Teng 1987). The backbone of the island is mainly composed of metamorphosed rocks, which are surrounded by Tertiary sedimentary rocks of the foothill zones. The western outskirts of the island are well developed tablelands and coastal alluvial-deltaic plains formed during the Quaternary. The Chianan coastal plain (Fig. 1) in southwestern Taiwan covers an area of ca. 2100 km2 and is a tectonically active subsiding depression formed at the foreland of the actively uplifting mountain belt on the east ( Liu et al. 1997). Carbon-14 dates show that sediment accumulation rates are rather high (~1 cm yr -1) and the upper-most 100-m thick layer was deposited during the last 10000 years. The present ground surface slopes westward with a very low gradient of < 3 m 10 km -1 and is characterized by continual sets of offshore bars and lagoons along the coast.
It has been found that the unconsolidated strata of this plain (more than 1000 m in thickness) are mainly composed of poorly sorted alternating beds of fine-grained clastic sediments such as clay, silt and fine sand (Oung et al. 2001). These sediments were deposited in mixed alluvial and delta environments such as fluvial flood plains, estuaries, lagoons, and shallow marines from the Late Pleistocene onwards (Wu 1999). These sediments were mainly derived from the very thick (~5000 m) sequence of slightly consolidated Pliocene marine mudstones (namely Kutingkeng Formation) exposed on the hills to the east (Ho 1986).
2. SAMPLING AND ANALYTICAL METHODS 2.1 Groundwater
At each monitoring site, a full-cored borehole was drilled first for obtaining subsurface lithological information, which was then used as the base for determining 2 ~ 4 depth ranges for installing screens for monitoring wells. Each well was linked through the screen to only a single aquifer. All these wells were established for water-quality sampling and subject to con-tinuous water-level monitoring.
collected for this study. At least three times the volume of stagnant water within the well casing was pumped out and discarded before taking samples. On-site measurements included pH, redox potential (Eh), and specific electric conductivity (EC). Of which, Eh and pH were determined by Sentron 1001 ® Eh-pH meter, while EC was measured by an Orion 130 ®. All samples were monitored in anaerobic flow cells during purging until stable readings were obtained. Water samples were collected for subsequent chemical and isotopic analysis only after pH and EC became stable, i.e., fluctuations of pH and relative EC were less than 0.1 and 5%, respectively.
Each water sample was divided into several portions according to the needs of chemical analyses. Waters for cation analyses were filtered through 0.45-µm acetate filters and acidified with nitric acid until pH= 2. Samples were then kept in ice boxes and delivered to the laboratory within 24 hours. Dissolved Fe and Mn were measured by atomic absorption spectroscopy using a Perkin-Elmer AAnalyst 100 ®. For arsenic analysis a flow injection hydride analysis system FIAS-400 ® was attached to the same spectrometer. Concentrations of SO42- and Cl -were determined by ion chromatograph with a Dionex-120 ®. About 50 liters of each water sample were collected for precipitating dissolved inorganic carbon (DIC) for 14C dating using the procedures described by Hackley et al. (1992) and Liu (1995). The uncertainties for As and SO42- are 3%, and that for Cl- is 2%.
2.2 Sediments
The sedimentary environments of the uppermost ~250-m thick deposits sampled were identified by sedimentary structures such as cross beddings, ripple marks, plane beddings, characteristic lithologies (such as rootlets and brownish to reddish paleo-soil) and fossils (such as mollusks, foraminifers, and calcareous nano-fossils) (Wu 1999). Terrestrial environments were characterized by paleo-soil, rootlets and having no marine fossils. In contrast, marine environments were characterized by foraminifers and nanofossils. The transitional environments, such as estuary, lagoon, and marsh between terrestrial and marine environments, were identified by brackish shell fossils including: Certhideospilla djadjariensis (Martens), Crassostrea gigas,
Placuna placenta, Bartillaria, and oysters. Peat layers and weathered variegated mud
frag-ments were often found in marsh environfrag-ments. Abundant marine fossils and trace fossils such as Ophiomorpha and Skolithos were often found in shallow open marine (shoreface) environ-ments (Reineck and Singh 1980).
