Changes in ammonium ion content and glutamine synthetase activity in
rice leaves caused by excess cadmium are a consequence of oxidative
damage
Hsiu-Fang Chien, Chuan Chi Lin, Jen-Wu Wang, Chien Teh Chen and Ching Huei Kao*
Department of Agronomy, National Taiwan University, Taipei, Taiwan, Republic of China; *Author for correspondence (e-mail: kaoch@ccms.ntu.edu.tw; tel: +886-2-23698159; fax: +886-2-23620879)Received 5 January 2001; accepted in revised form 14 March 2001
Key words: Ammonium ion, Cadmium, Glutamine synthetase, Lipid peroxidation, Oryza sativa
Abstract
Ammonium ion accumulation and the decrease in glutamine synthetase (GS) activity induced by CdCl2 were investigated in relation to lipid peroxidation in detached rice leaves. CdCl2 was effective in increasing ammo-nium ion content, decreasing GS activity and increasing lipid peroxidation. Free radical scavengers (glutathione, thiourea, sodium benzoate) and an iron chelator (2,2⬘-bipyridine) were able to inhibit the decrease in GS activity and ammonium ion accumulation caused by CdCl2and at the same time inhibit CdCl2-induced lipid peroxida-tion. Paraquat, which is known to produce oxygen radicals, decreased GS activity, increased ammonium ion con-tent, and increased lipid peroxidation. GS1 appears to be the predominant isoform present. Excess Cd caused a decrease in GS1 but not in GS2 in detached rice leaves. An increase in lipid peroxidation preceded ammonium ion accumulation and the decrease in GS1 activity. These results suggest that the decrease in GS activity and the accumulation of ammonium ions in detached rice leaves are a consequence of oxidative damage caused by ex-cess Cd.
Abbreviations: BP – 2,2⬘-bipyridine, DW – dry weight, FW – fresh weight, GS – glutamine synthetase, GS1 – cytosolic form of GS, GS2 – chloroplastic form of GS, GSH – reduced glutathione, MDA – malondialdehyde, PQ – paraquat, SB – sodium bezoate, TU – thiourea
Introduction
Cadmium is one of the most toxic heavy metals with no known biological function. Although Cd is a non-redox active metal, it was found to produce oxidative stress in plant tissues. Cd-increased lipid peroxidation has been demonstrated in Phaseolus aureus seedlings (Shaw 1995), Phaseolus vulgaris roots and leaves (Chaoui et al. 1997), Helianthus annuus leaves (Gal-lego et al. 1996a, 1996b), Pisum sativum shoot and root tissues (Lozano-Rodriguez et al. 1997), Solanum tuberosum tubers (Stroinski and Zielezinska 1997), and Festuca rubra seedlings (Wang et al. 1997). However, no peroxidation was found in Cd-treated plants and hairy roots of Daucus carota (Sanita di Toppi et al. 1998, 1999).
Ammonium ions are a central intermediate of ni-trogen metabolism (Miflin and Lea 1976). GS is the key enzyme in ammonia assimilation and catalyzes the ATP-dependent condensation of ammonium ions with glutamate to produce glutamine (Miflin and Lea 1976). A decline in GS activity has been shown in leaves subjected to water stress, when exposed to ex-cess Cu, and during dark-induced senescence (Backer et al. 1986; Chen and Kao 1998; Chen et al. 1997; Lin and Kao 1998; Peeters and Van Laere 1992; Tho-mas 1978), which may result in, at least in part, an accumulation of ammonium ions in leaves. In fact, various investigators have been able to show the ac-cumulation of ammonium ions in leaves during se-nescence and under water stress and excess Cu con-ditions (Chen and Kao 1998; Chen et al. 1997; Lin
and Kao 1998). It is generally believed that GS ac-tivity in plants is regulated at the transcriptional level (Edwards et al. 1990; Forde et al. 1989; Hirel et al. 1987; Roche et al. 1993; Sukanya et al. 1994; Walker and Cornzzi 1989). Aside from transcriptional regu-lation, GS activity in plants might also be regulated at the level of turnover. Oxidative modification of GS has been implicated as the first step in the turnover of GS in bacteria (Levine 1983; Rivett and Levine 1990). Stieger and Feller (1997) have shown that GS degradation in illuminated chloroplasts requires the function of the photosynthetic electron transport chain. Chloroplastic GS of wheat seedlings has been reported to be particularly prone to degradation under oxidative stress conditions (Palatnik et al. 1999). By incubating soybean root extracts enriched in GS in a metal-catalysed oxidation system to produce the hy-droxyl radical, Ortega et al. (1999) have shown that GS is oxidised and that the oxidised GS is inactive and more susceptible to degradation than nonoxidised GS. It is clear that GS degradation requires the pro-duction of free radicals.
