行政院國家科學委員會補助專題研究計畫
■
□
成 果 報 告 期中進度報告血管收縮素轉化酶 II–血管收縮素 1-7 途徑 (ACE2–Ang 1-7 axis) 在
心臟衰竭病程演進中扮演角色的探討
Studying the roles of angiotensin converting enzyme II – angiotensin 1-7
(ACE2–Ang 1-7) axis in the disease process of heart failure
計畫類別:
■ 個別型計畫 □ 整合型計畫
計畫編號:NSC
98-2313-B-009-002-MY3
執行期間:98 年 8 月 1 日 至 101 年 7 月 31 日
計畫主持人:
林 志 生
計畫參與人員:
吳希天(協同研究人員)
、李一良(博士後研究)
、關棠青
(博士班研究生)
、楊子慧(碩士班研究生)
、陳睦元(碩
士班研究生)
、廖燕秋(碩士班研究生)
成果報告類型(依經費核定清單規定繳交):
□精簡報告 ■完整報告
本成果報告包括以下應繳交之附件:
□赴國外出差或研習心得報告一份
□赴大陸地區出差或研習心得報告一份
■出席國際學術會議心得報告及發表之論文各一份
□國際合作研究計畫國外研究報告書一份
處理方式:除產學合作研究計畫、提升產業技術及人才培育研究計畫、
列管計畫及下列情形者外,得立即公開查詢
□涉及專利或其他智慧財產權,□一年□二年後可公開查詢
執行單位:
國立交通大學 生物科技學系
中 華 民 國 101 年 10 月 31 日
1
一、中文摘要
本研究計畫主要目的為探討在心臟疾病病程演進中血管收縮素轉化酶 II 與血管收 縮素 1-7 途徑(ACE2–Ang 1-7 axis)所扮演之角色,並瞭解在心臟組織與細胞中 ACE2 的表現調節機制,以及此調節機制與心臟重塑病程演進之關聯性。我們藉由報導基因技 術,分析了人類 ACE2 基因 5’端啟動子區域中受血管收縮素調控表現的重要序列。另外, 也利用 lentivirus 與 shRNA 技術探討 ACE2 過量表現與 ACE2 基因抑制對於心臟細胞及 其 MMP-2 表現之影響,藉此分析 ACE2 於心臟重塑病程中所扮演的角色。
在本研究中所得到之重要結果有:(1) 血管收縮素 II(Ang II)與 Ang 1-7 分別會經 由 AT1R 及 Mas 活化 ERK-MAPK 訊息傳遞途徑以刺激 ACE2 表現; (2) ace2 基因啟動子 的-516/-481 序列上之 5’-ATTTGGA-3’為特定轉錄因子-Ikaros 的結合位置並可藉此調控 ACE2 表現; (3) Ang II 會經由 AT1R 與其下游 ERK-MAPK 訊息傳遞鏈刺激 ace2 基因啟 動子的-516/+20 序列藉此調控 ACE2 表現; (4) TGF-β1 和 TNF-α 處理 HCFs 對於其 ace2 基因啟動子活性並無顯著影響; (5) ACE2 過量表現會造成 MMP-2 活性提昇,Ang II 及 Ang 1-7 分別可經由 AT1R 及 Mas 降低 MMP-2 活性表現。
以上研究成果顯示 angiotensin peptides 對於 ACE2 表現調節之影響性以及 ACE2 表 現調節與心臟重塑病程演進之關聯性。另外,本研究成功引進 ACE2 KO mice 且目前正 積極建立 ACE2 過量表現鼠種,這些研究成果相信對未來國內 ACE2 之相關研究發展能 有所助益。
關鍵詞:血管收縮素轉化酶 II、血管收縮素 1-7、血管收縮素 II、心臟疾病
Abstract
The aims of the this study is to investigate the roles of angiotensin converting enzyme II-angiotensin 1-7 (ACE2–Ang 1-7) axis in the disease process of heart and understand the regulatory mechanism of ace2 gene expression in cardiac cell and tissue. We have analyzed the promoter activity of human ace2 using luciferase report assay to identify regulatory elements of ace2 gene. In addition, we also revealed the relationship with ACE2, MMP-2 and heart remodeling in cardiac fibroblasts utilized the technology of lentivirus and shRNA.
The major results of this study are: (1) angiotensin II (Ang II)/AT1R and Ang 1-7/Mas signal pathways were via ERK-MAPK signal pathway to regulate ACE2 expression, respectively; (2) the sequences of 5’-ATTTGGA-3’ within -516/-481 domain of the ace2 promoter could regulate ACE2 expression and this sequences was the binding site of the transcription factor-Ikaros; (3) Ang II regulated ACE2 expression through AT1R to activate ERK-MAPK signal pathway to stimulate the -516/+20 domain within the ace2 promoter; (4) the ace2 promoter activity was not significant effect in HCFs treated with TGF-β1 and TNF-α;
2
(5) in HCFs, ACE2 overexpression enhanced MMP-2 activity, but Ang II/AT1R and Ang 1-7/Mas signal pathways were decreased MMP-2 activity.
