Marine Biology 116, 205-217 (1993)
Marine
;z, Biology
9 Springer-Verlag 1993Hydrography and distribution dynamics of larval and juvenile fishes
in the coastal waters of the Tanshui River estuary, Taiwan,
with reference to estuarine larval transport
W.-N. Tzeng, Y.-T. Wang
Department of Zoology, College of Science, National Taiwan University, Taipei, Taiwan 10617, Republic of China Received: 29 December 1992 / Accepted: 16 February 1993
Abstract.
Distribution dynamics of fish larvae and juve- niles in the co~/stal waters of the Tanshui River, Taiwan was studied fortnightly using surface horizontal tows with a larval net in daytime during the period from early April through early June 1991. Environmental factors, including water temperature, salinity, dissolved oxygen, pH, transparency and depth at sampling stations, were also monitored. A total of 10 737 fish eggs and 1387 indi- viduals, representing 43 families and 93 species, was col- lected during five cruises from 12 stations in the coastal waters. Most fish were estuarine-dependent marine spe- cies.Liza macroIepis, Ambassis gymnocephalus, Terapon
jarbua,
Mullidae and Gobiidae were the most dominant,making up 64.7% of the total catch. Early life stages, including egg, preflexion, flexion and postflexion larvae were abundant in surface samples. However, yolk-sac larvae were absent in the surface water, probably due to an ontogenetic behavioral shift as a consequence of a change in specific weight during early development. The species composition of fish larvae and juveniles was relat- ed to the microhabitats found in the coastal waters. The physico-chemical conditions, along with ontogenetic be- havior, played an important role in larval fish distribu- tion in the coastal waters.
Introduction
The coastal waters adjacent to the Tanshui River estuary make up a commercial fishing ground important for har- vesting the juveniles of engraulids and clupeids for local consumers, as well as anguillid elvers for cultivation. There are rare mangroves,
Kandelia candel
(Rhi- zophoraceae), in the lower estuary of the river (Chou et al. 1987), which provide plentiful organic detritus and a link to the food web of the estuarine-dependent marine fish population during their early life stages (McErlean et al. 1973, Haedrich and Haedrich 1974, Bell et al. 1984, Robertson and Duke 1987, Blaber and Milton 1990). Consequently, the study area functions as a nursery and feeding ground for the onshore-offshore migratory fish.Due to rapid economic and population growth, the river has been severely polluted, receiving domestic sewage from Taipei city and industrial wastewater from factories in the immediate area (Chou et al. 1987). Thus, the production of larval fishes in the Tanshui River estu- ary seems to be decreasing and the fishing grounds shift- ing seaward. In order to begin restoration of the river, a sewage and wastewater processing plant is now under construction at Bali. Treated wastewater will be dis- charged into the nearshore waters off the Tanshui River, a nursery ground for many commercially important fish- es. In order to evaluate the effect of this discharge on distribution and abundance of fish larvae and juveniles in the ecosystem, baseline information, including hydrogra- phy, fauna and flora of the estuary is essential. Accord- ingly, a multidisciplinary team of scientists was formed, supported by the National Science Council, Republic of China, to study the mangrove estuarine ecosystem in the Tanshui River (Chou and Bi 1990).
The temporal and spatial use of estuaries and nearshore waters by estuarine, marine, freshwater and estuarine-dependent marine species has been schemati- cally described by Deegan and Thompson (1985). A con- ceptual model of the transport of fish larvae and juve- niles between nearshore and estuarine nursery areas was proposed on the basis of physical processes, activity and behavior of the fish, and environmental cues (Boehlert and Mundy 1988). In addition, the movement and verti- cal distribution of the fish larvae and juveniles from off- shore to estuaries in relation to spawning mode and de- velopment of the fish were also suggested (Tanaka 1976). The occurrence, abundance and species composition of fish larvae and juveniles in the estuary and nearshore waters varies with the spawning season of the fish and their seasonal onshore-offshore migrations, as well as the physico-chemical conditions during estuarine transporta- tion (Blaber and Whitfield 1977 a, b, Weinstein 1979, Bell 1980, Blaber and Blaber 1980, Quinn, 1980, Yfifiez-Aran- cibia et al. 1980, Bell et al. 1984, Loneragan et al. 1986, Mukai 1987, Powell et al. 1989, Blaber and Milton 1990, Robertson and Duke 1990, Sebat6s 1990, Drake and Ari-
W.-N. Tzeng and Y.-T. Wang: Distribution dynamics of estuarine fishes as 1991 b). T h e e s t u a r i n e o r i e n t a t i o n o f t h e fish is d e p e n - d e n t o n s w i m m i n g a b i l i t y a n d t o l e r a n c e o f t h e fish t o t h e e x t r e m e s o f t h e e n v i r o n m e n t a l v a r i a b l e s , w h i c h differs c o n s i d e r a b l y a m o n g species ( K i n n e 1964, W h i t f i e t d et al. 1981), as well as b e t w e e n size a n d d e v e l o p m e n t a l s t a g e s o f e a c h species ( K i n n e 1964, H o l l i d a y 1971). T h e species c o m p o s i t i o n , s t r u c t u r e a n d s e a s o n a l d y - n a m i c s o f t h e l a r v a l a n d j u v e n i l e fish c o m m u n i t y in t h e m a n g r o v e e s t u a r y o f t h e T a n s h u i R i v e r h a s p r e v i o u s l y b e e n s t u d i e d ( W a n g et al. 1991, T z e n g a n d W a n g 1992). T h e p r e s e n t p a p e r a i m s to c l a r i f y t h e d i s t r i b u t i o n a n d a b u n d a n c e o f fish l a r v a e a n d j u v e n i l e s in r e l a t i o n to envi- r o n m e n t a l f a c t o r s in the c o a s t a l w a t e r s a d j a c e n t to the T a n s h u i R i v e r e s t u a r y .
Materials and methods
S t u d y a r e a
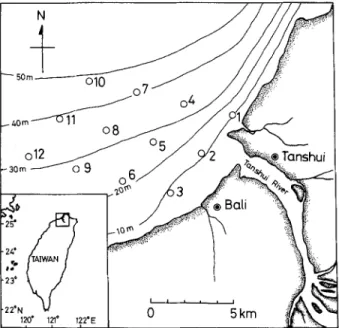
The Tanshui River, approximately 159 km long, is the largest river in northern Taiwan. The river flows through the Taipei basin and meets the sea at the town of Tanshui. The river mouth faces the shallow continental shelf in the northern part of the Taiwan Strait. The estuary belongs to a coastal plain estuary, its hydrography being greatly influenced by tidal currents. The direction of the tidal current is southward during flood and northward during ebb, with a tidal range in the river inlet from approximately 3.0 m at spring tide to 1.5 m at neap tide (Lee and Chu 1965). Twelve stations in the nearshore waters of the Tanshui River were selected for larval and juvenile fish sampling (Fig. 1). These stations were chosen because they will probably be affected by the treated wastewater discharged from the Bali wastewater processing plant.
The topography is deeper in the northern, offshore areas and shallower in the river inlet and in the southern part of the studied habitat. The depth of the sampling stations ranged from 10 to 50 m (Fig. 1).
206
Fig. 1. Map showing sampling stations (1-12) and isodepth in the nearshore waters of the Tanshui River, Taiwan
can reduce the sampling bias (e.g. Clutter and Anruku 1968, Smith and Richardson 1977, Omori and Hamner 1982, Leis 1986, Brander and Thompson 1989). However, sampling in the present study was not replicated because the environmental conditions changed rapid- ly due to tidal currents. In addition, stratified sampling was difficult due to the shallow waters. The difference between night and day collections, gear selection and the vertical distribution of the larvae will be studied in a separate paper. The present study emphasized the horizontal distribution of the fish larvae in relation to environ- mental factors.