In recent studies, we found that CdCl2 induced ammonium ion accumulation in detached rice leaves (Chien and Kao 2000). Evidence was also presented to show that CdCl2-induced ammonium ion accumu-lation in detached rice leaves is attributed to a de-crease in GS activity (Chien and Kao 2000). Since CdCl2 is known to induce lipid peroxidation in de-tached rice leaves (Chien et al. 2001), we examined the possible involvement of lipid peroxidation (oxi-dative stress) in regulating the decline of GS activity and accumulation of ammonium ions in detached rice leaves.
Materials and methods
Rice (Oryza sativa cv. Taichung Native 1) was cul-tured as described previously (Chen and Kao 1998). Briefly, rice seedling were planted on a stainless net floating on half-strength Johnson’s modified nutrient solution (pH 4.2) in a 500-ml beaker. The nutrient so-lution was replaced every 3 days. Rice plants were grown for 12 days in a greenhouse, where natural light was provided and the temperature was con-trolled at 30 °C during the day and at 25 °C at night. The apical 3 cm of the third leaf was used for the ex-periment. A group of ten segments was floated in a Petri dish containing 10 ml of test solutions. Unless otherwise indicated, 5 mM CdCl2was used.
Incuba-tion was carried out at 27 °C in the light (40
mol m−2s−1) for the time indicated.
For determination of Cd, detached rice leaves were dried at 65 °C for 48 h. Dried materials was ashed at 550 °C for 20 h. Ash residue was incubated with 31% HNO3 and 17.5% H2O2 at 70 °C for 2 h, and dis-solved in 0.1 N HCl. Cd was then quantified using an atomic absorption spectrophotometer (model AA-6800, Shimadzu, Kyoto). Malondialdehyde (MDA), routinely used as an indicator of lipid per-oxidation, was extracted with 5% (w/v) trichloroace-tic acid determined according to Heath and Packer (1968).
Ammonium ions were extracted by homogenising leaf segments in 0.3 mM sulphuric acid (pH 3.5). The homogenate was centrifuged for 10 min at 39 000 × g and the supernatant was used for determination of ammonium ions as described by Lin and Kao (1996). For extraction of GS, leaf segments were homoge-nised with 10 mM Tris-HCl buffer (pH 7.6, contain-ing 1 mM MgCl2, 1 mM EDTA and 1 mM 2-mercap-toethanol) using a chilled pestle and mortar. The ho-mogenate was centrifuged at 15 000 × g for 30 min and the resulting supernatant was use for determina-tion of GS activity. The whole extracdetermina-tion procedure was carried out at 4 °C. GS was assayed by the method of Oaks et al. (1980). The reaction mixture contained in a final volume of 1 ml was 80 mol Tris-HCl buffer, 40 mol L-glutamic acid, 8 mol ATP, 24mol MgSO4, and 16mol NH2OH; the fi-nal pH was 8.0. The reaction was started by addition of the enzyme extract and, after incubation for 30 min at 30 °C, was stopped by adding 2 ml 2.5% (w/v) FeCl3 and 5% (w/v) trichloroacetic acid in 1.5 M HCl. After centrifugation the absorbance of the super-natant was read at 540 nm. The definition of 1 U of GS activity is defined as 1 mol L-glutamate
␥-monohydroxamate formed per min. In order to sep-arate the activites of GS1 and GS2 isofroms in leaf extract, activities were also measured in the same conditions in the presence of 1 mM glucosamine-6-phosphate, a specific inhibitor of GS2 (Hirel and Gadal 1980).
Cd content was expressed per g dry weight (DW), ammonium and MDA contents and GS activity were expressed per g fresh weight. Absolute levels of each measurement varied among experiments because of seasonal effects. However, the patterns of responses to CdCl2 or PQ were reproducible. For all measure-ments, each treatment was repeated four times. All
experiments were repeated at least three times and yielded results consistent with the data reported here.