The results in this study reveal the ACE2 regulation of angiotensin peptides and the association of ACE2 with the pathological heart remodeling. In addition, we already introduce the ACE2 KO mice from MMRRC and try to established ACE2 overexpression mice. These achievements were advantageous in ACE2 research development in future.
Keywords: angiotensin-converting enzyme II, angiotensin II, angiotensin 1-7, heart disease
二、緣由與目的
自 2000 年血管收縮素轉化酶 II(angiotensin-converting enzyme II; ACE2)被發現 以來[1, 2],它在腎素-血管收縮素系統(Renin-angiotensin system; RAS)中所扮演的重要
角色,日漸受到心血管研究者所重視[3, 4]。由於 ACE2 可將血管收縮素 I(angiotensin I;
Ang I)與血管收縮素 II(angiotensin II; Ang II)降解為血管收縮素 1-9(angiotensin 1-9; Ang 1-9)與血管收縮素 1-7(angiotensin 1-7; Ang 1-7)[2, 5],其不僅能降低血液中 Ang II, 且已知 Ang 1-7 具有拮抗 Ang II 的生理功能[6, 7]。因此,ACE2 擔任平衡 RAS 中
ACE2/Ang 1-7/Mas 與 ACE/Ang II/AT1R 的重要角色以維持心血管、腎、肺及腦神經系 統的正常運作,並有效控制心[8, 9]、肺[10, 11]及肝[12, 13]的纖維化及結構重塑。
先前的文獻指出,ACE2 過量表現可保護 Ang II 所誘發之高血壓[14]與纖維化[8],並
改善心肌梗塞所造成之左心室重塑[15]。ACE2 缺乏則會加劇心臟重塑的發生以及心臟
收縮功能降低,進而引發心臟衰竭[16-18]。另外,Ang II 會經由 AT1R 誘發 NADPH oxidase
及 MMPs[18, 19],這些結果顯示 ACE2 於心臟重塑之病程演進中擔任一重要的保護角色,
但 ACE2 與心臟重塑相關之 MMP-2 及 MMP-9 之表現調節卻鮮少被探討。
除此之外,在分子生物學的層次上,ACE2 基因表達調控相關之研究[20]與一些心血
管疾病(例如心肌梗塞、心臟衰竭或心房顫動等)的發生,以及其在生理學和病理學上 的關聯性,都亟需被探討。因此,本研究將於分子生物學的層次,逐步探討 ACE2 基因 表現調控之相關機制、ACE2 所平衡之 ACE2/Ang 1-7/Mas 與 ACE/Ang II/AT1R 途徑以 及 ACE2 表現調控與 MMPs 表現之關聯性,並以細胞與動物模式進行佐證,藉此探討 ACE2 於心臟重塑病程中所負責之調控機制及其所扮演的角色。
3
三、研究成果與討論
3.1 Ang II 對人類心纖維細胞之 ACE2 表現的影響
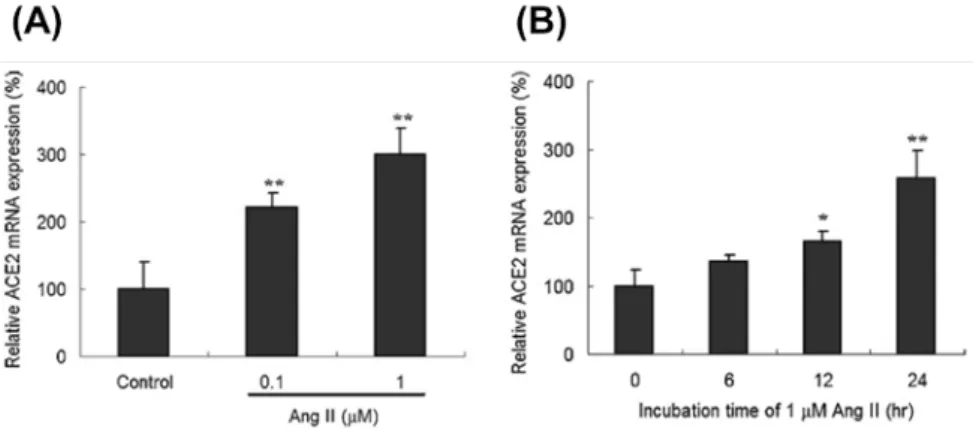
人類心臟纖維母細胞(human cardiac fibroblasts; HCFs)以不同濃度 Ang II 處理後, 可以測得 HCFs 內 ACE2 的 mRNA 表現量會隨著 Ang II 處理劑量的增加而有顯著的上 升(Fig. 1A)。在 1 µM 的 Ang II 處理下,ACE2 的 mRNA 表現量約提升三倍。此外, ACE2 的 mRNA 增加量與 Ang II 處理的時間長短則有正相關性(Fig. 1B)。
