slurry pH increases, the removal rate of polished titanium increases, but it decreases for polished aluminum. The removal rate of titanium was limited to its oxidation rate and aluminum was limited to its oxide dissolution rate.
© 2002 The Electrochemical Society. 关DOI: 10.1149/1.1449954兴 All rights reserved.
Manuscript submitted April 16, 2001; revised manuscript received October 19, 2001. Available electronically February 7, 2002.
Chemical mechanical polishing共CMP兲 has long been recognized as a promising technique for global planarization to delineate metal patterns for submicrometer integrated circuit共IC兲 processing.1-3 Alu-minum共Al兲 and its alloys have traditionally been used as multilevel interconnects and have emerged as the most important material for such applications. On the other hand, it is well known that titanium 共Ti兲 is an effective metal barrier.4The total process time for Al CMP
is also controlled by the removal of the Ti diffusion barrier. Little research has been devoted to the CMP of this barrier film. Since Ti is harder than Al, a lower Ti removal rate is therefore expected.
Because Al is soft and easily scratched, polishing on a softer pad has therefore been suggested to avoid severe surface damage, but this technique can produce unwanted pattern geometry effects like metal dishing and interlevel dielectric共ILD兲 erosion.5Therefore, a two-step CMP process has usually been implemented. For the first step, the overburden Al would be planarized for the step-high reduc-tion, be removed faster and uniformly, and stopped as Ti exposed. For the second step, both Al and Ti outside of trenches would be removed simultaneously and stopped as the ILD exposed. The con-trol of removal selectivity is therefore very critical in the second step. In the ideal case, Al, Ti, or TiN and ILD would be expected to remove at the same rate, or it would result in metal dishing, oxide erosion, and surface nonplanarity. Overpolishing for the complete removal of overburden metals outside of trenches would further in-duce surface nonplanarity.
Kaufman et al.6has proposed a desirable removal mechanism for metal CMP which involves the oxidation of metal to form passive surface oxide and dissolution of this metal oxide under polishing stress. No direct metal corrosion to form soluble metallic ions or direct mechanical abrasion on nonoxidized metal substrates would be allowed for CMP, and it would result in the issues of metal corrosion and surface scratching. The formation and dissolution of oxide passive film on a given metal surface would strongly depend on the oxidizing conditions in the aqueous media controlled by the slurry formulation.
One may obtain useful information regarding the thermodynami-cally stable oxidized species of Al and Ti metals in a given pH aqueous medium from Pourbaix diagrams,7but not enough for the complicated, dynamically removed and reformed metal oxide sur-face during the polishing due to the absence of any kinetic
informa-tion. Both Al and Ti are reactive metals that owe their corrosion resistance to a thin, protective, barrier oxide surface layer which is stable in air and in most aqueous solutions. Regarding material re-moval during the metal CMP, the properties of the metal oxide on the surface is the key issue. But there is the question of whether the overall removal rate is limited to the oxidation rates of metal to form metal passive oxide, or the dissolution rates of this passive oxide into the aqueous slurry. Furthermore, both the rate of metal oxida-tion and the rate of metal oxide dissoluoxida-tion is modulated by the mechanical stress during polishing, like pressure, platen rotation ve-locity, and abrasive size.
Both the chemical and mechanical interactions should be taken into account simultaneously to elucidate the complicated material removal during polishing. There are two possible approaches to modeling the CMP process. One is to describe the CMP process as chemically assisted abrasion based on the contact wear between the abrasives and the substrate. The material removal is proportional to the volume of plastic deformation of substrate on which abrasive stress is imposed. The most difficult point of this approach, in prac-tice, is how to determine the change of mechanical hardness of the chemically softened layer on substrate or abrasives in a given slurry chemical formulation. The other way is that the stress-modulated corrosion 共or dissolution兲 based on the metal corrosion under a given slurry formulation and polishing stress could be well charac-terized by modern electrochemical techniques such as dc polariza-tion and ac impedance methods. One may suspect that the overall removal could be contributed only from metal corrosion, not partly from abrasion wear. Removal by microdebris wearing could happen as if polishing with the larger particles on the harder pad, which is not an acceptable CMP process due to the severe surface damage. Therefore, it is reasonable and practical to explore metal CMP by in situ electrochemical corrosion measurements during the polishing.