D a t a a n a l y s i s
S a m p l i n g d e s i g n
Fish larvae and juveniles were collected fortnightly from the 12 stations in the nearshore waters of the Tanshui River, during the period from early April to early June 1991. The investigation period is consistent with the main fishing season of the larval and juvenile fish. Sampling was conducted using surface horizontal tows with a larval net during the daytime flood tide. The duration of sampling was pre-set at 5 min for each station at a speed of ca. 2 knots. The larval net was a modified Maruchi-D larval net, net mouth diameter 1.3 m, length 4.5 m and mesh size 0.5 x 0.5 mm (Nakai 1962). A flowmeter mounted in the net mouth recorded filtered water vol- ume. The fish collected were fixed immediately in 10% formalin seawater solution. Fish larvae and juveniles were identified to the lowest possible taxon, the developmental stages of the fish deter- mined according to Kendall et al. (1984).
The environmental factors were monitored during sampling. Temperature was measured with a mercury thermometer, salinity with a salinometer (WTW: microprocessor conductivity meter, Model: LF 196), DO (dissolved oxygen) with Winkler's method, pH with a pH meter (Coring: pH meter, M107), transparency with a secchi disc, and the depth at station was measured with an echo sounder.
Patchy distribution, gear selection, net avoidance, and diel ver- tical migration of the larvae in relation to ontogenetic behaviour of the fish, and their influence on the accuracy of the estimation of fish abundance, has frequently been debated by many researchers. It has been suggested that replicated and vertically stratified samplings
The density of fish eggs and larvae was calculated from the flow- meter reading, then standardized according to the number of fish per 1000 m 3 seawater filtrated. The homogeneous and heteroge- neous relationships of the environmental factors among stations were analyzed using multivariate analysis: principle coordinate analysis (PCA). To explain the zonation of the fish community according to habitat, the 12 stations were clustered and the domi- nant species ordinated based on species density data using PCA. For purposes of comparison, the similarities of species composition among stations was also calculated using Kimoto's (1976) overlap- degree index, and then clustered into different groups using the Mountford's (1962) method.
The community structure of fish larvae and juveniles was evalu- ated using Shannon-Weaver's species diversity (H') and Pielou's evenness (J') indices (Pielou 1966). The relationship between biotic and environmental factors was analyzed by canonical (Dillon and Goldstein 1984) and Spearman rank correlations (Siegel 1956).
Results P h y s i c o - c h e m i c a l c o n d i t i o n s T h e e n v i r o n m e n t a l f a c t o r s in t h e e s t u a r y c h a n g e d w i t h t i d a l c o n d i t i o n s a n d s e a s o n . H e n c e , e n v i r o n m e n t a l c o n - d i t i o n s n o t o n l y d i f f e r e d b e t w e e n t h e five s u r v e y s b u t a l s o a m o n g t h e 12 s t a t i o n s t h e m s e l v e s (Fig. 2).
W.-N. Tzeng and Y.-T. Wang: Distribution dynamics of estuarine fishes
I
28 2O 207 35 ~-~ 3o 25 ur3 20 9 April 8 + npfil t May 6 May 18 June 4 9 r r 10 g 6- ~ = ~7~ -" = - = Fig. 2. Temporal and spatial variations of tempera- ture, salinity, DO (dissolved oxygen), pH and trans- parency measured in the nearshore waters of the Tanshui River on 8 and 24 April, 6 and 18 May, and
1 2 3 4 5 6 7 8 9 10 11 12 4 June 1991
Stations
Water temperatures increased from 21 ~ on 8 April to 31 ~ on 4 June 1991. The temperatures in the southern stations were lower before and higher after 18 M a y (Stns 3, 6, 9, and 12) than in the northern and offshore stations o f the studied habitat. This indicated that seawater tem- peratures were more variable in the shallower areas.
Salinity varied with sampling dates and fluctuated be- tween the 12 stations (Fig. 2). Salinity was higher on 24 April and 18 M a y (greater than 33%o), moderate on 6 M a y (32 to 33%o) and less than 32%o at some o f the stations on 8 April and 4 June. The lower salinity was due to a strong river discharge, more greatly affecting the shallower, southern stations (Stns 3, 6, and 9). Therefore, the outflow of freshwater from the Tanshui River tended to be diverted to the southern part o f the studied habitat during flood tides.
D O also varied with sampling dates and stations, and ranged from 4.5 to 9.0 ml 1-1 (Fig. 2). This range was above the minimal requirements o f the fish. D O was
higher both during cold water mixing period (8 April) and during increased photosynthesis during the summer (6 June), but lower when affected by strong fluvial dis- charge, p H values were close to that o f normal seawater (8.1 to 8.3), their fluctuations following those o f DO. When D O increased, p H increased. DO and p H were related to biological activity and river discharge. Trans- parency was higher in offshore than in inshore waters but decreased with temperature and DO. This p h e n o m e n o n indicated that transparency was correlated to plant biomass, as well as being influenced by the turbidity load- ing in the estuary.
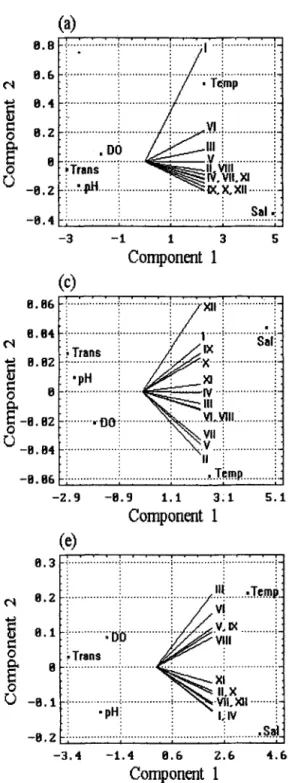
Relationships among the five environmental factors at the 12 stations in the nearshore waters were analysed by PCA ordination. They indicated that physico-chemical conditions at these stations were very dynamic, differing on all five successive surveys. The 12 stations were classi- fied into different groups according to the attributes of the five changing environmental factors (Fig. 3).
208
(0
o. e-I-.-!--..:.-.:...."-...:-..V:...:?(.
.--'-.-:--...-...'-
: / . . , ... i ... S - i ... :; ... :.i ... i .../~ :: -Y!
...o . ,
8 . . . O ;Trans " ~ 1 1 . VIII : ' ! _ . ~ I V . V I I . X I : ' C,..) - 8 , 2 .i..-:.t m ... L-....-.'r:'W-.~iX, X, Xti ... ::2... :: Sal .~: - 8 . 4 . . . 9 ... :'-;" , , - 3 - 1 1 3 5 C o m p o n e n t 1 (c)
8 8' f ~ : : : ~ ' " : ' ~ ? x "
~
i
i
+ " " " ~ 1
i
.i
0 O " III ! :: i i V i l l i... ...
i ... }
- e 86 ~i ... ...:...i... ... ::...,:.T.~.~... ... i.4
- 2 . 9 - 8 . 9 1 . 1 3 , 1 5 . 1C o m p o n e n t 1
(0
... ~ ... i i i .v~ =
i
! " 7 VIII ! ~::
. xi... ~
Trans i i ! ~ II.X ! -" ... :.- ... ~ , , ~ . ~ I . Xli ... :-:! "prli ~ I.~lV S {al
- ' l " " , " , , I , , ~ . . . i , , , I " - I . 4 0 , 6 Z . 6 4 , 6 C o m p o n e n t 1 8 , 3 : 8 . 2 8 . 1 8 rd - e . I - 8 , Z - 3 , 4
W.-N. Tzeng and Y.-T. Wang: Distribution dynamics of estuarine fishes
(b)
B. 12 .;....'....:...:...~....'....:...:...;...:...:...l...:....'....:...b .... .'.7..: ...... L
: : / i 1 " ~ "X. Xll : - i ' P H---2i ... ... ~ ... k"'" ! ... !- - 6 . 1 2 " ' ~ " " , . . . " . . . , " " T ' " , . . . " . . . . - 2 . 9 - B . 9 1 . 1 3 . 1 5. C o m p o n e n t 1(d)
8 . 1 5.!....'....:...:...::...:....:...:...i...:....'....:...~...:....'.,..;..:.