進一步探討此調控的現象和 AT1R 與 ERK-MAPK 之間的關係,利用專一性的藥物 -Valsartan(Val; AT1R 之抑制劑)及 PD98059(MEK 之抑制劑)以抑制 Ang II 下游的訊 息傳導分子。HCFs 預先以 1 µM 的 Val 或 10 µM 的 PD98059 處理後再與 Ang II 進行反 應。結果顯示,Val 的前處理可以抑制 Ang II 經由 AT1R 路徑對 ACE2 mRNA 表現量所 造成之影響,且 PD98059 的前處理也具有相同之結果(Fig. 2A)。除此之外,我們用 Western blot 探討 Ang II 影響 ACE2 表現時其 ERK-MAPK 訊息傳遞路徑分子之表現量變 化(Fig. 2B)。與 ACE2 mRNA 表現之結果相似,HCFs 與 Ang II 刺激後,能引起細胞 內 ACE2、p-MEK1/2 及 p-ERK1/2 等蛋白質大量表現(Fig. 2C∼2E)。當細胞以 Val 或 PD98059 預處理後再與 Ang II 反應時,原先因 Ang II 刺激所增加的 ACE2、p-MEK1/2 及 p-ERK1/2 等蛋白都會被抑制,然而單獨使用 Val 或 PD98059 處理 HCFs 對上述分子 的表現量並沒有影響(Fig. 2C∼2E)。
以免疫細胞螢光染色的方式對 HCFs 內 ACE2 的分佈與表現量進行研究,HCFs 以 Ang II 刺激後,其 ACE2 的螢光標定表現有明顯的增加,而此增加的 ACE2 螢光表現會 因 Val 的前處理而抑制(Fig. 3A)。此外,免疫細胞螢光染色的結果與之前 RT-PCR 及 Western blot 所得到的結果相似(Fig. 3B)。以上結果顯示 Ang II 可能是經由 AT1R 活化 下游之 ERK-MAPK 訊息傳導分子並提昇 ACE2 之表現量。
3.2 Ang 1-7 對人類心纖維細胞之 ACE2 表現的影響
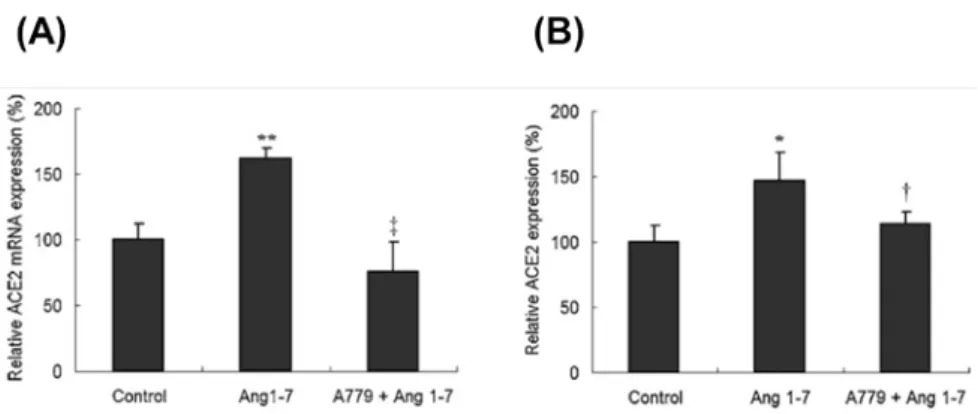
本研究同時也探討 Ang 1-7 是否對 HCFs 的 ACE2 表現有所影響。以 1 µM 的 Ang 1-7 對 HCFs 進行反應,結果顯示 HCFs 細胞內 ACE2 之 mRNA 及蛋白質表現量均有顯著的 增加(Fig. 4)。此外,利用 A779(Mas receptor 之抑制劑)進行預處理再以 Ang 1-7 刺 激 HCFs 發現 Ang 1-7 是經由 Mas receptor 調控 ACE2 之表現(Fig. 4)。此實驗結果進一 步以免疫細胞螢光染色的方式進行佐證也得到相同之結果(Fig. 5),以上結果顯示 Ang 1-7 是經由 Mas receptor 調控 ACE2 之 mRNA 及蛋白質表現。
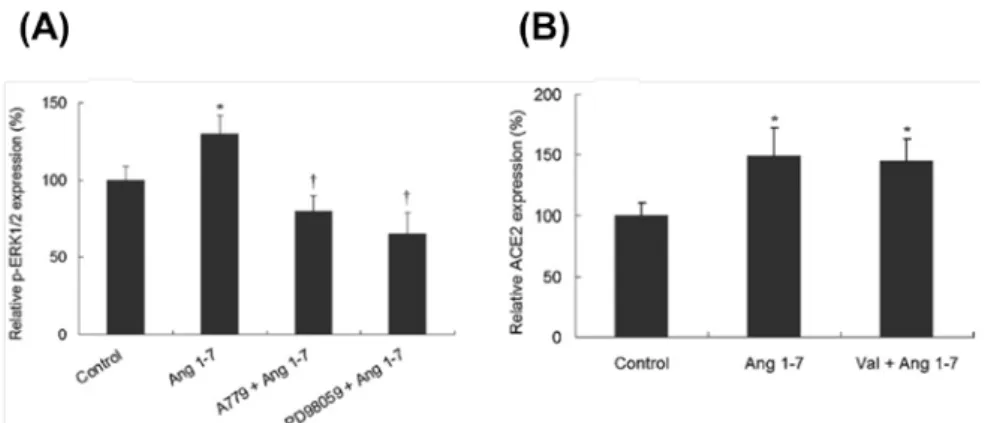
進一步以 A779 及 PD98059 探討 Ang 1-7/ERK-MAPK/ACE2 於 HCFs 之關聯性,結 果顯示 Ang 1-7 會活化 p-ERK1/2 之蛋白質表現且 A779 及 PD98059 的預處理可抑制此 結果之產生(Fig. 6A)。此外,HCFs 以 AT1R 的抑制劑 Val 進行預處理並不會抑制 Ang 1-7 刺激 ACE2 表現之結果(Fig. 6B),顯示 Ang 1-7 並非經由 AT1R 調控 ACE2 之表現,
4
乃是經由 Mas receptor 活化 p-ERK1/2 以提升 ACE2 之表現量。
3.3 ace2 基因啟動子中影響基因表現的序列探索
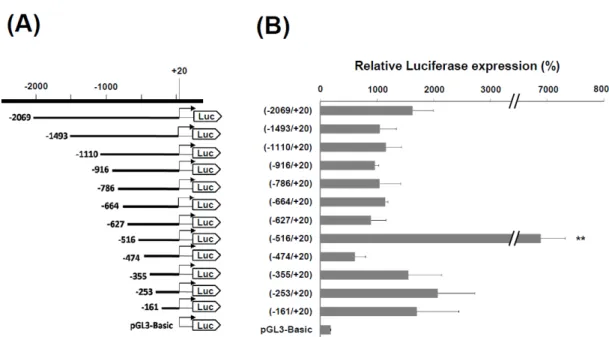
本研究為於分子層面探討 ACE2 之表現調控,藉由 PCR 自人類基因組 DNA 中增幅 2.1 kb(-2069/+20)的 ace2 基因啟動子 DNA 片段,並將此 DNA 片段建構至質體 pGL3-Basic 冷光報導基因(luciferase),以獲得 ace2 基因啟動子(-2069/+20)表現 luciferase 的表現載體。此外,也同時建構一系列不同長度之 5’端 ace2 基因啟動子的 DNA 表現載 體,包括-1493/+20、-1110/+20、-916/+20、-786/+20、-664/+20、-627/+20、-516/+20、 -474/+20、-355/+20、-253/+20 及-161/+20 等,這些載體用以轉染至 HCFs,據以探討不 同區段之 ace2 啟動子的活性(Fig. 7A)。啟動子活性分析的結果顯示,經-2069/+20 載 體轉染的 HCFs 中所表現的 luciferase 活性高於對照組 8.9 ± 2.0 倍,而在所有構築的表現 載體中,-516/+20 載體轉染的 HCFs 具有最高的 luciferase 活性,其為對照組的 36.2 ± 2.4 倍,分別為-2069/+20 與-474/+20 轉染組的 4.1 ± 0.3 及 10.9 ± 0.7 倍(Fig. 7B)。