Results and discussion
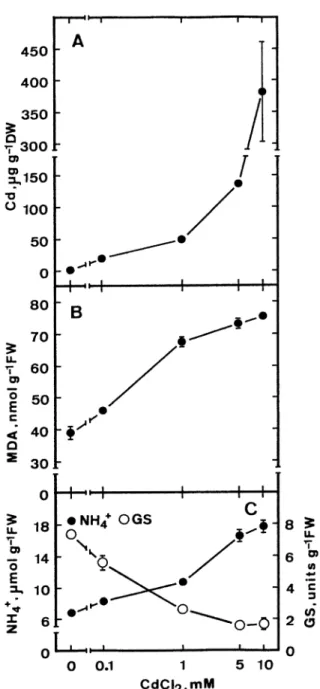
Increasing concentration of CdCl2from 0.1 to 5 mM progressively increased ammonium ion content in de-tached rice leaves in the light (Figure 1C). No further increase was observed at 10 mM CdCl2. Ammonium ion content increased ⬃ 2-fold in detached rice leaves treated with 5 mM CdCl2for 48 h in the light (Figure 1C). Ammonium ions are a central intermediate of nitrogen metabolism in plants (Miflin and Lea 1976). Ammonium ions are produced during nitrate assimi-lation, deamination of amino acids and photorespira-tion (Miflin and Lea 1976). The assimilaphotorespira-tion of am-monium ions requires GS (Miflin and Lea 1976). Re-cently, we reported that the decrease in GS activity but not the promotion of nitrate reduction is the source of Cd-induced ammonium ion accumulation in detached rice leaves (Chien and Kao 2000). We also showed that excess Cd increased lipid peroxidation in detached rice leaves (Chien et al. 2001). GS activity and MDA content were observed to decrease and in-crease, respectively, with the increase in CdCl2 con-centrations (Figure 1B and C). These results indicate that ammonium accumulation caused by CdCl2is at-tributed to the decline in GS activity and increase in lipid peroxidation. To be sure that the described changes in ammonium ion and MDA contents and GS activity were related to an increase in the leaf Cd content, Cd concentration was determined in detached rice leaves with various concentration of CdCl2. In-creasing concentration of CdCl2 progressively in-creased leaf Cd content (Figure 1A).
Lipid peroxidation is a free radical mediated pro-cess (Hirel and Gadal 1980). If lipid peroxidation is important in regulating the decrease in GS activity and the increase in ammonium ion content, then free radical scavengers, such as reduced glutathione (GSH), sodium benzoate (SB) and thiourea (TU), are expected to decrease MDA and ammonium ion con-tents and to increase GS activity. The results reported in Figure 2 demonstrated that this is, in fact, correct. Altough free radical scavengers are effective in reduc-ing ammonium ion content induced by CdCl2, ammo-nium ion contents are still high (Figure 2B). This is probably due to the fact that free radical scavengers are less effective in reducing lipid peroxidation (Fig-ure 2A) in detached rice leaves.
Superoxide can serve as a source to generate more active hydroxyl radicals by Haber-Weiss and Fenton reactions (Foyer et al. 1997). Transition metals, such as iron and copper, are able to accelerate Haber-Weiss and Fenton reactions (Naqni and Chance 1986). In a
Figure 1. Effect of CdCl2on the contents of Cd, malondialdehyde
(MDA) and ammonium ions and the activity of glutamine syn-thetase (GS) in detached rice leaves in the light. Detached rice leaves were incubated in solutions containing 0–10 mM CdCl2. All
measurements were made 48 h after treatment. Vertical bars repre-sent standard errors (n = 4). Only these standard errors larger than the symbol are shown.