: II1!-i ... .: ... 7....~....~.~,p...i.
W...
!i ... i ... 7 ~;/iixii
... ~ i ... : .. . . =~ ... i.!!;:D~ ~ , , i
: pit : . ~ . " ": . . . ! . . . IVi . . . i"ii...T,.,~
. . .
...~!:.~:.~! ... !.
" , v m . x . x , s . i - 8 , 8 9 : . . . : . . . , , , : . . . : I ... :- , . , i - 3 . 2 - 1 . 2 8 . 8 2 . 8 4 . 8C o m p o n e n t 1
8 . 8 8 ("t B.B4 0 B @~l'_B. 04 - 8 . 6 8 8 . 1 1 1"t '~ B.B7 O~ 8 . 8 3 0 ~ ' - 8 . 8 1 r.) - 8 . 8 5Fig. 3. Principle coordinate analysis for the ordina- tion of the 12 stations ([-XII) in response to the five environmental variables in the nearshore wa- ters of the Tanshui River, surveyed on: (a) 8 April; (b) 24 April; (c) 6 May; (d) 18 May; and (e) 4 June 1991. Temp: temperature; Sal: salinity; DO: dis- solved oxygen; Trans: transparency
The ordination o f the 12 stations as indicated f r o m the first c o m p o n e n t o f P C A in Fig. 3 a, surveyed on 8 April 1991 was positively correlated to t e m p e r a t u r e a n d salini- ty, and negatively correlated to D O , transparency and p H . But the ordination o f the 12 stations as indicated f r o m the 2nd c o m p o n e n t was slightly divergent, the ordi- nation o f the shallower stations (Stns 1, 3, 6) being posi- tively correlated to t e m p e r a t u r e and D O , and negatively correlated to salinity, p H and transparency; at the re- maining nine stations, the relationships were reversed. This situation changed in each o f the other four surveys (Fig. 3 b - e).
A b u n d a n c e and distribution o f fish eggs and larvae Fish eggs were m o r e a b u n d a n t at the deeper stations than at the shallower stations, except at Stn 1 on 8 April (Fig. 4). Fish eggs were p r o b a b l y spawned in the studied area or drifted with the current f r o m offshore into the studied area. Fish larvae were m o r e a b u n d a n t in the deeper offshore stations (Stns 11 and 12) than in the shallower, m o r e nearshore stations (Stns 1, 2, 3, and 6). This suggested that m o s t fish eggs and larvae p r o b a b l y originated f r o m the offshore-spawning marine species.
8 0 0 0 6 Z 9 N" 6 0 0 0 Z 4000 2 0 0 0
Eggs
9 April 8 April 24 9 May 6 M a y 18 209 800 1000 Larvae and j u v e n i l e s 1 2 3 4 5 6 7 8 9 10 11 12 8 0 0 600 4 0 0 200W-N. Tzeng and Y.-T. Wang: Distribution dynamics of estuarine fishes
Stations 10737
Fig. 4. Temporal and spatial variations in abundance of fish eggs and larvae collected in the nearshore wa- ters of the Tanshui River on 8 and 24 April, 6 and 18 May, and 4 June 1991
"0 "0 "6 6 Z 6OO 40O 2 0 0
Egg Yolk-sac Preflexion Flexion Postflexion Juvenile Young
larva larva larva larva Developmental
stages
Fig. ft. Relative abundance at different developmen- tal stages of the fish collected from the 12 stations in the nearshore waters of the Tanshui River on 8 and 24 April, 6 and 18 May, and 4 June 1991
Fish a b u n d a n c e associated with developmental stages In general, due to mortality, the n u m b e r o f fish gradually decreased f r o m egg to young. However, the n u m b e r o f yolk-sac larvae f r o m surface sampling in the studied area was dramatically less t h a n that o f the next developmental stage (Fig. 5, Table 1). The n u m b e r o f yolk-sac larvae was
significantly lower t h a n that o f preflexion, flexion, and postflexion larvae. This indicates that the a b u n d a n c e o f yolk-sac larvae does not fit the standard survival curve o f fish in the early stages. This discontinuous p h e n o m e n o n was p r o b a b l y due to ontogenetic behavioral changes. The specific weight o f yolk-sac larvae has been reported to be greater than that o f pelagic eggs (Tanaka 1990). Yolk-sac
210 W.-N. Tzeng a n d Y.-T. Wang: Distribution dynamics of estuarine fishes Table 1. Species composition, life stage and frequency occurrence
of fish larvae and juveniles collected from 12 stations in the nearshore waters of the Tanshui River estuary, surveyed on: 8 (A)
and 24 (B) April; 6 (C) and 18 (D) May; and 4 (E) June 1991. Ys: yolk-sac larva; Pr: preflexion larva; FI: flexion larva; Po: postflexion larva; Ju: Juvenile; Yg: young
Serial Species a n d family
n o .
