CORRELATIONS IN CETACEAN LIFE HISTORY TRAITS
全文
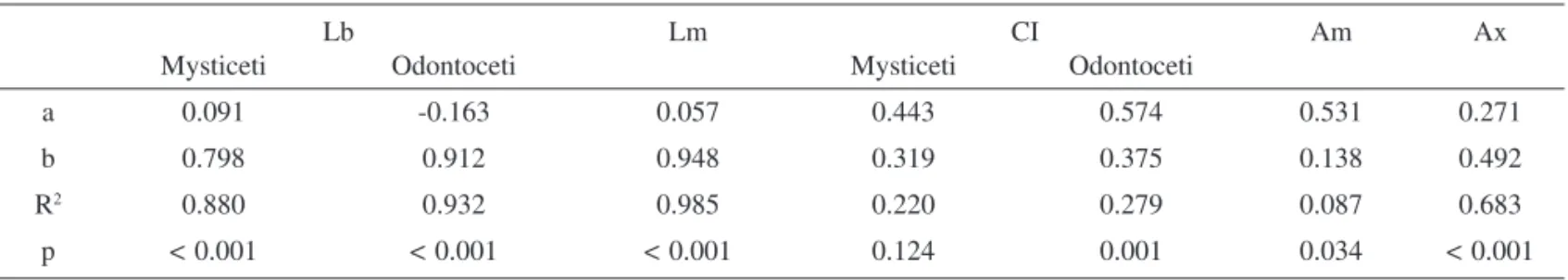
(2) Huang et al.: Correlations in cetacean life history traits Table 1. Effect (F-value) of Lx on Lb, Lm, CI, Am and Ax in cetaceans by GLM and significant tests between suborders Mysticeti and Odontoceti. Effect. Lb. Lm. CI. Am. Ax. Lx. 875.01. 1427.7. 12.93. 9.35. 35.74. Suborder. 9.75. 3.34NS. 11.46. 3.57NS. 0.053NS. NS: not significant at p > 0.05 Table 2. Coefficient of allometry regression to Lx in cetaceans, logY = a + blogLx, where Y represents Lb, Lm, CI, Am and Ax. The effect of suborder on Lb and CI were considered but excluding Lm, Am and Ax. Lb. Lm. Mysticeti. Odontoceti. a. 0.091. -0.163. b. 0.798. 0.912. R. 2. p. CI. Am. Ax. 0.574. 0.531. 0.271. 0.375. 0.138. 0.492. Mysticeti. Odontoceti. 0.057. 0.443. 0.948. 0.319. 0.880. 0.932. 0.985. 0.220. 0.279. 0.087. 0.683. < 0.001. < 0.001. < 0.001. 0.124. 0.001. 0.034. < 0.001. Bradford et al., 2006). Some studies have shown that s0 is strongly affected by the size at birth (Pontier et al., 1993; Beekman et al., 1999; Chambellant et al., 2003; McMahon & Hindell, 2003). In other words, the maternal size, the size at birth, the size and age at sexual maturity, the calving interval, and lifespan are the potential LHT that significantly affect reproductive fitness in cetaceans. The best way of obtaining first-hand LHT is to follow the fate of individuals and record the size and age at different stages of growth. However, this approach is constrained by the long lifespan of these organisms. Meta-analysis of existing databases is another alternative (Barreto & Rosas, 2006). Nonetheless, constructing a detailed database usually requires massive specimen collection from lethal operations. In cetaceans, many data come from fishery activities (Archer & Robertsona, 2004), incident stranding events, or both (Barreto & Rosas, 2006), which generally excludes the rare and endangered species. Unlike terrestrial mammals, many cetaceans are too large to be manipulated directly, or too long-lived to be monitored for generations. In addition, their strictly aquatic lifestyle makes them almost inaccessible to most researchers. Finding reliable, easy-to-use and, most of all, non-lethal ways to estimate LHT in cetaceans requires immediate attention, especially when some species are in decline, endangered or at the verge of extinction. The potential method to reliably extrapolate the LHT of undocumented species is to use the allometric relationships in LHT, expressed by: Y = aXb ’ logY = loga + b logX (Blueweiss et al., 1978) [2] where Y and X represent the correlated LHT, a is the allometric coefficient and b the allometric exponent (Blueweiss et al., 1978; Allaine et al., 1987; Stearns, 1992). However, directly using bi-variate regressions without accounting for potential confounding factors, especially body. size, may lead to over-simplified results (Stearns, 1992). Therefore, eliminating potential confounding effects before regressions is necessary. In this study, we seek to clarify the interactions in cetaceans’ LHT and propose reliable expressions. In these analyses, the variation between the two suborders of cetaceans, baleen whales (Mysticeti) and toothed whales (Odontoceti), is considered. We focus particularly on the estimation of Ax which is one of the most difficult LHT to access, requiring life-long tracking or a data from a huge number of dead, mature cetaceans.. MATERIALS AND METHODS Data collection. – We collected six LHT, i.e. female asymptotic length (Lx), length at birth (Lb), length at sexual maturity (Lm), age at sexual maturity (Am), calving interval (CI) and life