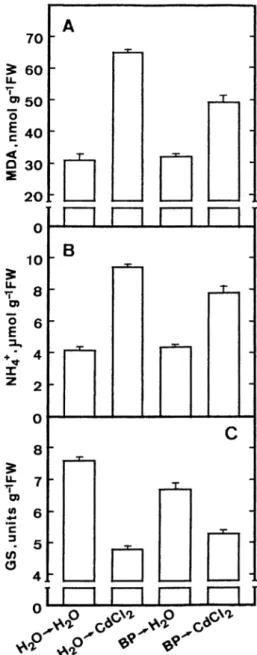
recent study (Chien et al. 2001), pretreatment of leaf segments with 2,2⬘-bipyridine (BP) or 1,10-phenan-throline, iron chelators, caused a reduction of CdCl2-induced increase in toxicity and MDA content. If lipid peroxidation plays a significant role in regulating am-monium ion accumulation and decline in GS activity, we also expect that pretreatment of leaf segments with BP would reduce the increase in ammonium ion
content and the decrease in GS activity caused by CdCl2. BP-pretreatment resulted in a reduction of CdCl2-induced lipid peroxidation (Figure 3A) and ammonium ion accumulation (Figure 3B) and CdCl2-induced decrease in GS activity (Figure 3C) in de-tached rice leaves. Thus, free radical generation is most likely involved in regulating ammonium ion
ac-Figure 2. Effect of free radical scavengers on the content of mal-ondialdehyde (MDA) and ammonium ions and the activity of glutamine synthetase (GS) in detached rice leaves in the light. The concentrations of CdCl2, thiourea (TU), sodium benzoate (SB) and
reduced glutathione (GSH) were 5 mM. All measurements were made 24 h after treatment. Vertical bars represent standard errors (n = 4).
Figure 3. Effect of 2,2⬘-bipyridine (BP) on the contents of malon-dialdehyde (MDA) and ammonium ions and the activity of glutamine synthetase (GS) in detached rice leaves. Detached rice leaves were pretreated with either water or 5 mM BP for 6 h in the light and then treated with either water or 5 mM CdCl2for 24 h in
cumulation and GS activity. To test further this pos-sibility, detached rice leaves were treated with a well known free-radical-generating chemical, paraquat (PQ). PQ treatment was observed to increase MDA content (Figure 4A) and at the same time increase ammonium ion content (Figure 4B) and decrease GS activity (Figure 4B) in detached rice leaves.
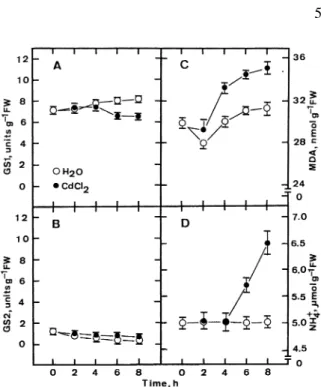
In green tissues of angiosperms the occurrence of two GS isoenzymes, GS1 and GS2, has been demon-strated. GS1 catalyses glutamine biosynthesis in the cytosol whereas GS2 is confined to the chloroplast (Lancien et al. 2000). Thus, it is of great interest to know the effect of excess Cd on the activities of GS1 and GS2 in detached rice leaves. By including 1 mM
glucosamine-6-phosphate, a specific inhibitor of the chloroplastic GS2 isoform (Hirel and Gadal 1980) in the assay medium, discrimination between the activi-ties of the two GS isoforms present in detached rice leaves was possible. It was found that GS1 appears to be the predominant isoform in detached rice leaves of the test variety (Figure 5A and B). It was also found that excess Cd (5 mM) caused a decrease in GS1 (Figure 5A) but had no effect on GS2 (Figure 5B), suggesting that the excess Cd-induced decrease in ex-tracted total GS activity was mainly due to the GS1 cytosolic isoform. Lutts et al. (1999) also observed that GS1 was the predominant isoform in leaves of rice (cv. I Kong Pao and Nona Bokra).
To test the causal relationship between lipid per-oxidation, ammonium ion level, and GS1 activity, de-tached rice leaves were incubated in distilled water and CdCl2(5 mM), respectively, for 2, 4, 6, and 8 h. Changes in MDA content, ammonium ion content, and GS1 activity were monitored. An increase in MDA content was observed at 4 h after CdCl2 treat-ment (Figure 5C), whereas ammonium ion accumu-lation (Figure 5D) and the decrease in GS1 activity (Figure 5A) occurred at 6 h after CdCl2 treatment. These results indicate that an increase in lipid peroxi-dation precedes ammonium ion accumulation and the decrease in GS1 activity. Clearly, the links between
Figure 4. Effect of paraquat (PQ) on the contents of malondialde-hyde (MDA) and ammonium ions and the activity of glutamine synthetase (GS) in detached rice leaves in the light. Detached rice leaves were incubated in solutions containing 0–50M PQ. All measurements were made at 48 h after treatment. Vertical bars re-present standard errors (n = 4). Only these standard errors larger than the symbol are shown.