The depositional environments and arsenic contents of sediments from the boreholes at Tainan (site CN-1) and Hsiaying (site CN-23) were studied in particular detail. Except the uppermost brownish-yellow soils (mostly ≤ 5 m thickness), the clay-silt-fine sand strata of the Chianan plain are light- to dark-grey in color, indicating that the sediments and groundwater were under reducing conditions. Accordingly, efforts were made to avoid sampling discernible oxidation portions such as anything yellowish in color due to exposure to the atmosphere. In the field, each core sediment sample for arsenic extraction analysis was pushed directly into a 30-cm long thick-walled plastic pipe, immediately sealed on both ends, and stored under 4°C. Each 100-g sample for chemical extraction analysis was taken from the middle inner part of a
core segment by scraping the outer sediments, and put into a high density polyethylene centrifuge tube. Then the samples were dried with a freeze-vacuum chamber before chemical extraction analysis. At present, there is no well accepted standard method for single or sequential extrac-tions of As in soil and sediments (e.g., Keon et al. 2001; Montperrus et al. 2002). In this study, arsenic associated with relatively mobile phases [Fe-Mn-(hydr)oxides and organic matter] in the sediments was extracted by the method developed by Ellwood and Maher (2003). Typically, 0.1 mg of sediment was weighed out into centrifuge tube to which 10 ml of a mixture (abbreviated as PHH solution) of 0.5 M of phosphoric acid and 0.1 M of hydroxylamine hydrochloride
(NH OH HCl2 ⋅ ) were added. Aliquots of the supernatant were removed and usually stored
overnight at 4°C until analysis.
In addition to the one-step extraction described above, the core sediments from CN-1 were also analyzed by the sequential extraction method proposed by Tessier et al. (1979) and Li et al. (1995). This was originally intended to reveal the amount of arsenic associated with: (1) easily exchangeable matter and carbonates, (2) Fe-Mn-(hydr)oxides, and (3) sulfide and organics. The first fraction was extracted by 1 M CH3COONa. A mixture of NH OH HCl2 ⋅ in 25% acetic acid, as an acidic and reductive solvent, is reasonably selective in extracting sec-ondary amorphous Fe and Mn(hydr)oxides and associated arsenic (Varsányi and Kovács 2006). This solvent also removed the elements weakly attached to organic matter. Finally, sulfide-associated arsenic and organics were extracted with a mixture of 0.02 M HNO3 , 30% H2O2 , and 3.2 M CH3COONH4 . The arsenic contents of the extraction solutions were also determined by atomic absorption spectroscopy mentioned above.
3. RESULTS AND DISCUSSION 3.1 Hydrogeological Framework
The hydrogeological framework of the Chinan plain is shown in Fig. 2. The directions of regional groundwater flows basically follow the three main streams of this plain. Based on regional lithology, the upper 250-m thick sediments are divided into four aquifer units (i.e., Aquifers 1 ~ 4), each of them comprises an aquifer and an overlying aquitard. The correlation of the aquifers along each profile was essentially based on the depth and partly on the litho-logic sequence because the strata are roughly horizontal. It is also obvious that the uppermost ~300-m thick sequence of mixed alluvial and marine strata was deposited in response to sea-level changes brought about by global glacial cycles during the late Quaternary as evidenced by 14C ages. From a regional hydrogeological point of view, coarser sediments of the aquifers represent higher energy environments during periods of regression or “low-stand” sea-levels, while sediments of the aquitards were mainly deposited during high sea-level stands when transgression occurred, especially during the last one between 12 and 6 Ka.
3.2 Distributions of Arsenic and Other Geochemical Parameters
The analytical results of Eh, pH, As, Fe, SO42-, and Cl- of the groundwater samples are shown in Fig. 3. The 0.1 mg L-1 of arsenic concentration, which was found to be the lower
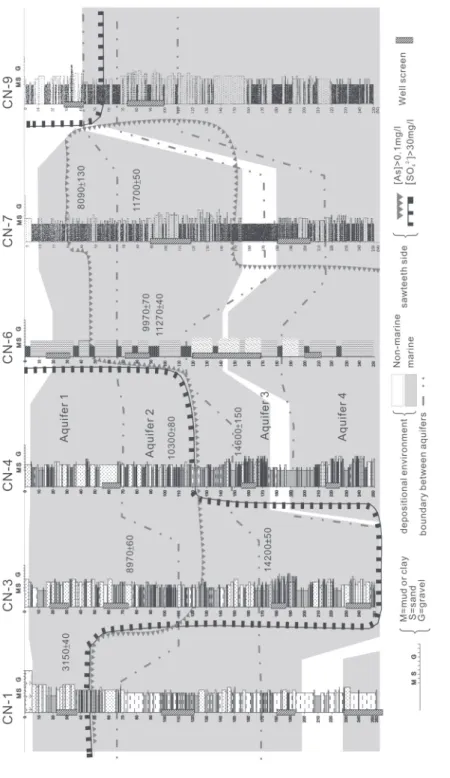
Fig. 2.