span (Ax), based on data sourced from scientific journals, scientific reports and biological books published by scientific societies. When data were provided as a range rather than a fixed value, the midpoint of the range was taken. When there was more than one record published for a species, we calculated the midpoint of each published value and took the average of these midpoints in our operation (Kovacs & Lavign, 1986; Allaine et al., 1987; Pontier et al.,1993; Silva, 1998; Trites & Pauly, 1998; Read, 2001; Schulz & Bowen 2004). Statistical analysis. – The correlations in LHT were first explored by the allometric relationship (expression [2]): logY = loga + b logX, where X was Lx and Y represented Lb, Lm, CI, AM and Ax. All LHT data were log-transformed and analysed by general linear model (GLM). The effect of suborders Mysticeti and Odontoceti on the allometries was also analysed by GLM. The applicability was set at R2 ≥ 0.50 (Blueweiss et al., 1978).. 286. 25_Huang_Pg 285-292.indd 286. 12/11/08 2:27:45 PM.
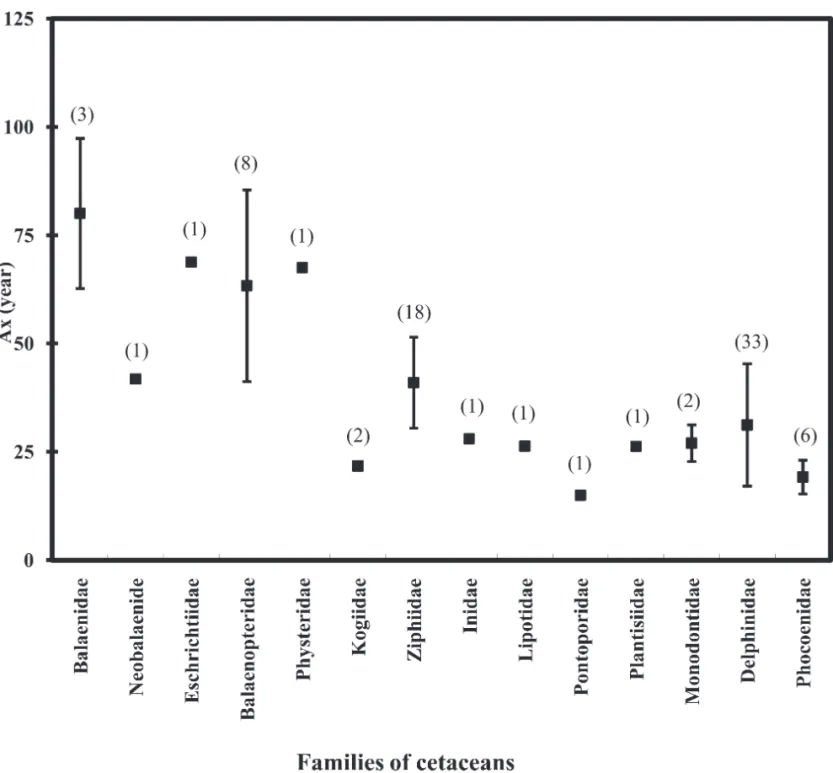
(3) THE RAFFLES BULLETIN OF ZOOLOGY 2008 Table 3. Correlation of residuals in life history traits after removing the confounding Lx. Lb. Lm. CI. Lm. 0.502. –. –. Am. CI. -0.099. -0.106. –. –. Am. -0.142. -0.007. 0.632. –. Ax. -0.074. -0.149. 0.509. 0.572. Table 4. The determination of Ax by stepwise GLM. The effect of CI was not significant and therefore removed, which yielded the generalized expression: logAx = -0.017 + 0.435 logLx + 0.463 logAm or Ax = 0.962 Lx0.435 Am0.463, R2=0.782.. Effect Constant Lx Am CI. F – 91.032 8.828 2.343. To further explore the interactions in LHT while avoiding the confounding effect of Lx, the correlation of residuals was analysed (Stearns, 1992). The residuals of each LHT (ε), except for Lx, was calculated by the following equation:. ε = log Y – (log a + b x logLx) (Stearns, 1992). [3],. where Y represents Lb, Lm, Am, CI and Ax. Finally, Pearson correlation coefficients were calculated with significance (p < 0.05) determined by Bonferroni tests.. RESULTS The GLM results indicate that Lx had significant effect on Lb, Lm, CI, Am and Ax in cetaceans (Table 1). However, the effect of suborder affected homogeneity significantly for both Lb and CI, but not for Lm, Am and Ax (Table 1). Therefore, the allometric regressions on Lb and CI were tested separately for different suborders, while Lm, Am and Ax were explored for cetaceans as a group. For Am and CI, their R2 values were too low (R2 = 0.086 and 0.279, respectively) (Table 2) to extrapolate to other undocumented species. Therefore, four general expressions were left to estimate Lb (within two cetaceans groups), Lm and Ax of cetaceans: logLb = 0.091 + 0.0796 logLx (Lb = 1.234 Lx0.796) for baleen whales [4], logLb = -0.163 + 0.932 logLx (Lb = 0.687 Lx0.932) for toothed whales [5], logLm = 0.057 + 0.948 logLx (Lm = 1.140 Lx0.948) [6], and logAx = 0.271 + 0.492 logLx (Ax = 1.865 Lx0.492). [7].. The residuals of Lb, Lm, CI, Am and Ax and their correlations were calculated (Table 3). Lb and Lm were significantly. p – < 0.001 0.005 0.135. Coefficeint –0.017 0.435 0.463 –. correlated with each other (r = 0.50, Bonferroni p = 0.001), but neither of them were correlated with CI, Am and Ax. We therefore conclude that size at birth and at sexual maturity do not have a direct effect on life span, calving interval, and age at sexual maturity in cetaceans. Furthermore, both CI (r = 0.509, Bonferroni p = 0.001) and Am (r = 0.572, Bonferroni p < 0.001) were significantly correlated with Ax. Nonetheless, they were also significantly correlated with each other (r = 0.632, Bonferroni p < 0.001) and