No. o f fish at life stage
Ys Pr F1 Po Ju Yg
Total no. of fish
Frequency o f occurrence (5 surveys at 12 stns)
Date Sum for %
all Stns Clupeidae 1 Sardinella melanura 1 2 sp. 3 Engraulidae 3 Stolephorus buceaneeri 1 24 3 4 Thryssa kammalensis Myctophidae 5 Benthosema pterotum 1 2 6 sp. 3 1 7 sp. 4 5 8 sp. 5 Synodontidae 9 Trachinocephalus myops 1 Bregmacerotidea 10 Bregmaceros nectabanus 1 11 Antennariidae sp. 1 12 Atherinidae sp. 1 2 13 Holocentridae sp. 1 7 1 14 Scorpaenidae sp. 2 1 Platycephalidae 15 sp. 2 1 16 sp. 3 1 17 sp. 4 1 C e n t r o p o m i d a e 18 Ambassis gymnocephalus i 62 5 19 Serranidae sp. 1 1 Teraponidae 20 Terapon jarbua 1 35 93 21 Priacanthidae sp. 1 1 A p o g o n i d a e 22 Apogon notatus 21 2 23 sp. 3 37 10 24 sp. 5 25 sp. 6 26 sp. 7 1 Sillaginidae 27 Sillago sihama 1 5 28 Sillago japonica 5 10 29 Sillago maculata Carangidae 30 Trachurus japonicus 12 1 31 Decapterus maruadsi 4 1 32 Scomberoides tol 2 26 Coryphaenidae 33 Coryphaena hippurus Formionidae 34 Formio niger 11 Menidae 35 Mene maculata 1 Leiognathidae 36 sp. 1 37 sp. 2 1 Nemipteridae 38 sp. 1 3 39 sp. 2 7 18 40 sp. 3 8 6 Gerreidae 41 Gerres abbreviatus 6 42 Gerres macrosoma 43 Caesionidae sp. 1 1 Sciaenidae 1 2 2 36 52 1 9 1 1 1 1 1 1 11 2 1 10 2 1 4 2 2 2 1 2 C 2 3.33 1 B 1 1.67 31 A B C 10 16.67 2 C 1 1.67 2 1 1 2 8 1 1 1 1 156 1 139 1 23 47 1 2 1 6 16 2 13 18 29 11 3 35 14 12 2 4 A D B D C D B C D C B B A B C D E B B C D D A B C A B C C C B B B C B C A B C C A C B B C 2 E 1 E 4 E 1 E D E E D E D E E D D E E D E D E 38 1 13 1 15 12 1 2 1 4 11 2 12 10 8 2 16 2 3.33 1.67 6.67 1.67 1.67 1.67 1.67 3.33 11.67 1.67 1.67 1.67 1.67 63.33 1.67 21.67 1.67 25.00 20.00 1.67 3.33 1.67 6.67 18.33 3.33 20.00 16.67 13.33 1.67 5.00 1.67 5.00 1.67 3.33 26.67 3.33 8.33 1,67 5.00
~i~ ~ ~ ~ ~ ~ ~ ~~ ~ ~ ~ ~ ... ~ 9 9 9 ~ ... ~ ~ ~ ~ ~ I~ ~ 9 9 ~ ~ ~i~c~ ~l~ ~ ~i ~ ~ ~ ~ ~Q i~IL~bi~ ~ ~j ~i~ ~ ~ O~l~t~L~ ... 0~ ~l~ ~i~ ~1 ~Pl iI~i~ ~l~ ~ ~l ~ ~ ~ll~ ~i~ ~ ~S ~ll~ ~ii~ ~ i~ ~j~ ~ L~ ~ ~ll ~I~1 4 ~ ~ ~ ~lJ j~ o~o~ t~ C~ C~
212
<
< ~ oo r~o ~
,-.M m a cr Z < < < 0 <<
<
-~ < 4- 4--I-I 4- +4-1 4-1 + 4-1 4-1 +1 4-1 -H 4-1 +l 4-1 4-1 4-1 + +1 4-1 4-1 4-1 4- 4-1 4-1 +l +1 4-1 4-1 4- 4-4-1 4- 4-1 4-1 4-1 +l 4-1 +l 4-1 4-1 +l 4-1 -I-I 4-1 +[ 4-1 4-1 4-1 4-1 4-1 4-1 4-1 +1 4-1 4-1 -I-I 4-1 "1 -H 4-1 4-1 4-1 4-1 4-1 t, 4-1 4-1 4- +1 4- 4-1 4-1 4-1 4-1 4-1 +1 4-1 +1 -H 4-1 +1 4-1 4-1 4-1 4-1 4-1 4-1 4-1 +1 +1 4-1 4-[ 4-1 4-1 4-1 4-1 4-1 4-1 ~ +1 -I-I 4-1 4-1 r.) +1 +1 +l +l +1 +1 + +1 +l +1 +1 +1 +l +1 +l +l +l + +1 +1 + + 1 + + + + l + l + +I 4-1 4-1 4-1 4-1 4-1 +l 4-1 +l 4-1 4-1 4-1 I 4-1 4-1 4-1 4- 4-1 4-1 +l +l +l +1 +1 +l + +1 +l +l +1 +l +1 +1 +1 +1 +l +l +1 +l +1 +1 +l +1 +1 + + +l +l +l +1 +l + + 1 + + + + l + l +l +l +l +1 +1 +l +1 +l +1 +l +1 +l +1 +[ +l +l +l +1 +l +l +l +l +l +l +l +l +l +1 +l +l +1 +l +l +1 +l +l +l +1 +1 +l +1 +1 +l +1 I +l +l +l +l +l +l +1 +1 +l +1 +l +l +1 +1 +l +l +l +1 +1 +1 +l +l +l +l +l +l I +1 +1 +1 *1 +1 + +1 +l +1 +l +l +1 +l +1 +l + 1 + 1 + +1+1 I +1 +l +1 +l +1 +1 +~ +1 +l +1 +l +l +1 +M + [ + [ + l + l +l +1 +1 + +1 +l +l +1 +1 +1 ,~ ~ ~=_ .~~ ~_~ -
N ~ ' ~ 2~ Z ~
~
W.-N. Tzeng and Y.-T. Wang: Distribution dynamics of estuarine fishes
larvae usually sink to the bottom at this stage. Therefore,
yolk-sac larvae were not as accessible to surface sam-
pling.
2~ e~ + o +lSpecies composition and retention
A total of 1387 individuals, representing 43 families and
93 species, was collected from 12 stations through five
surveys, during the period from early April to early June
1991 (Table 1). The number of fish captured was consis-
tent with their frequency of occurrence. The dominant
species was widely distributed among the 12 stations and
occurred with higher frequency in the five surveys. The
top five dominant taxa -
Liza macrolepis, Ambassis gym-
noeephalus, Terapon jarbua,
Mullidae and Gobiidae -
made up 64.7% of the total catch. Most species occurred
in low numbers and at an early life stage, indicating that
most species occurring in the area were short-term, tem-
porary residents or rare species (Bell et al. 1984). In con-
trast, the dominant species,
Liza macrolepis
and
Ambassis
gymnoeephalus,
may be long-term, temporary residents,
because of their higher frequency of occurrence in the
nearshore waters and the larger number of life history
stages in comparison with the other species. They may
live in the nearshore waters for extended periods as juve-
niles before leaving.
Ordination of station by species density
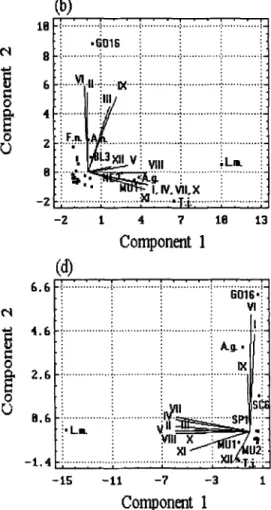
The 12 stations clustered by PCA according to the species
density data (Fig. 6), are similar to those clustered by
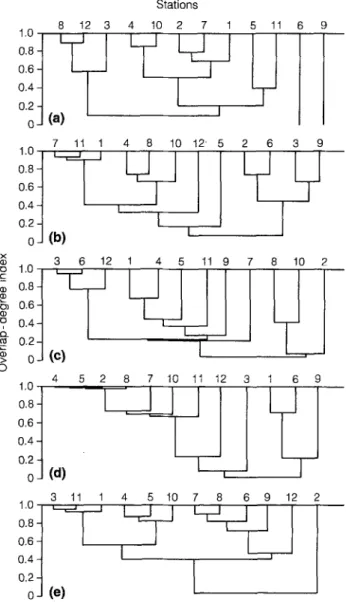
Kimoto's (1976) index and Mountford's (1962) method
(Fig. 7). The classification of the stations was not always
consistent between surveys (Fig. 6).