Profile of lithologic columns of the wells studied along the lines AA’ (a), BB’ (b), and CC
’ (c) shown in
Fig. 1; depositional environments (modified from Wu 1999) and hydrochemical analysis data are also shown. Depositional ages of sediments are shown in the right side of each lithologic column. The water samples from the sawteeth side have arsenic and sulfate concentrations greater than 0.1 and 30 mg L -1,
Fig. 2.
Fig. 2.
threshold for causing noticeable clinical symptoms of arsenic poisoning during the most seri-ous period (1940s ~ 1970s) of the disease, is used in this study to define high arsenic contents. The aquifers on the triangular sawteeth side in Fig. 2 have higher than 0.1 mg L-1 of arsenic. It is evident that almost all high-arsenic waters occur in the deeper and down-gradient (discharging) side of each profile. The water samples from shallow Aquifer 1 are low in arsenic content, generally lower than the previous drinking water standard (0.05 mg L-1). In contrast, water samples from the deeper aquifers (2, 3, and 4), except those from the eastern (recharging) areas, have arsenic concentrations much higher than 0.05 mg L-1. Similarly, an arbitrary value of 30 mg L-1, which is equivalent to ~1% of SO42- abundance of standard sea water (2650 mg L-1; Riley and Chester 1978), was used to define high SO42- in groundwater. Except for Aquifer 2 - 3 at site CN-3 and Aquifer 3 at site CN-10 where SO42- concentrations are slightly higher than 30 mg L-1 (i.e., 30 - 300 mg L-1), all the other high-SO
42- waters are found to occur only in Aquifer 1. Furthermore, it is worth pointing out that the SO42- (~2700 - 6000 mg L-1) and Cl -(~25000 - 45000 mg L-1) concentrations of Aquifer 1 are mostly higher than that of common open sea water (2650 and 19000 mg L-1, respectively; Figs. 4a, b). This is not surprising because Aquifer 1 was mainly deposited in a coastal marine environment where strong evaporation was very likely to have occurred. In fact, the present annual mean evaporation (~1600 mm yr -1) is remarkably greater than the annual mean rainfall (~1000 mm yr -1) on this coastal plain.
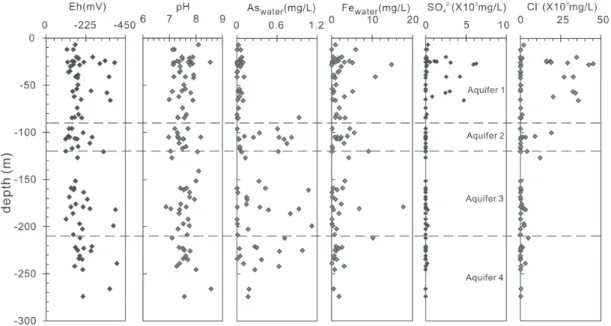
Fig. 3. Variation of Eh, pH, As, Fe, and SO42- concentrations vs. depth for ground-water from the Chianan plain.
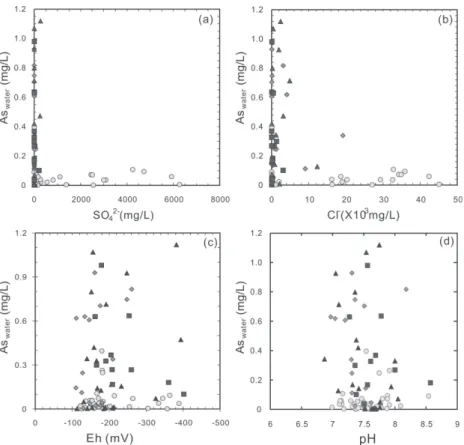
High As concentrations were demonstrated to occur only in deeper aquifers (> 80 m in depth). All the groundwater samples which have higher than 0.1 mg L-1 of As are low in SO
4 2-(mostly < 30 mg L-1; Fig. 4a) and Cl- (mostly < 2000 mg L-1; Fig. 4b) when compared with seawater. All the Eh values obtained are lower than -110 mV, indicating highly reducing conditions. In fact, all the water samples were considerably reducing as indicated by common presence of characteristic H2S odor and/or detectable amounts of HS- (TSC 2001). Most of the deep Aquifers (2 ~ 4) are even more reducing as evidenced by the presence of abundant CH4 gas during pumping and sampling. However, no consistent correlation was found between As concentrations and Eh values (Fig. 4c), suggesting As concentration is not only controlled by Eh but also by other factor(s) when the environment is reducing enough with Eh < 110 mV.