this required further exploration. By stepwise GLM, either forward or backward, the effect of CI on Ax was not significant (Table 4). That is, the seeming correlation between CI and Ax indeed comes from the confounding Am. Consequently, we presented another general proxy expression of Ax determination with a higher R2, Ax = 0.962 Lx0.435 Am0.463, R2 = 0.782. [8].. DISCUSSION Ax of 45 species has so far been documented. By expressions [7] and [8], we expand current knowledge about cetaceans’ lifespan to 79 species of 14 families (Fig. 1). In general, baleen whales and the sperm whale (Physeter macrocephalus) live longer, from 40 years for the pygmy right whale (Caperea maginata) to more than 100 years for the bowhead whale (Balaena mysticetus), than medium or small cetaceans, that live from 14 years for the harbour porpoise (Phocoena phocoena) to approximately 60 years for pilot whales (Globicephala macrorhychus and G. melaena). Cetaceans are phylogenetically divided into two suborders, Mysticeti for baleen whales and Odontoceti for toothed cetaceans. Baleen whales are quite different from toothed cetaceans in morphology, anatomy, body size, feeding niches, reproductive energetics and mating systems (Oftedal, 1997; Boness et al., 2002). Any potential variations in life. 287. 25_Huang_Pg 285-292.indd 287. 12/11/08 2:27:45 PM.
(4) Huang et al.: Correlations in cetacean life history traits histories between baleen and toothed cetaceans may therefore result from the differences in these traits. However, this study shows that when the confounding Lx is factored out, variations in five LHT (Lb, Lm, Am, CI and Ax) do not always associate with the phylogenetic difference, only Lb and CI are significantly different between baleen and toothed whales but Lm, Am and Ax are not. Factors potentially affecting length at birth (Lb) and calving interval (CI) Size at birth is determined by maternal investment during gestation (Kovacs & Lavigne, 1986, 1992; Pontier et al., 1993; Boltnev & York, 2001; Read, 2001; Gorman & Nager, 2004). Prenatal investment depends on the type and availability of the food resources (Leon & De Nobrega, 2000; Georges & Guinet, 2001). Baleen whales primarily feed on plankton while toothed whales feed on fishes, cephalopods and other marine mammals. For toothed whales, their feeding. may be primarily limited by time and efficiency rather than seasonality of available prey (Benoit-Bird, 2004). In contrast, the seasonality of prey availability is critical for baleen whales. The patterns of foraging during gestation are quite different between baleen and toothed whales. Baleen whales reduce their feeding success and nourish their offspring using their somatic reservoirs (Lockyer, 1984; Oftedal, 1997) while toothed cetaceans concurrently feed themselves during gestation (Yasui & Gaskin, 1986; Cheal & Gales, 1991; Kastelein et al., 1993, Kastelein et al., 2002), corresponding to two forms of reproductive energetics: “capital” and “income” breeders (Stearns, 1992; Jonsson, 1997; Bonnet et al., 1998). Capital breeders such as baleen whales acquire and store energy in advance of the reproduction. Conversely, income breeders such as toothed cetaceans elevate their feeding levels concurrently with the breeding to meet the cost of reproduction. The different slopes of allometries in Lb between baleen and toothed cetaceans may potentially come from a combination of their different reproductive energetics and their different diets.. Fig. 1. Mean and range ( ± SD) of longevity (Ax) in cetaceans. Numbers above each family represent number of available species.. 288. 25_Huang_Pg 285-292.indd 288. 12/11/08 2:27:45 PM.