此外, 依據上述的結果,我們推測在 5’端 ace2 基因啟動子的-516 與-481 序列之間有一重要序 列可影響 ace2 基因的表現。利用-2069/+20 載體為主體,建構-516/-481 序列移除與反置 的載體(Fig. 8A),再將之分別轉染至 HCFs。結果顯示-516/-481 序列移除與反置皆會 導致 ace2 基因啟動子活性的明顯下降(Fig. 8B),證實 5’端 ace2 基因啟動子的-516 與-481 序列之間有一可大幅影響 ace2 基因表現的重要序列。 3.4 ace2 基因啟動子轉錄因子的結合位序列分析 先前實驗已證實 5’端 ace2 基因啟動子的-516 與-481 序列之間有一可大幅影響 ace2 基因表現的重要序列,接著進一步分析是否有特定轉錄因子的結合位置於此 ace2 基因 啟動子的-516/-481 序列之間。利用資料庫 TFSEARCH 搜尋比對,顯示本區域內之 5’-ATTTGGA-3’序列為已知轉譯因子 Ikaros 的結合序列。因此,我們針對本序列設計一 系列的突變序列(Fig. 9A),並將這些突變序列建構於 luciferase 表現載體用以轉染 HCFs 細胞,並分析其啟動子活性。實驗結果顯示 5’-ATTTGGA-3’序列確實是影響 ace2 基因 表現的一段重要序列,無論是單點突變或雙點突變序列,皆會使啟動子活性顯著下降 (Fig. 9B)。
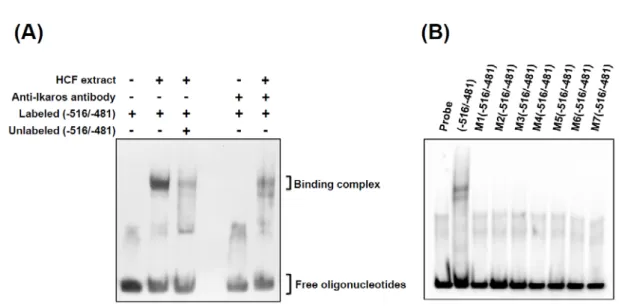
接著,利用 electrophoretic mobility shift assay(EMSA)分析以證實 HCFs 細胞核是 否存在會與 5’-ATTTGGA-3’結合的特定蛋白質。EMSA 結果顯示,僅 5’-ATTTGGA-3’ 序列與 HCFs 細胞核蛋白質有明顯的結合作用,HCFs 細胞核蛋白質與 Ikaros 抗體預處 理後,則會降低 HCFs 細胞核蛋白質與 5’-ATTTGGA-3’序列的結合作用(Fig. 10A),顯 示 5’-ATTTGGA-3’可能為 Ikaros 的結合區段。另外,將 5’-ATTTGGA-3’其他點突變之 序列與 HCFs 細胞核蛋白質反應,經 EMSA 分析後顯示點突變之序列與 HCFs 細胞核蛋 白質皆無明顯的結合作用(Fig. 10B)。以上結果證實 ace2 基因啟動子的-516/-481 序列 上之 5’-ATTTGGA-3’為特定轉錄因子-Ikaros 的結合位置並可藉此調控 ACE2 表現。
5
3.5 Ang II 對 ace2 基因的表現調控
先前的研究顯示 Ang II 會刺激 HCFs 以提升其 ACE2 之 mRNA 及蛋白質表現,依 據此結果本研究將探討 ace2 基因啟動子的-516/-481 序列與 Ang II 對 ace2 基因的表現調 節是否有關。經-516/+20 及-481/-516/+20 反置載體轉染的 HCFs,分別以不同濃度的 Ang II(0、0.1、1 及 10 µM)處理並測定其 luciferase 活性。結果顯示,經-516/+20 載體轉 染的 HCFs 以 Ang II 刺激後可顯著增加其 luciferase 活性,而以-481/-516/+20 載體轉染 的 HCFs 則否,顯示 Ang II 刺激 HCFs 會經由 ace2 基因啟動子的-516/+20 序列提昇 ACE2 表現(Fig. 11A)。此外,以 Val 或 PD98059 預處理可抑制 Ang II 刺激所造成之 luciferase 活性提昇(Fig. 11B)。上述研究結果顯示,Ang II 會經由 AT1R 與其下游 MEK 訊號傳 遞鏈刺激 ace2 基因啟動子的-516/+20 序列藉此調控 ACE2 表現。另外為了探討 ace2 基 因 5’端中-516/-481 序列與發炎反應間是否具有關聯性,我們分別以 TGF-β1 和 TNF-α 處理已轉染-516/+20 表現載體的 HCFs,並分析其 ace2 啟動子活性。實驗結果顯示, TGF-β1 和 TNF-α 處理對於其 ace2 基因啟動子活性並無顯著影響。 3.6 ACE2 過量表現與抑制對 HCFs 之影響 以 Lentivirus 表現外源基因或 shRNA 為一常見且有效之技術以探討單一蛋白過量表 現或抑制對細胞或實驗動物之影響[21, 22],本研究也利用此技術探討 ACE2 高表現與抑制 對 HCFs 之影響。本研究室構築 pCMV-hACE2 基因轉殖載體並轉染於大鼠心臟細胞株 H9c2 中,經測試結果確認 pCMV-hACE2 轉染之 H9c2 細胞可於 mRNA、蛋白質及酵素 活性測得高量之 ACE2 表現後,委託鑫品生醫科技建構 pVBI-TLC-ACE2 以生產 high titer lentivirus TLC-ACE2。