The pH values for the samples vary between 6.8 and 8.5. No significant correlation was found between As content and pH values (Fig. 4d), although a positive correlation between As and pH was previously reported (e.g., Huntsman-Mapila et al. 2006). Most of the Eh-pH data
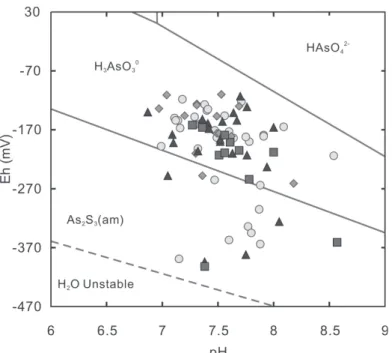
Fig. 4. Plot of As vs. SO42- (a), Cl- (b), Eh (c), and pH (d) for groundwater from the Chianan plain ( : aquifer 1; : aquifer 2; : aquifer 3; : aquifer 4).
points are located within the H3AsO3° stability field in the simplified Eh-pH diagram (Inskeep et al. 2002) for As-S-H2O system at 25°C based on the assumptions: SO42- = 10-3 M, H3AsO3° = 10-5 M (Fig. 5). Except for the water samples from sites CN-3, 10, and 22, the samples plotted within the amorphous-As2S3 domain of Fig. 5 are low in As as expected. Arsenic in both of H3AsO3 and As2S3 is of reducing form As (III), which is in agreement with the result that As in groundwater of this plain is present largely as As (III) (Chen et al. 1994).
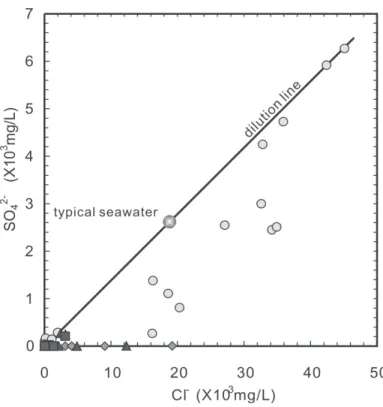
When molecular oxygen is not available, or when it has run up, decay of organic matter continues by a series of reactions that represent successively lower Eh values (Stumm and Morgan 1981). Sulfate reduction is a bacterial reaction in which bacteria use SO42- to oxidize organic matter to CO2. By contrast, chlorine ions are a relatively conservative constituent and are essentially not affected by biologica1 activity. Four of the nine groundwater samples which have higher Cl- concentration than the standard seawater lie on the dilution line of seawater in the SO42- vs. Cl- diagram (Fig. 6), indicating that reduction of sulfate in the four samples was negligible. On the contrary, data points for all the other high-chlorine (Cl- > 2000 mg L-1) groundwater samples lie below the dilution line, demonstrating the occurrence of sulfate-reduction reaction after deposition of sediments.
Fig. 5. Plot of Eh vs. pH for groundwaters from the Chianan plain in the
simpli-fied Eh-pH diagram for the As-S-H2O system at 25°C constructed by Inskeep et al. (2002). The SO42- content of the samples whose Eh-pH data points fall within the As2S3 (am: amorphous) field are also shown ( : aquifer 1; : aquifer 2; : aquifer 3; : aquifer 4).
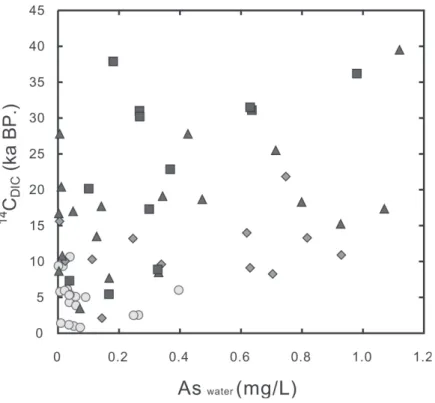
As stated above, SO42- concentrations can be reduced by microbial activity but Cl- is con-servative or biologically inert. The fact that all the high-arsenic water samples are low in Cl -content (< 2000 mg L-1), as mentioned above, strongly suggests that they are either original (or only slightly diluted) fresh formation water trapped in fluvial/estuarine environments or inter-stitial seawater remarkably diluted after deposition. Except for the few low-arsenic samples (i.e., sample sites CN-7, 18, 20, 24, 26, and 27) from the deep aquifers under the eastern recharging area, a slight positive correlation exists between arsenic content and mean residence time (14C
DIC ages) of groundwater (Fig. 7). This is in agreement with the conclusion made by Smedley and Kinniburgh (2002) and Varsányi and Kovács (2006) that a long residence time of groundwater plays an important role in As enrichment, probably implying that the rate of some As-related reactions is lower than the rate of groundwater flow. It is also worth mentioning that almost all 14CDIC ages for water in Aquifers 2, 3, and 4 vary between 10 and 40 Ka, and are about the same as their corresponding depositional ages of sediments. This implies that the groundwater flow rates are very low as expected from the hydrogeological characteristics. The
Fig. 6. Plot of Cl- vs. SO42- for groundwater from the Chianan plain. The data points for samples with Cl- contents greater than 15000 mg L-1 are shown ( : aquifer 1; : aquifer 2; : aquifer 3; : aquifer 4).