(5) THE RAFFLES BULLETIN OF ZOOLOGY 2008 For capital breeders, availability and seasonality of potential food may have important effects on CI (Stearns, 1992; Jonsson, 1997). In baleen whales, Reeves et al. (2001) reported an inverse relationship between CI and marine productivities, however, body size had little effect on CI, consistent with our own findings. On the other hand, organisms in resource-rich and predictable environments usually use income strategies, coupled with long CI to reduce their daily energy expenditure (Johnsson, 1997; Dufour & Sauther, 2002). Organisms with large body size generally have longer CI (Blueweiss et al., 1978; Stearns, 1983), potentially due to longer growth time, longer period of maternal dependence, and skewing to K-strategy end of life histories (Allaine et al., 1987). The social parental care system, especially when the alloparent helper in a cooperative system is included (Geffen et al., 1996), sometimes results in extra-long CI. In toothed whales, such as the killer whale (Orcinus orca) and the sperm whale (Physter macrocephalus), have complex maternal social linkages and very long CI (Best et al., 1984; Perrin & Reilly, 1984; Whitehead, 1996; Whitehead et al., 2004; Foote 2008). Nonetheless, high sociability is not necessarily associated with large body size. For example, the beaked whales—toothed whales comparable in size to killer whales—do not have high sociability (Gowans et al., 2001) and seldom have long CI. Furthermore, many smallsized mammals also feature alloparental-care reproductive systems, further complicating and reducing the correlation of CI to body size. Therefore, the statistically significant correlation between CI and body size in toothed cetaceans may be the joint result of body size, reproductive energetics and sociability. Factors adjusting lifespan (Ax) The potential Ax is determined by both intrinsic and extrinsic mortality. The asymptotic size is one of the most direct and decisive parameters, either in theoretical approaches (Charnov, 1991) or in empirical approaches (as this study). Life history theory predicts low mortality will result in long Ax and large body size (Charnov, 1991; Stearns 1992). However, the evolution of body size is also shaped by phylogeny, resource availability and environmental conditions (Brown et al., 1993; Diniz-Filho & Veira, 1998; Symonds & Elgar, 2002; Savage et al., 2004; West et al., 2004). When the effect from phylogeny is factored out, organisms that live in resource-rich and environment-stable patches are usually large and have a long lifespan. The age at sexual maturity is another important parameter in determining Ax (Charnov, 1991; Stearns, 1992). When organisms attain sexual maturity and start to reproduce, their mortality increases and Ax shortens due to aging after conception. Though organisms with large size generally have low mortality, long lifespan and, perhaps, high reproductive fitness, it is usually accompanied with late sexual maturity (George et al., 1999). When environment stability is low or the resource distribution and availability is stochastic, life histories of early sexual maturity, small body size, high mortality and short Ax should increase the probability of successful reproduction.. The Ax is not only affected and constrained by intrinsic parameters but is also determined by extrinsic mortality. For cetaceans, extrinsic mortality comes from fishery bycatches, ship collisions (Kraus, 1990) and mother-calf separation (Noren & Edwards, 2007), for example. The fluctuation and availability of potential food also has profound effects on extrinsic mortality. For toothed cetaceans, the availability of their potential prey, rather than their feeding efficiency, will have a decisive effect on the daily energetic maintenance (Benoit-Bird, 2004). Even a shortage of food for one to two days can have catastrophic effects on their lives. On the other hand, for baleen whales, characterized by their huge body size and non-continuous feeding, short-term fluctuation and availability of food seemingly has less effect on mortality because of huge body size and associated fat reservoirs. However, the distribution and amount of their potential prey, primarily plankton, is