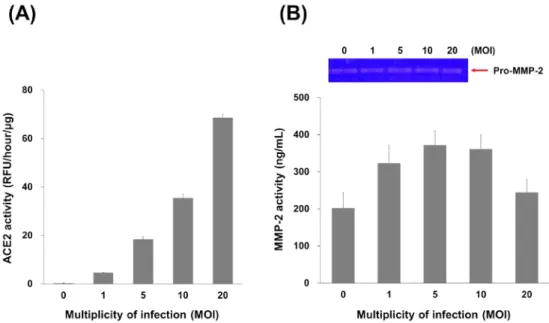
HCFs 以不同 multiplicity of infection(MOI)之 TLC-ACE2 進行感染後並檢測其 ACE2 及 MMP-2 活性。相較於未感染之 HCFs,以 1、5、10 及 20 MOI 感染之 HCFs 其 ACE2 活性分別提昇 20、78、151 及 292 倍(Fig. 12A),MMP-2 活性分別為 1.6、1.8、1.8 及 1.2 倍(Fig. 12B)。此結果顯示 ACE2 之過量表現會隨著 MOI 的提昇而隨之提高,MMP-2 活性則會提高後逐漸趨緩。
相較於 ACE2 過量表現,本研究也以 shRNA 探討 ACE2 抑制對 HCFs 之影響。本 研究室自中央研究院 National RNAi Core Facility Platform 引進 ACE2 shRNA
(TRCN0000046693 - TRCN0000046697 )並製備完成其 ACE2-shRNA/lentivirus。HCFs 同 時以 TLC-ACE2 及 ACE2 shRNA (TRCN0000046693 - TRCN0000046697 )於 5 MOI 進行 感染,相較於僅以 TLC-ACE2 感染之 HCFs,以#46693 至#46697 共感染之 HCFs 其 ACE2 活性分別降低至 53%、 7%、25%、17%及 5%,顯示 TRCN0000046697 具有最佳之 ACE2 抑制率。此外,利用 TLC-ACE2 及 TRCN0000046697 共感染也證實 TLC-ACE2 感染 HCFs 所造成之 MMP-2 活性提昇是由於 ACE2 過量表現所導致。
6
MOI 感染後得到 ACE2 過量表現之 HCFs/ACE2,將此 HCFs/ACE2 分別以 Val 及 A779 預處理後再分別以 Ang II 及 Ang 1-7 刺激並檢測其 MMP-2 活性表現。結果顯示 ACE2 過量表現會提昇 HCFs 之 MMP-2 活性,Ang II 及 Ang 1-7 會降低 ACE2 過量表現所造成 之 MMP-2 活性,而 Val 及 A779 預處理會抑制 Ang II 及 Ang 1-7 對 HCFs/ACE2 其 MMP-2 活性表現之影響(Fig. 13)。此結果表示 ACE2 過量表現會造成 MMP-2 活性提昇,Ang II 及 Ang 1-7 分別可經由 AT1R 及 Mas 降低 MMP-2 活性表現。
3.7 ACE2 基因剔除小鼠
目前可供研究之 ACE2 KO mice 為 B6;129S5-Ace2tm1Lex/Mmcd (MMRRC:31665), 本研究室自美國 MMRRC 小鼠種原庫引進此 ACE2 KO mice,並委託國家實驗動物中心 成功繁衍此基因缺陷鼠。由於 ACE2 基因為染色體 X-linked 之緣故,因此 ACE2 KO mice
可分為具半合子之公鼠(hemi),及同合子(homo)或異合子(hetero)的母鼠共三種,
經由 genotyping 及性別區分可分辨此三類 ACE2 KO mice。
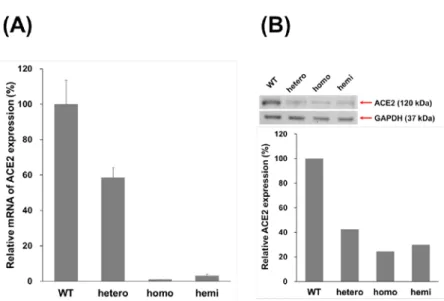
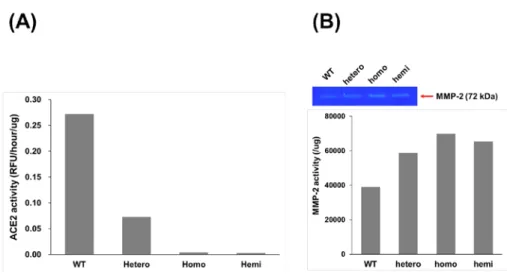