arsenic contents (> 0.1 mg L-1) of the two samples from site CN-27 (i.e., Aquifers 1 and 3) are higher than that of the neighboring aquifers. This may be attributed to the more complicated topographic relief inferred from the unconformity (e.g., site CN-28; Fig. 2) between the Ho-locene strata and its underlying late Pleistocene basement in the northeastern part.
Many studies have shown that hydroxyl groups on the surface of many minerals are the most abundant and reactive adsorption sites, and consequently, oxides and hydroxides (Fe, Mn, and Al) have a strong affinity for As (V) under an oxic environment (e.g., Pierce and Moore 1982; Bowell 1994).
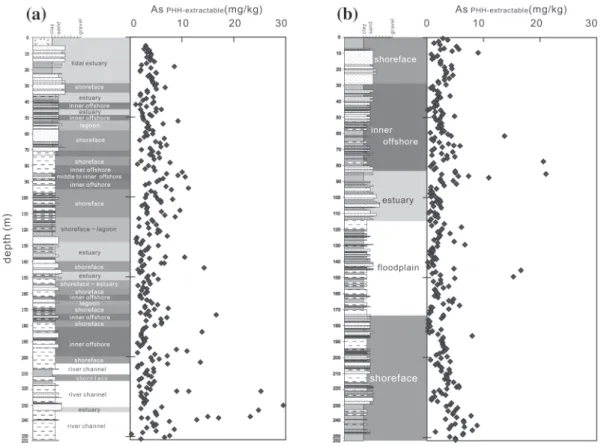
Detailed results on lithology, arsenic concentrations extracted by the mixture of phosphoric acid and hydroxylamine hydrochloride (PHH solution), and depositional environments for the core sediments from site CN-1 and site CN-23 are shown in Fig. 8. Apparently, no preferred relationship between extractable arsenic concentrations and different depositional facies can be inferred.
It was noted that oxidation of mineral sulfides can be very rapid leading to the formation of iron oxide and this would cause misleading results (Breit et al. 2006). Despite the efforts we
Fig. 7. Plot of 14CDIC ages vs. As for groundwater from the Chianan plain ( : aquifer 1; : aquifer 2; : aquifer 3; : aquifer 4).
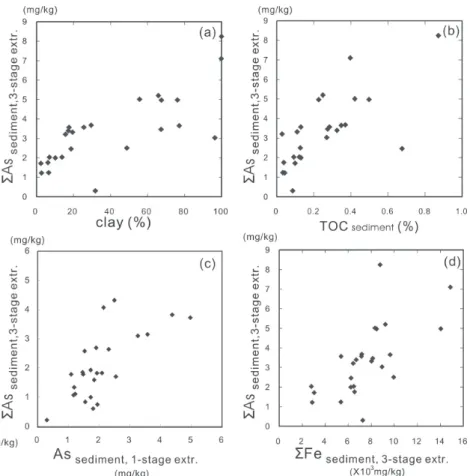
made to keep the reduced sediment anaerobic, brief contact with the atmosphere did occur. Ngiam and Lim (2001) and Gómez-Ariza et al. (1999, 2000) also pointed out the limitations of sequential extraction. Accordingly, we summed up the arsenic contents obtained from the three-stage sequential extraction scheme for each sample (Fig. 9a) to operationally quantify the total extractable arsenic in the easily exchangeable, Fe-(hydr)oxides, sulfides and organic matter phases (Fig. 9b). In fact, the sequentially extracted total arsenic content is fundamen-tally the same as that obtained from PHH extraction for samples collected from the same sample (Fig. 9c) and the amount of arsenic extracted from organic matter is negligible as compared with that from Fe-(hydr)oxides. In short, mobile arsenic in the sediments of the Chianan plain was demonstrated to be dominantly associated with clay-sized Fe-(hydr)oxides. Although there exists a good correlation between total extractable arsenic and Fe in sedi-ments (Fig. 9d), no simple relationship was found between the contents of As and Fe in
ground-Fig. 8. Column profiles of arsenic contents extracted by a mixture of phosphoric
acid and hydroxylamine hydrochloride (PHH) for core sediments from CN-1 (A) and CN-23 (B).