According to species composition of fish larvae and
juveniles, the 12 stations surveyed on 8 April can be clas-
sified into three groups: A (Stns I, II, IV, VII and X), B
(Stns V and XI) and C (Stns III, VIII and XII). The
dominant species in each group was different. Group A
consisted mainly of
Scomberoides tol
and
Stolephorus
buccaneeri;
Group B of Gobiidae sp. 1, Apogonidae sp. 3;
and Group C of Callionimidae sp. 2 and Blenniidae sp. 3,
respectively. Group A was mainly made up of pelagic
fishes, while Groups B and C were mainly benthic fishes
(Fig. 6a). Similarly, the same stations surveyed on 24
April were classified into only two groups: A (Stns I, IV,
V, VII, VIII, X, XI and XII) and B (Stns II, III, VI and
IX). The dominant species in the stations changed,
Group A dominated by
Liza macrolepis
and
Teraponjar-
bua;
and Group B by Gobiidae sp. 16 and
Formio niger,
respectively (Fig. 6 b). The stations of the other three sur-
veys can also be classified into different groups, in which
the dominant species again changed (Figs. 6 c - e ) . These
changes of station groupings and dominant species indi-
cated that species composition of fish larvae and juveniles
in the nearshore waters changed rapidly. This phe-
nomenon was probably due to the seasonal succession of
the species and/or environmental conditions.
W.-N. Tzeng and Y.-T. Wang: Distribution dynamics o f estuarine fishes 0 0 0 C4 0 0 0 C4 0
t
o 0 7.5 5.5 3.5 1,5 -8.5 -2.5 6.9 4.9 2.9 0.9 -1.1 -3.1 7 . 3 5 . 3 3 . 3 1.3 - 8 . ? - 2 . 7 - 1(a)
9 .. .. .. . . . . . , .... . . . : . . . , . . . ; ... i - .: ... ~..., s ... :. viii x l i III i: ... : ... ~ ... i ...-
t~--~'
.,t3{
i:
U ;;iiiiii iiiiiiiiiiiiiiiii'il
...
... :::: ... ii
5 8 . 5 2 . 5 4 . 5 6 . 5Component I
@)
, , . , . , , , . , . . . , . . . . . ~ . . . , . . .i
~
' ~ ~
; . . . ; . . . I V , . ; . . . : . . . , . . .:: x~
i
i
i
i-"A:ii:.~t'i
... i ... .! ... .i ...
i ... i ... i ... ~ l , ~ - - i i ~-,lt-.:...: . . . .' ... " ... : ... ': : .~." VI : :.,L m. " i " U ; ... ;'"':'"i'~ Ir'''.'''';''''.'''':''', -" .'", -2 4 7 18 13Component
1
(e)
.~.,.:..:...!...:...:...!..,.'.,.:,..i..:...:...!.,.:...:...'...'...:... : ,G016 :i IX ::i / ,~ i i ! i
: N E 2 " ~ I ' ~ ; I V l I I i i i i '~ " / / ~ , : / ' VII i i i i :t;_.~~ ... i ... :: ... i ...i-
9 !\Q~pt.xi
!
:: A.g i ' i , " \ ~ l , X l i ! i , ' !:~.,~.~.,..~,,,...~ ... :...:...:...i ... :...i.
2 5 8 11 14 17Component I
r 0 0 0 C4 0 0 U 18 8 6 4 2 8 -2 6.6 4.6 2,6 B.6 -1.4 -15r
- L..'...:....!....:,.. :... L..'...'.,..!...',.. :....!...:....'....' :! ,Gols :: . , : . . . ; . . . . . . . . . : . . . = . ;i : i i "iw""iiii:~iJl~!S-..i.. ...;
... i . . . !i ! , ~ Y l l l i ~LI i - i g ~ ~ : ~ ... i ... i ... i ': " " : lU1"~-- I. N . VII. X : :,i...-:...:....i..,:....:,...~.:...---T-.i:-...:.-..i.-..:.,.-.-.-.i-
- 2 1 4 7 18 13Component
1
(d)
ff":::ff'::!:":b:i~iii~.il::
!
i
i
w::
!
i
i
i
li
i i, i j : : : XI " ~ i "_':__'/MU2i l -i...:...:.-.i.--...-..:...:...:;....:.: ... ,~t;".:~.:,.i-I - 1 1 - 7 - 3 1Component 1
Fig. 6. Principle coordinate analysis for the ordina- tion o f the 12 stations ( I - X I I ) in response to spe- cies density, surveyed on: (a) 8 April; (b) 24 April; (c) 6 May; (d) 18 May; and (e) 4 June 1991. D o m i - nant species: Ambassis gymnocephalus (A.g.);
Apogon notatus (A.n.); A p o g o n i d a e sp. 3 (AP3); Blenniidae sp. 3 (BL3); Callionymidae sp. 2 (CA2);
Decatperus maruadsi (D.m.); Formio niger (F.n.);
Gerres abbreviatus (G.a.); Gobiidae sp. 16 (GO16);
Liza macrolepsis (L.m.); Mullidae sp. 1 (MU1); Mullidae sp. 2 (MU2); Nemipteridae sp. 2 (NE2);
Stolephorus buccaneeri (S.b.); Stephanolepis cirrhifer
(S.c.); Sciaenidae sp. 6 (SC6); Sphyraenidae sp. 1 (SP1); Scomberoides tol (S.t.); Terapon jarbua (T.j.)
213
Relationships between biotic and abiotic factors A canonical correlation of the relationships between biot- ic and abiotic factors indicated that the abundance offish eggs and larvae as well as species diversity indices were influenced by multiple abiotic factors (Fig. 8). The first two canonical correlations between six abiotic and five biotic factors were both significant (r 1 = 0.7298, DF = 30, Z12=74.917 P<0.00001; r2=0.5959, D F = 2 0 , ~2 2 =
36.88, O.Ol<P<O.05). In the first canonical variate, abundance of fish egg and larvae, and species diversity index (H') were positively correlated with temperature, DO, depth and transparency, but negatively correlated with salinity and pH, as well as number of species and
species evenness index (J'). While in the second canonical variate, the relationship between biotic and abiotic fac- tors was more complicated. Larval abundance was posi- tively correlated with all the abiotic factors except pH, and negatively correlated with egg abundance and num- ber of species (Fig. 8).
Furthermore, the community variables and abun- dance of dominant species in relation to each of the abi- otic factors for each of the five surveys were conducted by Spearman rank correlation (Table 2). The community structure was more complicated with increasing pH, depth, transparency and the distance from the coast. Of the 18 selected species, half were significantly correlated with the abiotic factors. The abundance of most domi-
214
x -(3 0 8 12 3 1,0 0.6 0.4 0.2 0 (a) 7 11 1 4 8 10 1.0 i08 t 1
0.6 l 0.4 I 0.2 [ o (b) 3 6 12 1.008t
0.6 0.4 02 0 (C) 4 5 2 1.0t I
0,6 0,4 0.2 o(d)
3 11 1 4 5 101~ t '
J
0.6 0.4 I 0.2 0 (e) Stations 4 10 2 7 1 5 11 I 12' 5 2 6 3 9 1 4 5 11I
8 10 8 7 10 11 I2 3 I I 6 9 7 8 6 9 IL
12 2Fig. 7. Clustering of the 12 stations by Mountford's (1962) method based on Kimoto's (1976) overlap-degree index (C~) of species composition between stations, surveyed on: (a) 8 April; (b) 24 April; (c) 6 May; (d) 18 May; and (e) 4 June 1991
nant species was positively correlated to depth and loca-
tion of the station.
Ambassis gymnocephalus,
Nemipteri-
dae, Mullidae and
Liza macrolepis
tended to live in low
salinity water. Fish abundance was not significantly cor-
related with DO, probably due to the fact that DO was
not lower than the required threshold of the fish. The
positive correlation between fish egg and larval abun-
dance, as well as the depth and location of the station,
suggested that most fish larvae and juveniles in the
nearshore waters of the Tanshui River originated from
offshore.