Figure 5. Time courses of the CdCl2effect on GS1 and GS2 ac-tivities, MDA contents, and ammonium ion contents in detached rice leaves. Detached rice leaves were incubated in water or 5 mM CdCl2. Vertical bars represent standard errors (n = 4).
CdCl2 treatment, lipid peroxidation, ammonium ion accumulation and GS activity are well established. The results reported here suggest that the decreases in GS activity and the increases in ammonium ion content demonstrated in detached rice leaves are a consequence of oxidative damage caused by excess Cd. Our results are in agreement with the suggestion that GS is prone to degradation under oxidative stress conditions (Palatnik et al. 1999).
Acknowledgements
This work was supported by grant NSC 89-2312-002-222 from the National Science Council of the Repub-lic of China.
References
Backer T.W., Hoppe M. and Foch P.H. 1986. Evidence for the par-ticipation of dissimilatory processes in maintaining high carbon fluxes through the photosynthetic carbon reduction and oxida-tion cycles in water-stressed bean leaves. Photosynthetica 20: 153–157.
Chaoui A., Mazhouri S., Ghorbal M.H. and Ferjani E.E. 1997. Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Sci. 127: 139–147.
Chen L.-M. and Kao C.H. 1998. Relationship between ammonium accumulation and senescence of detached rice leaves caused by excess copper. Plant Soil 200: 169–173.
Chen S.J., Hung K.T. and Kao C.H. 1997. Ammonium accumula-tion is associated with senescence of rice leaves. Plant Growth Regul. 21: 195–201.
Chien H.-F. and Kao C.H. 2000. Accumulation of ammonium in rice leaves is response to excess cadmium. Plant Sci. 156: 111– 115.
Chien H.-F., Wang J.-W., Lin C.C. and Kao C.H. 2001. Cadmium toxicity of rice leaves is mediated through lipid peroxidation. Plant Growth Regul. 33: 205–213.
Edwards J.W., Walker E.L. and Coruzzi G.M. 1990. Cell specific expression in transgenic plants reveals nonoverlapping roles for chloroplast and cytosolic glutamine synthetase. Proc. Natl. Acad. Sci. 87: 3459–3463.
Forde B.G., Day H.M., Turton J.F., Shen W.-J., Cullimore J.V. and Oliver J.E. 1989. Two glutamine synthetase genes from
Phaseolus vulgaris L. display contrasting developmental and
spatial pattern of expression in transgenic Lotus corniculatas plants. Plant Cell. 1: 391–401.
Foyer C.H., Lopez-Delgado H., Dat J.F. and Scott I.M. 1997. Hy-drogen peroxide and glutathione-associated mechanism of ac-climatory stress tolerance and signaling. Physiol. Plant 100: 241–254.
Gallego S.M., Benavides M.P. and Tomaro M.L. 1996a. Effect of heavy metal ion excess on sunflower leaves: evidence for in-volvement of oxidative stress. Plant Sci. 121: 151–159. Gallego S.M., Benavides M.P. and Tomaro M.L. 1996b. Oxidative
damage caused by cadmium in sunflower (Helianthus annuus L.). Phyton. 58: 41–52.
Gutteridge J.M.C., Rowley D.A. and Halliwell B. 1981. Superox-ide-dependent formation of hydroxyl radical in the presence of iron salts. Biochem. J. 199: 263–265.
Heath R.L. and Packer L. 1968. Photoperoxidation in isolated chlo-roplasts. I. Kinetics and stoichiometry of fatty acid peroxida-tion. Arch. Biochem. Biophys. 125: 189–198.
Hirel B. and Gadal P. 1980. Glutamine synthetase in rice: A com-parative study of the enzymes from roots and leaves. Plant Physiol. 66: 619–623.
Hirel B., Bouet C., King B., Layzell D., Jacobs F. and Verma D.P.S. 1987. Glutamine synthetase genes are regulated by ammonia provided externally or by symbiotic nitrogen fixation. EMBO J. 6: 1167–1171.
Lancien M., Gadal P. and Hodges M. 2000. Enzyme redundancy and the importance of 2-oxoglutarate in higher plant ammo-nium assimilation. Plant Physiol. 123: 817–824.