largely dependent on the primary productivity of ocean systems. Large-scale environment changes, such as those predicted with global climate change, will alter the distribution and seasonality of their potential food (Neira & Arancibia, 2004; Richardson & Schoeman, 2004; MacLeod et al., 2005), potentially affecting their mortality and the consequent Ax. In some situations, extrinsic mortality may also affect the intrinsic parameters and further reduce Ax. When a stock or species is over-exploited, compensation effects result in early maturation, shortening Am, reducing asymptotic size and finally shortening Ax (Lockyer, 1984; Kasuya, 1991; Chivers & Demaster, 1994; Kasuya, 1999). For example, the Am declined from 10 to 6 years in the fin whale (Balaenoptera physalus), 11.4 to 7 years in the sei whale (B. borealis) and 14 to 6 years in the minke whale (B. acutorostrata) (Lockyer, 1984). Unfortunately, the extent to how over-exploitation affects the asymptotic size or the Ax which in cetaceans is unknown. In future studies examining life histories and stock persistence of cetaceans, the net effect from extrinsic mortality, especially from anthropogenic mortality, on Lx and Am should be monitored because they both are fundamental to cetacean lifespan.. Empirical CI and AM are required Over-extrapolating the observed regressions, especially those which are statistically significant but with low R2 (< 0.50), such as CI and Am of the cetaceans in this study, should be viewed with caution (Blueweiss et al., 1978). That is, only through direct measurements, either non-lethal or lethal, can identify CI and Am of the remaining undocumented species. This work requires immediate attention because many of these species are rare and/or endangered. Longterm fishery activities should be continuously monitored, not only for evaluating if the annual removal exceeds the PBR (potential biological removal) (Wade, 1998), but also because it provides first hand data on CI and Am with minimal lethal specimen collection. This work should given highest priority in future studies, not only to better cetacean LHT but also to ensure the long-term existence of these ocean giants.. 289. 25_Huang_Pg 285-292.indd 289. 12/11/08 2:27:47 PM.
(6) Huang et al.: Correlations in cetacean life history traits ACKNOWLEDGEMENTS Our thanks go to the anonymous reviewers for critically reading the manuscript and offering valuable suggestions. This study was funded by Ministry of Education (96529001A4, 97529002C6) and National Science Council of Taiwan (NSC 95-2621-B-019-004).. LITERATURE CITED Aguilar, A. & A. Borrell, 1994. Reproductive transfer and variation of body load of organochlorine pollutants with age in fin whales (Balaenoptera physalus). Archives of Environmental Contamination and Toxicology, 27: 546–554. Allaine, D., D. Pontier, J. M.Gaillard, J. D. Lebreton, J. Trouvilliez & J. Colbert, 1987. The relationship between fecundity and adult body weight in homeotherms. Oecologia, 73: 478–480.. Bradford, A. L., P. R. Wade, D. W. Weller, A. M. Burdin, Y. V. Ivashchenko, G. A. Tsidulko, G. R. VanBlaricom & R. L. Brownell Jr., 2006. Survival estimates of western gray whales Eschrichtius robustus incorporating individual heterogeneity and temporary emigration. Marine Ecology Progress Series, 315: 293–307. Brown, J. H., P. A. Marquet & M. L. Taper, 1993. Evolution of body size: Consequences of an energetic definition of fitness. American Naturalist, 142: 573–584. Chambellant, M., G. Beauplet, C. Guinet & J.-Y. Georges, 2003. Long-term evaluation of pup growth and preweaning survival rates in subantarctic fur seals, Arctocephalus tropicalis, on Amsterdam Island. Canadian Journal of Zoology, 81: 1222–1232. Charnov, E. L., 1991. Evolution of life history variation among female mammals. Proceedings of the National Academy of Sciences of the United States of America, 88: 1134–1137. Charnov, E. L., 2001. Reproductive efficiencies in the evolution of life histories. Evolutionary Ecology Research, 3: 873–876.. Archer, F. I. & K. M. Robertsona, 2004. Age and length at weaning and development of diet of pantropical spotted dolphins, Stenella attenuata, from the Eastern Tropical Pacific. Marine Mammal Science, 20: 