Discussion and conclusion
Species composition and their origin
A total of 1387 individuals, representing 43 families and
93 species, was captured in the nearshore waters of the
W-N. Tzeng and Y.-T. Wang: Distribution dynamics of estuarine fishes 1.0 oJ 0.5 0 > 0 E -0.5 0 c {") -1 - 1 . 5 9 No. of species 9 pH 9 j, 9 Salinity gs DO Transparency 9 n 9 9 Depth Temperature H' 9 Larvae I -0.5 0.5 Canonical variate 1
Fig. 8. Ordination of the biotic and abiotic factors by first two
canonical variates. J': Pielou's evenness index; H': Shannon-
Weaver's species diversity index
Tanshui River during the period from April to June 1991
(Table 1). The larval fish community is comparable with
the fish fauna existing in other tropical mangrove estuar-
ine ecosystems (Blaber et al. 1985, Robertson and Duke
1990).
Liza macrolepis, Ambassis gymnocephalus, Ter-
apon jarbua,
Mullidae and Gobiidae are most dominant
and frequently occur in the estuary. They are estuarine-
dependent marine and estuarine species (Deegan 1989)
and their retention in the estuary was longer than for the
other species. The remaining marine species, e.g., Myc-
tophidae,
Coryphaena hippurus,
and
Scomber japonicus,
occurred in low numbers and their larvae occasionally
drifted with the tidal current into the estuary (Table 1).
No freshwater species was found in the present study
(Table 1), probably due to the salinity (Fig. 2). Freshwa-
ter eel,
Anguilla japonica,
elvers are abundant at night-
time in the study area during upstream migration in win-
ter; however, they were not caught during the investigat-
ed period because the sampling was conducted during
daylight.
Fish larvae and juveniles in the estuary were classified
into permanent residents, long- and short-term tempo-
rary residents and rare species according to their number
and duration in the estuary (Bell et al. 1984). Most of the
fish captured seemed to be temporary residents or rare
species, because the number of individuals of most spe-
cies was fewer than four per sample (Table 1). Most fish
larvae and juveniles in the nearshore waters of the Tan-
shui River were spawned in adjacent marine waters, their
eggs and larvae passively drifting with the current into
the study area, where they stayed through the early stages
of their life history. On the other hand, Gobiidae are
estuarine spawning and may remain in the estuary
throughout their life cycle. There are three major migra-
tion patterns by which fish use estuarine systems for re-
production and juvenile feeding (Deegan and Thompson
1985), viz.: (1) saltwater spawning, followed by immigra-
tion of the larvae into an estuary; (2) estuarine spawning,
in which the larvae remain, for the most part, within an
estuary; (3) freshwater spawning, followed by the down-
stream drift or swimming of larvae and juvenile fish into
an estuary.
W-N. Tzeng and Y.-T. Wang: Distribution dynamics of estuarine fishes
Offshore (
) Nearshore
Estuary
Sea surface|
Egg
/ Postlarva /
*-1early stage "-~'
*-1
(nighttime) r -a"
Surface * [ 'I'
layer t
In ~1' I
II
I I
/
~
I Rising I I
Sinking
I I
I I
Pro,a v l "-t
Ilate
stage[
"-t
.... ;'~
~-" [(daytime)
L lifetTpe
;
1
Bottom
layer
Sea bottom
Pelagic life (Passive)
Pelagic life (Active)
Pelagic life
Dermersai life
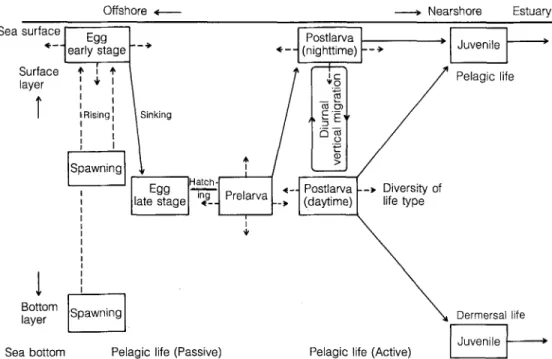
215
Fig. 9. Schematic diagram show-
ing onshore movement of fish
larvae and juveniles in relation
to their ontogenetical behavioral
changes and life-type (redrawn
from Tanaka 1976)
To classify the species listed in Table 1 into the differ-
ent migratory patterns, detailed information about the
life history of the fish was necessary. This information
was very limited except for some commercially important
species, e.g.
Scomber japonicus, Anguilla japonica
and
Terapon jarbua
(Miu et al. 1990).
Larvae fish abundance in relation to estuarine transport
and behavioral ontogeny
Transportation of fish larvae from offshore into the
nearshore waters and further into estuarine nursery areas
is influenced both by physical processes, as well as the
fishes' activity and behavior (Tanaka 1976, 1985, 1990,
Weinstein et al. 1980, Boehlert and Mundy 1988, Drake
and Arias
1991
a, b). This process of transportation is
important in determining the occurrence and abundance
of fish eggs and larvae in the nearshore waters. Many
studies indicate that the buoyancy of fish eggs changes
during early developmental stages (Russell 1976,
Coombs 1981, Tanaka 1981, Coombs et al. 1985, Tanaka
1990). Pelagic fish eggs passively drift with the current
into the estuary. Upon reaching the yolk-sac stage, their
specific weight increases and thus the larvae sink to sub-
surface layers, so that the yolk-sac larvae are not accessi-
ble to surface sampling. This behavior could prevent the
larvae from being transported away from suitable habi-
tats. When the larvae develop to the post flexion stage,
their gas bladder is completely formed and they can per-
form diel vertical movement and active migration. Then,
the larvae can adjust their buoyancy to allow selective
tidal stream transport, using currents to move into an
estuary (Kuwahara and Suzuki 1984, Boehlert and
Mundy 1988). After the juvenile stage, the fish differenti-
ated into pelagic and benthic forms. Benthic forms settle
down and do not drift with the tidal current (Fig. 9).
Therefore, most benthic species beyond the juvenile stage
were not caught by surface sampling, and the number of
benthic species, e.g.
Terapon jarbua,
MuUidae, Apogo-
nidae, Nemipteridae, Gobiidae, and Callionymidae,
gradually decreased with increasing developmental
stages (Table 1). In contrast, the pelagic forms, e.g.
Am-
bassis gymnocephalus
and
Liza macrolepis,
which are es-
tuarine-dependent pelagic species, were abundant and
stayed longer in the nearshore waters (Table 1). These
facts indicated that ontogenetically behavioral change as-
sociated with developmental stage may play an impor-
tant role in determining the abundance of the fish larvae
in the nearshore waters (Fig. 5).
Distribution and abundance of fish larvae in relation
to abiotic factors
The physico-chemical characteristics of the water in the
studied area were significantly different in each of the five
surveys (Figs. 2, 3). This indicates that the estuarine envi-
ronment is very dynamic. The shallower area of the stud-
ied habitat was influenced by freshwater, lowering its
salinity (Fig. 2). The abundance and species composition
of fish larvae and juveniles also changed with the physico-
chemical conditions (Table 2). At the shallow water sta-
tions, the fish community was dominated by benthic spe-
cies, e.g. Callionymidae, Blenniidae, and Gobiidae. In
contrast, the deep-water stations of the study area were
dominated by nearshore pelagic species, e.g.
Ambassis
gymnocephalus, Liza macrolepis, Coryphaena hippurus,
Formio niger, Scomber japonicus, Stephanolepis cirrhifer,
Carangidae, and Engraulidae, as well as mesopelagic fish,
Myctophidae (Fig. 6).