進一步探討 ACE2 KO mice 其心臟組織之 ACE2 及 MMP-2 表現量,wild-type、 hetero、homo 及 hemi mice 分別犧牲並取其心臟組織進行蛋白質萃取,所萃取之蛋白質 分別以 real time PCR、western blot 及 ACE2 activity 進行 ACE2 表現測定。結果顯示 ACE2 之 mRNA 表現(Fig. 14A)、蛋白質表現(Fig. 14B)及活性表現(Fig. 15A)於不同 ACE2 KO mice 間所測得之 ACE2 表現量皆具有相同趨勢,相較於 WT mice,hetero mice 之 ACE2 表現量約降低 50%,而 homo 及 hemi mice 之 ACE2 表現量約降低 90%。另外, MMP-2 活性測定顯示,與 WT mice 相比,hetero、homo 及 hemi mice 其 MMP-2 活性分 別提昇了 1.5 倍、1.8 倍及 1.7 倍,顯示於小鼠心臟組織中 ACE2 抑制會提昇其 MMP-2 活性(Fig. 15B)。
3.8 ACE2 過量表現小鼠
由於國外並無販賣 ACE2 overexpression mice,本研究室構築 pCMV-hACE2 基因轉 殖載體並轉染於大鼠心臟細胞株 H9c2 中,經測試結果確認 pCMV-hACE2 轉染之 H9c2 細胞可於 mRNA(Fig. 16A)、蛋白質(Fig. 16B)及酵素活性(Fig. 16C)測得高量之 ACE2 表現,委託台大基因體醫學研究中心,進行 ACE2 過量表現鼠種之建立。
四、結論
在人類的心肌細胞與纖維母細胞可測得 ACE2 的表現,但也有研究指出於新生鼠的
心臟纖維母細胞內僅能測得少量的 ACE2 mRNA,但無法測得 ACE2 之酵素活性[23]。在
7 理由有三:第一,心臟纖維母細胞乃是心肌中最主要的細胞類型[24];第二,心臟纖維母 細胞之 AT1R 數量多於心肌細胞[25];第三,心臟重塑的特徵在於心臟纖維母細胞的增生 與不正常之細胞外基質代謝所導致的心臟纖維化[26, 27],且於心臟中細胞外基質的堆積主 要是由心臟纖維母細胞所負責。 由於心臟纖維母細胞於心臟中是一種非常普遍的細胞類型,且其對於調節正常的心 肌功能扮演著重要的角色,因此本研究主要在探討 angiotensin peptides 對調節心臟纖維 母細胞之 ACE2 表現,以及 ACE2 表現與 gelatinase 之關聯性,藉此探討 ACE2 於心臟 重塑中所扮演的角色。本研究中所得到之重要結果有:(1) Ang II 與 Ang 1-7 分別會經由 AT1R 及 Mas 活化 ERK-MAPK 訊息傳遞途徑以刺激 ACE2 表現; (2) ace2 基因啟動子的 -516/-481 序列上之 5’-ATTTGGA-3’為特定轉錄因子-Ikaros 的結合位置並可藉此調控 ACE2 表現; (3) Ang II 會經由 AT1R 與其下游 MEK 訊號傳遞鏈刺激 ace2 基因啟動子的 -516/+20 序列藉此調控 ACE2 表現; (4) TGF-β1 和 TNF-α 處理 HCFs 對於其 ace2 基因啟 動子活性並無顯著影響; (5) ACE2 過量表現會造成 MMP-2 活性提昇,Ang II 及 Ang 1-7 分別可經由 AT1R 及 Mas 降低 MMP-2 活性表現。另外,本研究成功引進 ACE2 KO mice 且目前正積極建立 ACE2 過量表現鼠種,待 ACE2 過量表現鼠種繁衍成功,此將會是國 內首批 ACE2 過量表現及 ACE2 KO mice,並提供國內 ACE2 相關研究一重要之工具。 依據上述研究成果我們得知 angiotensin peptides 可調控 ACE2 表現,並藉由 ACE2 降解 Ang II 得到 Ang 1-7 以維持 RAS 之平衡調控。另外,ACE2 過量表現會造成 MMP-2 活性提昇,Ang II 及 Ang 1-7 分別可經由 AT1R 及 Mas 降低 MMP-2 活性表現,此結果 顯示 Ang II、Ang 1-7 及 ACE2 皆會影響 MMP-2 活性表現,顯示其於心臟重塑中扮演重 要之調控角色。心臟組織中 ACE2 之表現調節可能是一種針對 Ang II 濃度不正常增加所 產生之重要的心臟生理保護反應,依據以上研究結果,我們認為心臟中不正常的 ACE2 表現調節可能與心房顫動、心肌缺血、高血壓等心臟的病理生理過程有很高的相關性。
五、相關文獻發表及專利
文獻發表:
1. Lin CS*, Pan CH, Wen CH, Yang TH, Kuan TC. 2010. Regulation of angiotensin converting enzyme II by angiotensin peptides in human cardiofibroblasts. Peptides 31:1334-1340. (IF=2.434, 114/261 in PHARMACOLOGY & PHARMACY)