232–245.. Cheal, A. J. & N. J. Gales, 1991. Body mass and food intake in captive breeding bottlenose dolphins, Tursiops truncatus. Zoo Biology, 10: 451–456.. Barlow, J. & P. J. Clapham, 1997. A new birth-interval approach to estimating demographic parameters of humpback whales. Ecology, 78: 535–546.. Chivers, S. J. & D. P. Demaster, 1994. Evaluation of biological indices for three eastern Pacific dolphin species. Journal of Wildlife Management, 58: 470–478.. Barreto, A. S. & F. C. W. Rosas, 2006. Comparative growth analysis of two populations of Pontoporia blainvillei on the Brazilian coast. Marine Mammal Science, 22: 644–653.. Clapham, P., J. Barlow, M. Bessinger, T. Cole, D. Mattila, R. Pace, D. Palka, J. Robbins & R. Seton, 2003. Abundance and demographic parameters of humpback whales from the Gulf of Maine, and stock definition relative to the Scotian Shelf. Journal of Cetacean Research and Management, 5: 13–22.. Beekman, S. P. A., B. Kemp, H. C. M. Louwman & B. Colenbrander, 1999. Analyses of factors influencing the birth weight and neonatal growth rate of Cheetah (Acinonyx jubatus) cubs. Zoo Biology, 18:129–139. Bejder, L., A. Samuels, H. Whitehead, N. Gales, J. Mann, R. Connor, M. Heithaus, J. Watson-Capps, C. Flaherty & M. Krutzen, 2006. Decline in relative abundance of bottlenose dolphins exposed to long-term disturbance. Conservation Biology, 20: 1791–1798.. Dans, S. L., M. K. Alonso, S. N. Pedraza & E. A. Crespo, 2003. Incidental catch of dolphins in trawling fisheries off Patagonia, Argentina: Can populations persist? Ecological Applications 13: 754–762. Diniz-Filho, J. A. & C. M. Veira, 1998. Patterns and processes in body size evolution of South American carniovores. Revista Brasileira de Biologia, 58: 649–657.. Benoit-Bird, K. J., 2004. Prey caloric value and predator energy needs: foraging predictions for wild spinner dolphins. Marine Biology, 145: 435–444.. Dufour, D. L. & M. L. Sauther, 2002. Comparative and evolutionary dimensions of the energetics of human pregnancy and lactation. American Journal of Human Biology, 14: 584–602.. Best, P. B., A. Brandao & D. S. Butterworth, 2001. Demographic parameters of southern right whales off South Africa. Journal of Cetacean Research and Management (Special Issue) 2: 161–169.. Ferguson, S. H. & J. W. Higdon, 2006. How seals divide up the world: environment, life history, and conservation. Oecologia, 150: 318–329.. Best, P. B., P. A. S. Canham & N. Macleod, 1984. Patterns of reproduction in sperm whales, Physter macrocephalus. Report of the International Whaling Commission (Special Issue 6): 51–79.. Fernandez, A., J. F. Edwards, F. Rodriguez, A. E. de los Monteros, P. Herraez, P. Castro, J. R. Jaber, V. Martin & M. Arbelo, 2005. Gas and fat embolic syndrome involving a mass stranding of beaked whales (Family Ziphiidae) exposed to anthropogenic sonar signals. Veterinary Pathology, 42: 446–457.. Blueweiss, L., H. Fox, V. Kudzma, D. Nakashima, R. Peters & S. Sams, 1978. Relationships between body size and some life history parameters. Oecologia, 37: 257–272.. Foote, A. D., 2008. Mortality rate acceleration and post-reproductive lifespan in matrilineal whale species. Biology Letters 4: 189–191.. Boltnev, A. I. & A. E. York, 2001. Maternal investment in northern fur seals (Callorhinus ursinus): Interrelationships among mothers’ age, size, parturition date, offspring size and sex ratios. Journal of Zoology, 254: 219–228.. Geffen, E., M. E. Gompper, J. L. Gittleman, H. K. Luh, D. W. MacDonald & R. K. Wayne, 1996. Size, life-history traits, and social organization in the Canidae: A reevaluation. American Naturalist, 147: 140–160.. Boness, D. J., P. J. Clapham & S. L. Mesnick, 2002. Life History and Reproductive Strategies. In: Hoelzel, A. R. (eds.), Marine Mammal Biology: an Evolutionary Approach. Wiley-Blackwell Science Ltd. Pp. 278-324.. George, J. C., J. Bada, J. Zeh, L. Scott, S. E. Brown, T. O’Hara & R. Suydam, 1999. Age and Growth estimates of bowhead whales (Balaena mysticetus) via aspartic acid racemization. Canadian Journal of Zoology, 77: 571–580.. Bonnet, X., D. Bradshaw & R. Shine, 1998. Capital versus income breeding: An ectothermic perspective. Oikos, 83: 333–342.. 290. 25_Huang_Pg 285-292.indd 290. 12/11/08 2:27:47 PM.