The extent of onshore migration of fish is dependent
on their tolerance to extremes of environmental variables
(Kinne 1964, De Veen 1978, Whitfield et al. 1981,
216
C l a r i d g e a n d P o t t e r 1983, T o n g i o r g i et al. 1986, B o e h l e r t a n d M u n d y 1988, Tosi et al. 1988). T h e a b u n d a n c e s o f d o m i n a n t species, Liza macrolepis a n d Ambassis gymno- cephalus, w e r e n e g a t i v e l y c o r r e l a t e d w i t h s a l i n i t y ( T a b l e 2), i n d i c a t i n g t h a t l o w s a l i n i t y m a y act as a cue to g u i d e these fish l a r v a e a n d j u v e n i l e s to i n s h o r e n u r s e r y g r o u n d s ( H u g h e s 1969, Y o u n g a n d C a r p e n t e r 1977). H o w e v e r , this p h e n o m e n o n m a y be d i f f e r e n t b e t w e e n species. S o m e species c a n n o t t o l e r a t e w i d e s a l i n i t y r a n g e s in t h e e s t u a r y a n d e m i g r a t e d u r i n g l a t e r life stages. T h u s , t h e y a p p e a r e d in l o w n u m b e r s a n d a t v e r y e a r l y s t a g e s o f t h e i r life cycle ( T a b l e 1). I n c o n c l u s i o n , the c o m m u n i t y s t r u c t u r e o f fish l a r v a e a n d j u v e n i l e s in this e s t u a r i n e a r e a w a s v e r y c o m p l e x b u t c o n s i s t e d m a i n l y o f e s t u a r i n e - d e p e n d e n t m a r i n e species. T h e i r l a r v a e d r i f t e d w i t h the c u r r e n t i n t o t h e e s t u a r y s h o r t l y a f t e r h a t c h i n g . N o t o n l y p h y s i c a l f a c t o r s , b u t a l s o the d e v e l o p m e n t a n d o n t o g e n e t i c b e h a v i o r o f t h e fish, p l a y e d a n i m p o r t a n t r o l e in t h e i r t r a n s p o r t a t i o n i n t o t h e e s t u a r y .
Acknowledgements. This study was conducted with the financial support of the National Science Foundation, Republic of China (Contract No. NSC80-0421-B002-05z). We are grateful to Mr. Y. C. Chen, Mr. T. Chen-Yang and Mr. Y. F. Shen for their help with field collections and laboratory work, two anonymous reviewers for helpful comments on the manuscript and Miss S. MacLeod for reading the English text.
Literature cited
Bell, J. D. (1980). Aspects of the ecology of fourteen economically important fish species in Botany Bay, New South Wales, with special emphasis on habitat utilization and a discussion of the effects of man-induced habitat changes. M. Sc. Thesis, Mac- quarie University, New South Wales, Australia
Bell, J. D., Polard, D. A., Burchmore, J. J., Pease, B. C., Middleton, M. J. (1984). Structure of a fish community in a temperate tidal mangrove creek in Botany Bay, New South Wales. Aust. J. mar. Freshw. Res. 35:33-46
Blaber, S. J. M., Btaber, T. G. (1980). Factors affecting the distribu- tion of juvenile estuarine and inshore fish. J. Fish Biol. 17: 143-162
Blaber, S. J. M., Milton, D. A. (1990). Species composition, commu- nity structure and zoogeography of fishes of mangrove estuaries in the Solomon Islands. Mar. Biol. 105:259-267
Blaber, S. J. M., Whitfield, A. K. (1977a). The feeding ecology of juvenile Mugilidae in southeast African estuaries. Biol. J. Linn.
Soc. 9:227-284
Blaber, S. J. M., Whitfield, A. K. (1977b). The biology of the
burrowing goby Croilia mossambica Smith (Teleostei: Gobi-
idae). Envir. Biol. Fish. 1:197-204
Blaber, S. J. M., Young, J. W, Dunning, M. C. (1985). Community structure and zoogeographic affinities of the coastal fishes of the Dampier Region of North-western Australia. Aust. J. mar. Freshw. Res. 36:247-266
Boehlert, G. W, Mundy, B. C. (1988). Roles of behavioral and physical factors in larval and juvenile fish recruitment to estuar- ine nursery areas. Am. Fish. Soc. Symp. 3 : 5 1 - 6 7
Brander, K., Thompson, A. B. (1989). Die1 differences in avoidance of three vertical profile sampling gears by herring larvae. J. Plankton Res. 11(4): 775-784
Chou, C. H., Bi, C. C. (1990). Dynamic distribution of nutrients and variation of environmental factors in Tamshui estuary
W.-N. Tzeng and Y.-T. Wang: Distribution dynamics of estuarine fishes ecosystem. Proc. natn. Sci. Coun., Taiwan, Republic of China (B: Life Sciences) 14(3): 131-141
Chou, C. H., Chang, F. C., Hwang, Y. S. (1987). Ecological research of mangrove forests: a review. In: Chou, C. H., Peng, C. I., Chaw, S. M. (eds.) Plant resources and conservation in Taiwan. Society for Wildlife and Nature, Republic of China, p. 23-48 Claridge, P. N., Potter, I. C. (1983). Movements, abundance, age
composition and growth of bass, Dicentrarehus labrax, in the Severn River estuary and inner Bristol Channel. J. mar. biol. Ass. U. K. 63:871-879
Clutter, R. L, Anruku, M. (1968). Avoidance of samplers in zooplankton sampling. Monogr. oceanogr. Methodol. (UNES- CO) 2 : 5 7 - 7 6
Coombs, S. H. (1981). A density-gradient column for determining the specific gravity of fish eggs, with special reference to eggs of mackerel Scomber scombrus. Mar. Biol. 63: 10l - 106
Coombs, S. H., Fosh, C. A., Keen, M. A. (1985). The buoyancy and vertical distributions of eggs of sprat (Sprattus sprattus) and pilchard (Sardina pilchardus). J. mar. biol. Ass. U.K. 65: 461- 474
Deegan, L. A. (1989). Nekton, the free-swimming consumers. In: Day, J. W, Hall, C. A. S., Kemp, W M., Yfifiez-Arancibia, A. (eds.) Estuarine ecology. Wiley-Interscience, New York, p. 377- 437
Deegan, L. A., Thompson, B. A. (1985). The ecology of fish com- munities in the Mississippi River deltaic plain. In: Yhfiez-Aran- cibia, A. (ed.) Fish community ecology in estuaries and coastal lagoons: towards an ecosystem integration. Editorial Universi- taria, UNAM-PUAL-ICML, Mexico, D.E, p. 35-56
De Veen, J. E (1978). On selective tidal transport in the migration of North Sea plaice (Pleuronectes platessa) and other flatfish species. Neth. J. Sea Res. 12:115-147
Dillon, W R., Goldstein, M. (1984). Multivariate analysis - meth- ods and applications. Wiley-Interscience, New York
Drake, P., Arias, A. M. (1991 a). Ichthyoplankton of a shallow coastal inlet in south-west Spain: factors contributing to coloni- zation and retention. Estuar. cstl. Shelf Sci. 32:347-364 Drake, P., Arias, A. M. (1991 b). Composition and seasonal fluctu-
ations of the ichthyoplankton community in a shallow tidal channel of Cadiz Bay (S. W Spain). J. Fish Biol. 39:245-263 Haedrich, R. L., Haedrich, S. O. (1974). A seasonal survey of the
fishes in the Mystic River, a polluted estuary in downtown Boston, Massachusetts. Estuar. cstl mar. Sci. 2 : 5 9 - 7 3 Holliday, F. G. T. (1971). Salinity: fishes. In: Kinne, O. (ed.) Marine
ecology, Vol. 2. Wiley-Interscience, Sydney, p. 997-1033 Hughes, D. A. (1969). Responses of salinity change as a tidal trans-
port mechanism of the pink shrimp Penaeus duroarum. Biol. Bull. mar. biol. Lab., Woods Hole 153:505-526
Kendall, A. W, Jr., Ahlstrom, E. H., Moser, H. G. (1984). Early life history stages of fishes and their characters. In: Moser, H. G., Richards, W J., Cohen, D. M., Fahay, M. P., Kendall, A. W Jr., Richardson, S. L. (eds.) Ontogeny and systematics of fishes. Spec. Publ. No. 1. Am. Soc. Ichthyol. Herpetol. Allen Press Inc., Lawrence, Kansas, p. 11-22
Kimoto, S. (1976). Studying methods of community structure of animals - I. Deversity and species composition. Kyau-ritu Press, Tokyo (in Japanese)
Kinne, O. (1964). The effects of temperature and salinity on marine and brackish water animals - II. Salinity and temperature salin- ity combinations. Oceanogr. mar. Biol. A. Rev. 2:281-339 Kuwahara, A., Suzuki, S. (1984). Diurnal changes in vertical distri-
butions of anchovy eggs and larvae in the western Wakasa Bay. Bull. Jap. Soc. scient. Fish. 50:1285-1292
Lee, C. W., Chu, T. Y. (1965). A general survey of Tanshni River and its tributary estuaries. Rep. Inst. Fish. Biol. Taipei 2(1): 34-45 Leis, J. M. (1986). Vertical and horizontal distribution offish larvae near coral reefs at Lizard Island, Great Barrier Reef. Mar. Biol. 90:505-516
Loneragan, N. R., Potter, I. C., Lenanton, R. C. J., Caputi, N. (1986). Influence of environmental variables on the fish fauna of the deeper waters of a large Australian estuary. Mar. Biol. 94:631-641
W.-N. Tzeng and Y.-T. Wang: Distribution dynamics of estuarine fishes 217 McErlean, A. J., O'Connor, S. G., Mihursky, J. A., Gibson, C. I.