Levine R.L. 1983. Oxidative modification of glutamine synthetase. I. Inactivation is due to loss of one histidine residue. J. Biol. Chem. 258: 11823–11827.
Lin C.C. and Kao C.H. 1996. Disturbed ammonium assimilation is associated with growth inhibition of roots in rice seedlings caused by NaCl. Plant Growth Regul. 18: 133–138.
Lin J.-N. and Kao C.H. 1998. Water stress, ammonium, and leaf senescence in detached rice leaves. Plant Growth Regul. 26: 165–169.
Lozano-Rodriguez E., Hernandez L.E., Bonzy P. and Charpena-Ruiz R.O. 1997. Distribution of cadmium in shoot and root tis-sue of maize and pea plants: physiological disturbances. J. Exp. Bot. 306: 123–128.
Lutts S., Majerus V. and Kinet J.-M. 1999. NaCl effects on proline metabolism in rice (Oryza sativa) seedlings. Plant Physiol. 105: 450–458.
Miflin B.J. and Lea P.J. 1976. The pathway of nitrogen assimila-tion in plants. Phytochemistry 15: 873–885.
Naqni A. and Chance B. 1986. Reactive oxygen intermediates in biochemistry. Annu. Rev. Biochem. 55: 127–166.
Oaks A., Stulen J., Jines K., Winspea M.J. and Booesel I.L. 1980. Enzymes of nitrogen assimilation in maize roots. Planta 148: 477–484.
Ortega J.L., Roche D. and Sengupta-Gopalan C. 1999. Oxidative turnover of soybean root glutamine synthetase. In vitro and in vivo studies. Plant Physiol. 119: 1483–1495.
Palatnik J.F., Carrillo N. and Valle E.M. 1999. The role of photo-synthetic electron transport in the oxidative degradation of chloroplastic glutamine synthetase. Plant Physiol. 121: 471– 478.
Peeters K.M.U. and Van Laere A.J. 1992. Ammonium and amino acid metabolism in excised leaves of wheat (Triticum aestivum) senescing in the dark. Physiol. Plant 84: 243–249.
Rivett A.J. and Levine R.L. 1990. Metal-catalyzed oxidation of
Escherichia coli glutamine synthetase: correlation of structural
Roche D., Temple S.J. and Sengupta-Gopalan C. 1993. Two classes of differentially regulated glutamine synthetase genes are ex-pressed in the soybean nodule: a nodule-specific class and a constitutively expressed class. Plant. Mol. Biol. 22: 971–983. Sanita di Toppi L., Lamberdi M., Pazzagli N., Cappugi G., Durante
M. and Gabbrielli R. 1998. Response to cadmium in carrot in vitro plants and cell suspension cultures. Plant Sci. 137: 119– 129.
Sanita di Toppi L., Lamberdi M., Pecchioni N., Pazzagli N., Du-rante M. and Gabbrielli R. 1999. Effects of cadmium stress on hairy root of Dacus carota. J. Plant Physiol. 154: 385–391. Shaw B.P. 1995. Effects of mercury and cadmium on the activities
of antioxidative enzymes in the seedlings of Phaseolus aureus. Biol. Plant. 37: 587–596.
Stieger P.A. and Feller U. 1997. Requirements for light-stimulated degradation of stromal protein in isolated pea (Pisum sativum L.) chloroplasts. J. Exp. Bot. 48: 1639–1645.
Stroinski A. and Zielezinska M. 1997. Cadmium effect on hydro-gen peroxide, glutathione and phytochelatins levels in potato tuber. Acta Physiol. Plant. 19: 127–135.
Sukanya R., Li M.-G. and Snastad D.P. 1994. Root- and shoot-spe-cific responses of individual glutamine synthetase genes of maize to nitrogen and ammonium. Plant Mol. Biol. 26: 1935– 1946.
Thomas H. 1978. Enzymes of nitrogen mobilization in detached leaves of Lolium temulentum during senescence. Planta 142: 161–169.
Walker E.L. and Cornzzi G.M. 1989. Developmentally regulated expression of the gene family for cytosolic glutamine syn-thetase in Pisum sativum. Plant Physiol. 191: 702–708. Wang Y.S., Luo G.H. and Kwan K.M.F. 1997. Peroxidation
dam-age of oxygen free radicals induced by cadmium to plant. Acta Bot. Sin. 39: 526–533.