In this work, electrochemical impedance spectroscopy 共EIS兲 analyses of Al and Ti during polishing or in static conditions was performed to evaluate the influence of H2O2 and slurry pH on the
surface passivation of Al and Ti. From these results, the control of removal selectivity between Al and Ti by means of slurry formula-tion is discussed.
Experimental
The special designed apparatus based on the traditional three-electrode rotating-disk configuration for in situ CMP measurement is schematically shown in Fig. 1. The electrochemical cell consisted *Electrochemical Society Active Member.
of a metal cylinder as the rotating working electrode and a Pt mesh net and an Hg/HgSO4 half-cell electrode as the counter and
refer-ence electrodes, respectively. The three-electrode electrochemical cell was connected to an EG&G lock-in amplifier model 5301 and potentiostat model 273, and immersed in the slurry with a Rodel Politex regular E polishing pad at the bottom. The working elec-trodes were made of a Ti共99.99%兲 and Al 共1% Si-0.5% Cu兲 cylinder embedded in epoxy resin (1 cm2); only one side of the cylinder was exposed to the slurry.
For EIS measurements, the polishing condition was fixed at a 45 rpm rotational speed and 4 psi downward pressure, the amplitude of the perturbation was⫾10 mV, and the frequency varying from 0.1 Hz to 100 kHz at 6 steps/dec. The slurry used in the experiments contained 0.5 M citric acid and 5 vol % phosphoric acid as the pH buffer solution, 0.05m Al2O3 abrasive particles of 5 wt %, and
0-10 vol % H2O2as the main oxidizer. The slurry pH was adjusted
from 2 to 6 by adding KOH. Zview version 2.1a computer software was used for the curve fitting and analysis of the impedance.
Polishing experiments were carried out on a Westech 372M pol-isher with a Rodel Politex regular E polishing pad and Rodel R200-T3 carrier film. For the Al polishing process, the polishing parameters of pressure, platen and carrier rotary speeds, back pres-sure, and slurry flow rate were set to 4 psi, 60 and 65 rpm, 2 psi, and 150 mL/min, respectively. In the Ti polishing step, back pressure and slurry flow rate were the same as the Al polishing process, with platen and carrier rotary speeds adjusted to 45 and 42 rpm, respectively.
Results and Discussion
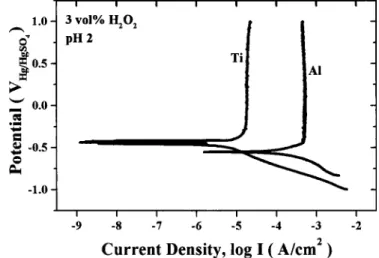
EIS for Ti without abrasion.—Figure 2 shows the potentiody-namic polarization curves for Ti and Al in a slurry of 3 vol % H2O2
at pH 2. It is evident from these polarization curves that both Ti and Al show an excellent passivation behavior and the corrosion poten-tial for Ti is considerably nobler than that for Al by 125 mV.
As exposed into the slurry of pH 2 without the addition of H2O2,
the EIS of Ti shows the presence of a stable thin metal oxide layer formed on the surface, as shown in Fig. 3. Furthermore, the imped-ances only increase slightly with exposure time, indicating that this oxide is quite stable after 20 min. In contrast, the impedance de-creases in the presence of H2O2; the higher the H2O2is added, the
more the impedance decreases, as shown in Fig. 4. The diameter of the frequency loop in the absence of H2O2 is significantly larger
than those in H2O2-added slurry. This implies that the polarization
resistance (RP) of the Ti oxide decreases in the presence of H2O2.
The fitted Rpand capacitance (C) in terms of a single time-constant
model composed of a RC parallel circuit are listed in Table I. Typi-cally, a two time-constant model is adopted to represent the system of metal oxide passivation; one time-constant is associated with the oxide and the other with the electrical double layer formed on the oxide surface. However, the double layer response at higher fre-quencies is negligible in comparison with the whole surface oxide, so the impedances responding at the frequencies we have studied in the range from 0.1 Hz to 100 kHz共as observed in Fig. 3 and 4兲 can be considered as the oxide on metal surface.