water (Fig. 10a). This suggests that Fe and As contents were further modified after the two elements were released into water. We found that the SO42- vs. Fe diagram of groundwater (Fig. 10b) shows a characteristic pattern similar to that of As vs. Fe. It has been well known that redox conditions are important in controlling the chemistry of metals ions and solids (for example, Fe2+ and Fe2O3), species or solids containing sulfur (for example, SO42-, H2S, and FeS2), and dissolved gases containing carbon (for example, CH4). There is a general trend of abundance in the above-mentioned species as the environment becomes more reducing (Appelo and Postma 1994). Based on the diagrams of As vs Fe and SO2- vs. Fe, the groundwater samples
Fig. 9. Plot of (a) total 3 stage-extractable arsenic vs. proportion of clay-size
fraction; (b) total 3-stage-extractable As vs. total organic matter; (c) total 3 stage-extractable arsenic vs. 1 stage-extractable arsenic; and (d) total 3 stage-extractable arsenic vs. total 3 stage-extractable iron in sediments from the Chianan plain.
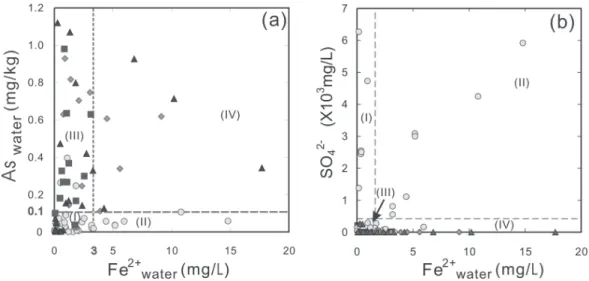
analyzed can be generally classified into four groups with increasing reduction potential (Chen 2007; Fig. 11). Group I samples are very low in As and Fe, but high in SO42-. Most of them were drawn from the oxic or less reductive shallow Aquifer 1, but this group also includes those low-SO42- samples drawn from the aquifers in the eastern recharge areas. Group II samples are low-medium in As, but high in Fe and SO42-. No positive correlation was found between As and Fe contents, probably because arsenic (V) reduction would normally be expected to occur after Fe3+ reduction but before SO42- reduction (Smedley and Kinniburgh 2002). Group III samples are relatively very high in As, but very low in Fe and SO42- due to the precipitation of iron sulfides. Dissolved As concentrations remain high because As is complexed by dissolved humic substances (Lu 1990b; Chen et al. 1994). Group IV samples are high in As and Fe, but very low in SO42- because all SO42- was reduced. It is noted that the concentrations of As, Fe, and SO42- of these four groups fundamentally reflect different stages in the sequence of reduc-tion processes of groundwater as aquifer depth increases (Fig. 11). Of course, a few samples are transitional in terms of the characterization of As occurrences.
4. CONCLUSIONS
This study demonstrates that all the arsenic concentrations of the analyzed groundwater sam-ples from the Chianan coastal plain exceed 0.01 mg L-1, with the highest value up to ~1.2 mg L-1. Almost all the high arsenic waters (> 0.10 mg L-1) occur only in deep aquifers (> 50 m) deposited
Fig. 10. Plot of As vs. Fe for groundwater from the Chianan plain. The water
sam-ples are classified into four groups (I ~ IV) as is also shown in Fig. 11 in terms of relative contents of As and Fe2+ (a), and relative SO
before the Holocene transgression and are low in SO42- (mostly < 2 mg L-1) under highly reducing conditions (Eh < -110 mV). The amounts of acid extractable arsenic show positive correlation with the proportion of clay-sized Fe-(hydr)oxides in the sediments. The concentrations of As, Fe, and SO42- of the water samples analyzed can be classified into four groups, which reflect developing stages in the sequence of reduction processes of common groundwater. It is sug-gested that the reductive dissolution of As-rich Fe-(hydr)oxides is the major source of As in the groundwater, but the contents of SO42-, dissolved humic substances and residence time of groundwater, and the proportion of clay-sized Fe-(hydr)oxides, are also responsible for dis-solved As in the Chianan coastal plain. The characteristic occurrences of As and humic sub-stances were in turn caused by the relatively low flow-rate and long residence time, as indicated by relatively old 14C
DIC ages, of groundwater in fine-grained sediments deposited in the flat coastal plain.
Acknowledgements The authors gratefully acknowledge Prof. Y. N. Shieh of Purdue
University, Indiana, USA, Prof. Y. G. Chen of National Taiwan University and anonymous reviewers for their useful comments and improvement of the manuscript. We also thank the Central Geological Survey, Ministry of Economic Affairs (ROC) for providing sediment core samples and related geological information, and the Groundwater Exploitation and
Conserva-Fig. 11. Schematic diagram of the distribution
of As concentrations in the groundwater of the Chianan plain, with respect to the variation of some indicative species in the sequence of reduction processes for groundwater (Appelo and Postma 1994). The designation of composition or redox zonations I ~ IV correspond to that of Fig. 10.
financially by National Science Council, Republic of China (NSC94-2116-M-002-004).