(7) THE RAFFLES BULLETIN OF ZOOLOGY 2008 Georges, J.-Y. & C. Guinet, 2001. Prenatal investment in the subantarctic fur seal, Arctocephalus tropicalis. Canadian Journal of Zoology, 79: 601–609. Gorman, H. E. & R. G. Nager, 2004. Prenatal developmental conditions have long-term effects on offspring fecundity. Proceedings of the Royal Society Biological Sciences Series B, 271: 1923–1928. Gowans, S., H. Whitehead, S. K. Hooker, 2001. Social organization in northern bottlenose whales, Hyperoodon ampullatus: Not driven by deep-water foraging? Animal Behaviour, 62: 369–377. Jefferson, T. A., 2000. Population biology of the Indo-Pacific humpbacked dolphin in Hong Kong waters. Wildlife Monographs, 144: 1–65. Jonsson, K. I., 1997. Capital and income breeding as alternative tactics of resource use in reproduction. Oikos, 78: 57–66. Kastelein, R. A., J. McBain, B. Neurohr, M. Mohri, S. Saijo, I. Wakabayashi & P. R. Wiepkema, 1993. The food consumption of Commerson’s dolphins (Cephalorhynchus commersonii). Aquatic Mammals, 19: 99–121.. 39degreeS). Journal of Experimental Marine Biology and Ecology, 312: 349–366. Noren, S. R. & E. F. Edwards, 2007. Physiological and behavioral development in delphinid calves: Implications for calf separation and mortality due to tuna purse-seine sets. Marine Mammal Science, 23: 15–29. Oftedal, O. T., 1997. Lactation in whales and dolphins: Evidence of divergence between baleen- and toothed-species. Journal of Mammalian Gland Biology Neoplasia, 2: 205–230. Parra, G. J., P. J. Corkeron & H. Marsh, 2004. The Indo-Pacific humpback dolphin, Sousa chinensis (Osbeck, 1765), in Australian waters: A summary of current knowledge. Aquatic Mammals, 30: 197–206. Perrin, W. F. & S. B. Reilly, 1984. Reproductive parameters of dolphins and small whales of the Family Delphinidae. Report of the International Whaling Commission (Special Issue 6): 97–133. Pontier, D., J.-M. Gaillard, D. Allaine, 1993. Maternal investment per offspring and demographic tactics in placental mammals. Oikos, 66: 424–430.. Kastelein, R. A., N. Vaughan, S. Walton & P. R. Wiepkema, 2002. Food intake and body measurements of Atlantic bottlenose dolphins (Tursiops truncatus) in captivity. Marine Environmental Research, 53: 199–218.. Read, A. J., 2001. Trends in the maternal investment of harbour porpoises are uncoupled from the dynamics of their primary prey. Proceedings of the Royal Society Biological Sciences Series B, 268: 573–577.. Kasuya, T., 1991. Density dependent growth in North Pacific Ocean sperm whales. Marine Mammal Science, 7: 230–257.. Reeves, R. R., R. Rolland & P. J. Clapham, 2001. Causes of reproductive failure in North Atlantic Right Whales: new avenues of research. Reports of a workshop held 26–28 April 2000, Falmouth, Massachusetts. Northeast FisherScience Center Reference Documents 01-16; 46 p. Available from: National Marine Fisheries Service, 166 Water St., Woods Hole, MA 02543-1026.. Kasuya, T., 1999. Review of the biology and exploitation of striped dolphins in Japan. Journal of Cetacean Research and Management, 1: 81–100. Kovacs, K. M. & D. M. Lavigne, 1986. Maternal investment and neonatal growth in Phocid seals. Journal of Animal Ecology, 55: 1035–1052. Kovacs, K. M. & D. M. Lavigne, 1992. Maternal investment in Otariid seals and walruses. Canadian Journal of Zoology, 70: 1953–1964. Kraus, S. D., 1990. Rates and potential causes of mortality in North Atlantic right whales (Eubalaena glacialis). Marine Mammal Science, 6: 278–291. Leon, J. A. & J.R. De Nobrega, 2000. Comparative statics of joint reproductive allocation. Journal of Theoretical Biology, 205: 563–579. Lockyer, C., 1984. Review of baleen whale (Mysticeti) repduction and implications for management. Report of International Whaling Commission (Special Issue 6): 27–50. MacLeod, C. D., S. M. Bannon, G. J. Pierce, C. Schweder, J. A. Learmonth, J. S. Herman & R. J. Reid, 2005. Climate change and the cetacean community of north-west Scotland. Biological Conservation, 124: 477–483. Mann, J., R. C. Connor, L. M. Barre & M. R. Heithaus, 2000. Female reproductive success in bottlenose dolphin (Tursiops sp.): life history, habitat, provisioning, and group-size effects. Behavioral