The fitted capacitance (C) of passivation oxide decreases slightly with increasing H2O2concentration共Table I兲. This could result as a
more porous metal oxide film forms, in contrast to the denser oxide film formed without H2O2. Porous oxide passivation formed in
H2O2 would also contribute to the lower polarization resistance Figure 1. Schematic of the in situ electrochemical measurement system.
Figure 2. Potentiodynamic polarization curve for Ti and Al in a slurry of 3 vol % H2O2at pH 2.
Figure 3. Nyquist plots for Ti experiencing various time intervals of immer-sion in a slurry of pH 2 without the addition of H2O2.
(Rp). This fact suggests that higher H2O2 concentrations would
cause higher oxidation rates due to the formation of more porous oxide.8-10Similar results were also obtained for Al.11
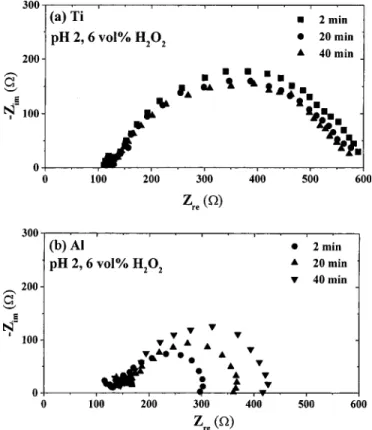
EIS for Ti with abrasion.—The EIS of in situ measurements during polishing Ti in a slurry of 6 vol % H2O2at pH 2 is shown in
Fig. 5a. The impedances do not change with time for abrasion and are much smaller than those without abrasion as shown in Fig. 4. This means that the oxide could be almost removed by abrasion and may reach a dynamic equilibrium between the oxidation formation and the abrasion removal. Similar results were obtained for polish-ing Ti with 3-10 vol % H2O2 slurry at pH 2. In other words, the
overall Ti removal rate is limited to its oxidation to form oxide, that is, more H2O2added into the slurry to increase the Ti corrosion rate,
so as to the removal rates of polishing Ti.
Figure 5b shows the EIS of the in situ measurements of polishing Al in the slurry with 6 vol % H2O2at pH 2. The impedance (RP)
increases with abrasion time, indicating that the passive oxide exists and would not be removed completely, instead of growing with abrasion time. As a consequence, the Al removal rate is limited to the oxide dissolution. However, if H2O2is lower than 3 vol %, the
impedance does not change with abrasion time. In an ideal metal CMP process, the metal being polished should be oxidized fast enough to be equal to the acceptable removal rate.12Thus, a balance between the mechanical abrasion and the chemical reaction is be-lieved to be the key issue for the metal CMP process. At low con-centration, H2O2 increases the oxidation rate, resulting in an
in-crease of the removal rate; however, for Al, excessive H2O2
concentration decreases the removal rate, since the oxidation rate exceeds the mechanical abrasion rate.
In this study, the formation of metal oxide passivating the metal plays a crucial role for Al and for Ti CMP. In a given aqueous corrosion environment, metal being polished could be oxidized to form soluble species, i.e., dissolution, or to form metal oxide through the passivation.
If dissolution dominates, removal rate is limited to the oxidation rate of metal being polished and behaves like wet etching. This is an unfavored condition for planarization and the surface damage, like roughness and scratches, and would be obtained due to direct abra-sion on the soft metal substrate. In addition, no stable oxide after polishing would lead to severe metal corrosion for post-CMP clean-ing. This is the typical problem for Cu CMP.13
Figure 6. The removal rates of polishing Al and Ti with the slurry formu-lated with 3 vol % H2O2at various slurry pH values.
Figure 4. Nyquist plots for Ti in a slurry of pH 2 with various H2O2
con-centrations after 2 min immersion.
Table I. Fitted parameters for Ti immersion in a slurry of pH 2 with various H2O2concentrations.
Parameters
H2O2 0 vol % 3 vol % 6 vol % 10 vol %
Capacitance, C共F兲 33.9 29.5 23.7 22.5
Resistance, Rp共k⍀兲 3615.7 53.4 36.3 22.9
Figure 5. Nyquist plots after various time intervals of abrasion in a slurry of 6 vol % H2O2at pH 2:共a兲 Ti and 共b兲 Al.