REFERENCES
Ahmed, K. M., P. Bhattacharya, M. A. Hasan, S. H. Akhter, M. Alam, M. A. H. Bhuyian, M. B. Imam, A. A. Khan, and O. Sracek, 2004: Arsenic enrichment in groundwater of the alluvial aquifers in Bangladesh: An overview. Appl. Geochem., 19, 181-200.
Anawar, H. M., J. Akai, K. Komaki, H. Terao, T. Yoshioka, T. Ishizuka, S. Safiullah, and K. Kato, 2003: Geochemical occurrence of arsenic in groundwater of Bangladesh sources and mobilization processes. J. Geochem. Explor., 77, 109-131.
Appelo, C. A. J., and D. Postma, 1994: Geochemistry, Groundwater and Pollution. Balkema, A. A., Rotterdam, the Netherlands, 535 pp.
Bowell, R. J., 1994: Sorption of arsenic by iron oxides and oxyhydroxides in soils. Appl.
Geochem., 9, 279-286.
Breit, G. N., J. C. Yount, M. N. Uddin, A. A. Muneem, H. A. Lowers, R. L. Driscoll, and J. W. Whitney, 2006: Compositional Data for Bengal Delta Sediment Collected from Bore-holes at Srirampur, Kachua, Bangladesh: US Geological Survey Open-File Report 2006-1222, 51 pp.
Chen, C. J., T. L. Kuo, and M. M. Wu, 1988: Arsenic and cancers. Lancet, 414-415.
Chen, K. P., and H. Y. Wu, 1962: Epidemiologic studies on blackfoot disease: 2. A study of source of drinking water in relation to the disease. J. Formosan Med. Assoc., 61, 611-618.
Chen, K. Y., 2007: Migration and Enrichment of Arsenic in Groundwater in the Chianan Coastal Plain, Southwestern Taiwan. Ph.D. Thesis, National Taiwan University, Taipei, Taiwan, ROC, 164 pp.
Chen, S. L., S. R. Dzeng, M. H. Yang, K. H. Chiu, G. M. Shieh, and C. M. Wal, 1994: Arsenic species in groundwaters of the Blackfoot disease areas, Taiwan. Environ. Sci. Technol.,
28, 877-881.
Ellwood, M. J., and W. A. Maher, 2003: Measurement of arsenic species in marine sediments by high-performance liquid chromatography-inductively coupled plasma mass spec-trometry. Anal. Chem. Acta., 477, 279-291.
Gómez-Ariza, J. L., I. Giráldez, D. Sánchez-Rodas, and E. Morales, 1999: Metal readsorption and redistribution during the analytical fractionation of trace elements in oxic estuarine sediments. Anal. Chim. Acta, 399, 295-307.
Gómez-Ariza, J. L., I. Giráldez, D. Sánchez-Rodas, and E. Morales, 2000: Comparison of the feasibility of three extraction procedures for trace metal partitioning in sediments from south-west Spain. Sci. Total Environ., 246, 271-283.
Hackley, C. K., C. L. Liu, and D. D. Coleman, 1992: 14C dating of groundwater containing microbial CH4 . In: Long, A., and R. S. Kra (Eds.), Proceedings of the 14th International 14C Conference, Radiocarbon, 34, 686-695.
mobility in water supplies. In: Frankenberger, W. T. Jr. (Ed.), Environmental Chemis-try of Arsenic, Marcel Dekker Publ., New York, USA, 155-181.
Ho, C. S., 1986: An introduction to the geology of Taiwan: Explanatory text of the geologic map of Taiwan. Cent. Geol. Survey, Taiwan.
Huntsman-Mapila, P., T. Mapila, M. Letshweny, P. Wolski, and C. Hemond, 2006: Charac-terization of arsenic occurrence in the water and sediments of the Okavango Delta, NW Botswana. Appl. Geochem., 21, 1376-1391.
Inskeep, W. P., T. R. McDermott, and S. Fendorf, 2002: Arsenic (V) / (III) Cycling in soils and natural waters: chemical and microbiological processes. In: Frankenberger, W. T. Jr. (Ed.), Environmental Chemistry of Arsenic, Marcel Dekker Publ., New York, USA, 183-215.