Ecology, 11: 210–219. McMahon, C. R. & M.A. Hindell, 2003. Twinning in southern elephant seals: the implications of resource allocation by mothers. Wildlife Research 30: 35–39. Metcalf, C. J. E. & S. Pavard, 2006. Why evolutionary biologists should be demographers. Trends in Ecology and Evolution, 22: 205–212. Neira, S. & H. Arancibia, 2004. Trophic interactions and community structure in the upwelling system off Central Chile (33-. Richardson, A. J. & D. S. Schoeman, 2004. Climate impact on plankton ecosystems in the Northeast Atlantic. Science, 305: 1609–1612. Savage, V. M., J. F. Gillooly, J. H. Brown, G. B. West & E. L. Charnov, 2004. Effects of body size and temperature on population growth. American Naturalist, 163: 429–441. Schulz, T. M. & W. D. Bowen, 2004. Pinniped lactation strategies: Evaluation of data on maternal and offspring life history traits. Marine Mammal Science, 20: 86–114. Silva, M., 1998. Allometric scaling of body length: elastic or geometric similarity in mammalian design. Journal of Mammalogy, 79: 20–32. Small, R. J. & D. P. Demaster, 1995. Survival of five species of captive marine mammals. Marine Mammal Science, 11: 209–226. Stearns, S. C., 1983. The influence of size and phylogeny on patterns of covariation among life-history traits in the mammals. Oikos, 41: 173–187. Stearns, S. C., 1992. The Evolution of Life Histories. Oxford University Press, Oxford Symonds, M. R. E. & M. A. Elgar, 2002. Phylogeny affects estimation of metabolic scaling in mammals. Evolution, 56: 2330–2333. Trites, A. W. & D. Pauly, 1998. Estimating mean body masses of marine mammals from maximum body lengths. Canadian Journal of Zoology, 76: 886–896. Wade, P. R., 1998. Calculating limits to the allowable humancaused mortality of cetaceans and pinnipeds. Marine Mammal Science, 14: 1–37.. 291. 25_Huang_Pg 285-292.indd 291. 12/11/08 2:27:48 PM.
(8) Huang et al.: Correlations in cetacean life history traits West, G. B., J. H. Brown & B. J. Enquist, 2004. Growth models based on first principles or phenomenology? Functional Ecology, 18:188–196.. Yasui, W. Y. & D. E. Gaskin, 1986. Energy budget of a small cetacean: the harbour porpoise Phocoena phocoena L. Ophelia, 25: 183–198.. Whitehead, H., 1996. Babysitting, dive synchrony, and indications of alloparental care in sperm whales. Behavioral Ecology and Sociobiology, 38: 237–244.. Ylitalo, G. M., C. O. Matkin, J. Buzitis, M. M. Krahn, L. L. Jones, T. Rowles & J. E. Stein, 2001. Influence of life-history parameters on organochlorine concentrations in free-ranging killer whales (Orcinus orca) from Prince William Sound, AK. Science of the Total Environment, 281: 183–203.. Whitehead, H., L. Rendell, R. W. Osborne & B. Wursig, 2004. Culture and conservation of non-humans with reference to whales and dolphins: review and new directions. Biological Conservation, 120: 427–437.. 292. 25_Huang_Pg 285-292.indd 292. 12/11/08 2:27:48 PM.
(9)
數據

相關文件
In the inverse boundary value problems of isotropic elasticity and complex conductivity, we derive estimates for the volume fraction of an inclusion whose physical parameters
Robinson Crusoe is an Englishman from the 1) t_______ of York in the seventeenth century, the youngest son of a merchant of German origin. This trip is financially successful,
fostering independent application of reading strategies Strategy 7: Provide opportunities for students to track, reflect on, and share their learning progress (destination). •
Strategy 3: Offer descriptive feedback during the learning process (enabling strategy). Where the
Akira Hirakawa, A History of Indian Buddhism: From Śākyamuni to Early Mahāyāna, translated by Paul Groner, Honolulu: University of Hawaii Press, 1990. Dhivan Jones, “The Five
There are existing learning resources that cater for different learning abilities, styles and interests. Teachers can easily create differentiated learning resources/tasks for CLD and
Microphone and 600 ohm line conduits shall be mechanically and electrically connected to receptacle boxes and electrically grounded to the audio system ground point.. Lines in
• Thresholded image gradients are sampled over 16x16 array of locations in scale space. • Create array of