If oxidation dominates, removal rate would be limited to both the oxidation rate of metal and the dissolution rate of the oxide under the polishing stress. For polishing Ti and Al with hydrogen peroxide (H2O2) formulation, Ti removal is limited by the oxidation step to
the formation of TiO2. On the other hand, Al could be oxidized fast
and form Al2O3through the passivation of Al. The removal of Al is
limited to the dissolution step of Al2O3as a result of passivation.
Effect of pH.—If Ti could be removed faster than Al, overpolish-ing of Al would be shortened and the result of dishoverpolish-ing could be reduced. The removal selectivity can be changed by means of pol-ishing with various slurry pH values. Figure 6 shows that as slurry pH increases the removal rate of Al decreases, while that of Ti increases. At slurry pH⭓ 4, the removal rate of Ti is notably faster than that of Al, and in the range of slurry pH 3.0-3.5 the Al/Ti removal selectivity is expected to be unity. However, the unity re-moval selectivity of blanket Al/Ti is undesirable for a practically polished patterned wafer. Indeed, the removal of Ti outside the trenches on the larger oxide features would be slower than those of dense Al interconnecting wires, and result in Al dishing as overpol-ishing to remove Ti completely.
Figures 7a and b are Nyquist plots for Ti and Al without abrasion as varying with slurry pH with the addition of 3 vol % H2O2after 2
min of immersion. These plots indicate that the electrochemical re-actions are different but depend on the slurry pH. Figure 7a shows clearly that impedance decreases in slurry pH 6. On the contrary, for Al, as shown in Fig. 7b, impedance increases as slurry pH increases to 5 but drops at pH 6, exhibiting that the passivation on Al would be maximized between pH 5 and 6. Consequently, increasing the slurry pH enhances the dissolution rate of Ti but decreases the dis-solution rate of Al.
An in situ measurement for Ti in a slurry with addition of 3 vol % H2O2at pH 4 is shown in Fig. 8a. The EIS spectra do not change
with time of abrasion appreciably, and similar results are also ob-tained for slurry pH from 2 to 6. The dissolution rate of TiO2 is
faster than oxidation rate of Ti. On the other hand, the result of impedance for Al increases with time of abrasion, as shown in Fig. 8b, indicating that the oxides are not removed completely, becoming stable and dense and difficult to remove. Hence, the oxide dissolu-tion rate is lower than the oxidadissolu-tion rate of Al. Then the by-products Figure 7. Nyquist plots of the specimens without abrasion in a slurry of 3
vol % H2O2at various pH values after 2 min immersion:共a兲 Ti and 共b兲 Al.
Figure 8. Nyquist plots for Ti after different time intervals of abrasion in a slurry of 3 vol % H2O2at pH 4:共a兲 Ti and 共b兲 Al.
passivation; Al removal rate is limited to the oxide dissolution. 4. With an increase of the slurry pH, impedance decreases for Ti but increases for Al. Correspondingly, increasing the slurry pH value enhances the Ti removal rate but decreases the Al removal rate. The Al/Ti removal selectivity is expected to be unity in the slurry pH 3.0-3.5 range with 3 vol % H2O2.
Acknowledgment
The authors are grateful for support of this work by the National Science Council共NSC兲 of Republic of China under grant NSC 89-2218-E-007-005.
als, 10, 166共1989兲.
10. J. Pan, D. Thierry, and C. Leygraf, Electrochim. Acta, 41, 1143共1996兲. 11. J. W. Hsu, S. Y. Chiu, I. C. Tung, Y. L. Wang, B. T. Dai, M. S. Tsai, M. S. Feng, and
H. C. Shih, in Chemical Mechanical Planarization in IC Device Manufacturing III, R. L. Opila, I. Ali, Y. A. Arimoto, Y. Homma, C. Reidsema-Simpson, and K. B. Sundaram, Editors, PV 99-37, p. 311, The Electrochemical Society Proceedings Series, Pennington, NJ共1999兲.
12. L. M. Cook, J. F. Wang, D. B. James, and A. R. Sethuraamn, Semicond. Int., 141
共Nov 1995兲.
13. A. Beverina, H. Bernard, J. Palleau, J. Torres, and F. Tardif, Electrochem.
Solid-State Lett., 3, 156共2000兲.
14. J. M. Steigerwald, S. P. Murarka, D. J. Duquette, and R. J. Gutmann, Mater. Res.