Kao, T. M., and S. R. Kao, 1954: Studies on the cause of a particular dry gangrene. J. Formosan
Med. Assoc., 53, 272. (the Abstract)
Keon, N., C. H. Swartz, D. J. Brabander, C. Harvey, and H. F. Hemond, 2001: Validation of an arsenic sequential extraction method for evaluating mobility in sediments. Environ.
Sci. Technol., 35, 2778-2784.
Li, X. D., B. J. Coles, M. H. Ramsey, and I. Thornton, 1995: Sequential extraction of soils for multi-element analysis by ICP-AES. Chem. Geol., 124, 109-123.
Liu, T. K., 1995: Estimating flow and recharge rates of groundwater in western Taiwan using radiocarbon and tritium. Proceedings of the 15th International 14C Conference, Radiocarbon,
37, 531-542.
Liu, T. K., Q. C. Sung, K. Y. Chen, Z. L. Pi, C. H. Yang, and P. H. Cheng, 1997: Tectonic subsidence and uplift in the Zeikang-Hopi area of southwestern Taiwan since the late Pleistocene. J. Geol. Soc. China, 40, 155-165.
Lu, F. J., C. K. Yang, and K. H. Lin, 1975: Physico-chemical characteristics of drinking water in blackfoot disease endemic areas in Chia-I and Tainan Hsiens. J. Formosan Med.
Assoc., 74, 596-605.
Lu, F. J., 1990a: Blackfoot disease: Arsenic or humic acid? Lancet, 336, 115-116.
Lu, F. J., 1990b: Fluorescent humic substances and blackfoot disease in Taiwan. Appl.
Organometall. Chem., 4, 191-195.
Mandal, B. K., and K. T. Suzuki, 2002: Arsenic round the world: A review. Talanta, 58, 201-235. Montperrus, M., Y. Bohari, M. Bueno, A. Astruc, and M. Astruc, 2002: Comparison of ex-traction procedures for arsenic speciation in environmental solid reference materials by high-performance liquid chromatography-hydride generation-atomic fluorescence spectroscopy. Appl. Organometall. Chem., 16, 347-354.
Ngiam, L. S., and P. E. Lim, 2001: Speciation patterns of heavy metals in tropical estuarine anoxic and oxidized sediments by different sequential extraction schemes. Sci. Total
Environ., 275, 53-61.
Oung, J. N., Y. D. Lin, S. Y. Lin, Q. C. Sung, T. K. Liu, and C. H. Yang, 2001: Organic geochemistry of sediments in the southeastern coastal plain, Taiwan. West. Pacific Earth
Sci., 1, 373-390.
Pedersen, H. D., D. Postma, and R. Jakobsen, 2006: Release of arsenic associated with the reduction and transformation of iron oxides. Geochim. Cosmochim. Acta, 70, 4116-4129.
Reineck, H. E., and I. B. Singh, 1980: Depositional Sedimentary Environments, 2nd Ed., Springer-Verlag, New York, USA.
Riley, J. P., and R. Chester (Eds.), 1978: Chem. Oceanogr., 2nd Ed., Academic Press, New York.
Smedley, P. L., and D. G. Kinniburgh, 2002: A review of the source, behavior and distribu-tion of arsenic in natural waters. Appl. Geochem., 17, 517-568.
Stumm, W., and J. J. Morgan, 1981: Aquatic Chem. 2nd Ed., Wiley-Interscience, New York, USA.
Teng, L. S., 1987: Stratigraphic record of the late Cenozoic Penglai Orogeny of Taiwan. Acta
Geol. Taiwan., 25, 205-224.
Tessier, A., P. G. C. Campbell, and M. Bisson, 1979: Sequential extraction procedure for speciation of particular trace metals. Anal. Chem., 51, 844.
TSC (Taiwan Sugar Company), 2001: Groundwater Quality by the Taiwan Groundwater Moni-toring Network (3/5). Taiwan Water Resource Bureau.
Tseng, W. P., W. Y. Chen, J. L. Sung, and J. S. Chen, 1961: A clinical study of Blackfoot disease in Taiwan: An endemic peripheral vascular disease. Mem. Coll. Med., Natl. Taiwan Univ., 3, 1-8.
Varsányi, I., and L. O. Kovács, 2006: Arsenic, iron and organic matter in sediments and groundwater in the Pannonian Basin, Hungary. Appl. Geochem., 21, 949-963.
Wu, L. C., 1999: Depositional environments and stratigraphic correlation of sediments in the Chianan plain, SW Taiwan. Final Report, Cent. Geol. Surv., MOEA, ROC.
Chen, K. Y., and T. K. Liu, 2007: Major factors controlling arsenic occurrence in the ground-water and sediments of the Chianan coastal plain, SW Taiwan. Terr. Atmos. Ocean.