2. Chang CC, Kuan TC, Hsieh YY, Ho YJ, Sun YL, Lin CS*. 2011. Effects of diosgenin on
myometrial matrix metalloproteinase-2 and -9 activity and expression in ovariectomized rats. International Journal of Biological Sciences 7:837-847. (IF=2.699, 158/289 in BIOCHEMISTRY & MOLECULAR BIOLOGY)
8
3. Kuan TC, Yang TH, Wen CH, Chen MY, Lee IL, Lin CS*. 2011. Identifying the
regulatory element for human angiotensin-converting enzyme 2 (ACE2) expression in human cardiofibroblasts. Peptides 32:1832-1839. (IF=2.434, 114/261 in
PHARMACOLOGY & PHARMACY)
4. Chuang YC, Huang WT, Chiang PH, Tang MC, Lin CS*. 2012. Aqueous zymography
screening of matrix metalloproteinase activity and inhibition based on colorimetric gold nanoparticles. Biosensors and Bioelectronics 32:24-31. Feb (IF=5.602, 1/27 in ELECTROCHEMISTRY)
5. Chin-En Kuo, Sheng-Fu Liang*, Shao-Sheng Lu, Tang-Ching Kuan, and Chih-Sheng
Lin*. 2012. Estimation and Prediction of Drug Therapy on The Termination of Atrial Fibrillation by Autoregressive Model with Exogenous Inputs. IEEE Transactions on Information Technology in Biomedicine (In press)
Conference:
1. Wen CH, Pan CH, Lin CS*. 2009. Expression of angiotensin-converting enzyme II is dependent on angiotensin II and angiotensin 1-7 in human cardiofibroblasts.
Seventeenth Symposium on Recent Advances in Cellular and Molecular Biology, Feb. 12, Pingtung, Taiwan.
2. Wen CH, Pan CH, Yang TH, Lin CS*. 2009. Modulation of angiotensin II and angiotensin 1-7 on angiotensin-converting enzyme II expression in human
cardiofibroblasts. The 24 th Joint Annual Conference of Biomedical Science, March 21-22, Taipei, Taiwan.
3. Lin CS*, Yang TH, Wen CH, Pan CH. 2010. Regulation of angiotensin converting enzyme II by angiotensin peptides in human cardiofibroblasts. ESC (Europe Society Cardiology) Congress 2010, July 27 – August 1, Stockholm, Sweden.