(1973). Abundance, diversity and seasonal patterns of estuarine fish populations. Estuar. cstl Shelf Sci. 1:19-36
Miu, T. C., Lee, S. C., Tzeng, W. N. (1990). Reproductive biology of Terapon jarbua from the estuary of Tanshui River. J. Fish. Soc. Taiwan 17(1): 9-20
Mountford, M. D. (1962). An index of similarity and its application to classificatory problem. In: Murphy, P. W. (ed.) Progress in soil zoology. Butterworths, London, p. 43-50
Mukai, T. (1987). Effects of micro-scale in situ environmental gradi- ents concerning water qualities on the structure of the phyto- plankton community in a coastal embayment. Estuar. cstl Shelf Sci. 25:447-458
Nakai, Z. (1962). Apparatus for collecting macroplankton in the spawning survey of iwashi (sardine, anchovy and round herring) and others. Bull. Tokai reg. Fish. Res. Lab. 9:221-237 Omori, M., Hamner, W. M. (1982). Patchy distribution of
zooplankton: behavior, population assessment and sampling problems. Mar. Biol. 72:193-200
Pielou, E. C. (1966). The measurement of diversity in different types of biological collections. J. theor. Biol. 13:131-144
Powell, A. B., Hoss, D. E., Hettler, W. E, David, S. P., Wanger, S. (1989). Abundance and distribution of ichthyoplankton in Flor- ida Bay and adjacent waters. Bull. mar. Sci. 44:35-48 Quinn, N. J. (1980). Analysis of temporal changes in fish assem-
blages in Serpentine Creek, Queensland. Envir. Biol. Fish. 5: 117-133
Robertson, A. I., Duke, N. C. (1987). Mangroves as nursery sites: comparisons of the abundance and species composition of fish and crustaceans in mangroves and other nearshore habitats in tropical Australia. Mar. Biol. 96:193-205
Robertson, A. I., Duke, N. C. (1990). Mangrove fish-communities in tropical Queensland, Australia: spatial and temporal patterns in densities, biomass and community structure. Mar. Biol. 104: 369-379
Russell, F. S. (1976). The eggs and planktonic stages of British marine fishes. Academic Press, London
Sebat~s, A. (1990). Changes in the heterogeneity of mesoscale distri- bution patterns of larval fish associated with a shallow coastal haline front. Estuar. cstl shelf Sci. 30:131-140
Siegel, S. (1956). Nonparametric statistics for the behavioral sci- ences. McGraw-Hill, New York
Smith, P. E., Richardson, S. L. (1977). Standard techniques for pelagic fish egg and larva surveys. EA.O. Fish. tech. Pap. 175: 1-100
Tanaka, K. (1976). Ecological consideration on meeting and parting of fish eggs and larvae. Bull. Jap. Soc. Fish. Oceanogr. 28: 79-89 (in Japanese)
Tanaka, M. (1981). Feeding and survival in marine fish larvae - V. Vertical distribution and migration of eggs and larvae. Aquabi- ology, Tokyo 16:379-386 (in Japanese)
Tanaka, M. (1985). Factors affecting the inshore migration of pe- lagic larval and demersal juvenile red sea bream Pagrus major to a nursery ground. Trans. Am. Fish. Soc. 114:471-477 Tanaka, Y. (1990). Change in the egg buoyancy of Japanese an-
chovy, Engraulisjaponicus during embryonic development. Nip-
pon Suisan Gakk. 56:165
Tongiorgi, P., Tosi, L., Balsamo, M. (1986). Thermal preferences in upstream migrating glass-eels of Anguilla anguilta (L.). -J. Fish Biol. 28:501-510
Tosi, L., Sala, L., Sola, C., Spampanato, A., Tongiorgi, P. (1988). Experimental analysis of the thermal and salinity preferences of glass-eels, Anguilla anguilla (L.), before and during the upstream migration. J. Fish Biol. 33:721-733
Tzeng, W. N., Wang, Y. T. (1992). Structure, composition and sea- sonal dynamics of larval and juvenile fish community in the mangrove estuary of Tanshui River, Taiwan. Mar. Biol. 113: 481-490
Wang, Y. T., Tzeng, W. N., Lee, S. C. (1991). A preliminary study on species composition and seasonal abundance of fish eggs and larvae from the coastal waters adjacent to the Tanshui River estuary, Taiwan, J. Fish. Soc. Taiwan 18(1): 7-20
Weinstein, M. P. (1979). Shallow marsh habitats as primary nurs- eries for fishes and shellfish, Cape Fear River, North Carolina. Fish. Bull. U.S. 77:339-357
Weinstein, M. P., Weiss, S. L., Hodson, R. G., Gerry, L. R. (1980). Retention of three taxa of postlarval fishes in an intensely flushed tidal estuary. Fish. Bull. U.S. 78:419-435
Whitfield, A. K., Blaber, S. J. M., Cyrus, D. P. (1981). Salinity ranges of some southern African fish species occurring in estuar- ies. S. Afr. J. Zool. 16:151-155
Y~tfiez-Arancibia, A., Linares, E A., Day, J. W. Jr. (1980). Fish community structure and function in Terminos Lagoon, a trop- ical estuary in the southern Gulf of Mexico. In: Kennedy, V. S. (ed.) Estuarine perspecitves. Academic Press, New York, p. 465 -482
Young, P. C., Carpenter, S. M. (1977). Recruitment of postlarval penaeid prawns to nursery areas in Moreton Bay, Queensland. Aust. J. mar. Freshwat. Res. 28:745-773