4. Wu HT, Sie SS, Chen CY, Kuan TC, CS Lin*. 2012. Curcumin and genistein inhibit TNF-alpha-inducted matrix metalloproteinase-9 (MMP-9) expression by repressing NF-kappa B-mediated transcription. Frontiers in CardioVascular Biology 2012 (Europe Society Cardiology), Mar 30–Apr 1, London, United Kingdom. Cardiovascular Research 93 (Suppl. 1):S53.
5. Lin CS*, Lai SC, Kuan TC, Chen MY, Wu ST. 2012. Activity and expression profiling of matrix metalloproteinases (MMPs) and tissue inhibitors of matrix
metalloproteinases (TIMPs) in the hearts with doxorubicin-induced cardiomyopathy. Frontiers in CardioVascular Biology 2012 (Europe Society Cardiology), Mar 30–Apr 1, London, United Kingdom. Cardiovascular Research 93(Suppl. 1):S121.
9
6. Kuan TC, Chen MY, Yang TH, Wu HT, Lin CS*. 2012. A regulatory element within the promoter of angiotensin-converting enzyme 2 (ace2) gene responsible for angiotensin II stimulation. Frontiers in CardioVascular Biology 2012 (Europe Society Cardiology), Mar 30–Apr 1, London, United Kingdom. Cardiovascular Research 93(Suppl. 1):S93. 專利:
1. Lin CS, Chuang YC, Chen SH. Sensing platform. (Pending IPA-ROC: 98118317) 2. Lin CS, Chuang YC, Chen SH. Sensing platform. (Pending US: 12/567,192)
六、參考文獻
1. Tipnis SR, Hooper NM, Hyde R, et al. 2000. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275:33238-43.
2. Donoghue M, Hsieh F, Baronas E, et al. 2000. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 87:E1-9.
3. Castro-Chaves P, Cerqueira R, Pintalhao M, et al. 2010. New pathways of the renin-angiotensin system: the role of ACE2 in cardiovascular pathophysiology and therapy. Expert Opin Ther Targets 14:485-96.
4. De Mello WC. 2011. Novel aspects of angiotensin II action in the heart. Implications to myocardial ischemia and heart failure. Regul Pept 166:9-14.
5. Vickers C, Hales P, Kaushik V, et al. 2002. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 277:14838-43. 6. Clark MA, Diz DI and Tallant EA. 2001. Angiotensin-(1-7) downregulates the
angiotensin II type 1 receptor in vascular smooth muscle cells. Hypertension 37:1141-6. 7. Grobe JL, Mecca AP, Lingis M, et al. 2007. Prevention of angiotensin II-induced cardiac
remodeling by angiotensin-(1-7). Am J Physiol Heart Circ Physiol 292:H736-42. 8. Huentelman MJ, Grobe JL, Vazquez J, et al. 2005. Protection from angiotensin
II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp Physiol 90:783-90.
9. Dong B, Yu QT, Dai HY, et al. 2012. Angiotensin-converting enzyme-2 overexpression improves left ventricular remodeling and function in a rat model of diabetic
cardiomyopathy. J Am Coll Cardiol 59:739-47.
10. Shenoy V, Ferreira AJ, Qi Y, et al. 2010. The angiotensin-converting enzyme
2/angiogenesis-(1-7)/Mas axis confers cardiopulmonary protection against lung fibrosis and pulmonary hypertension. Am J Respir Crit Care Med 182:1065-72.
10
abrogates bleomycin-induced lung injury. J Mol Med (Berl) 90:637-47.
12. Paizis G, Tikellis C, Cooper ME, et al. 2005. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut 54:1790-6.
13. Osterreicher CH, Taura K, De Minicis S, et al. 2009. Angiotensin-converting-enzyme 2 inhibits liver fibrosis in mice. Hepatology 50:929-38.
14. ez-Freire C, Vazquez J, Correa de Adjounian MF, et al. 2006. ACE2 gene transfer attenuates hypertension-linked pathophysiological changes in the SHR. Physiol Genomics 27:12–9.
15. D´ıez-Freire, J. V´azquez, M. F. Correa De Adjounian et al., 2006. ACE2 gene transfer attenuates hypertension-linked pathophysiologicalchanges in the SHR. Physiological Genomics 27:12–9.
16. Crackower MA, Sarao R, Oudit GY, et al. 2002. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 417:822-8.
17. Yamamoto K, Ohishi M, Katsuya T, et al. 2006. Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension 47:718-26.
18. Bodiga S, Zhong JC, Wang W, et al. 2011. Enhanced susceptibility to biomechanical stress in ACE2 null mice is prevented by loss of the p47(phox) NADPH oxidase subunit. Cardiovasc Res 91:151-61.
19. Kassiri Z, Zhong J, Guo D, et al. 2009. Loss of angiotensin-converting enzyme 2
accelerates maladaptive left ventricular remodeling in response to myocardial infarction. Circ Heart Fail 2:446-55.
20. Chou CF, Loh CB, Foo YK, et al. 2006. ACE2 orthologues in non-mammalian vertebrates (Danio, Gallus, Fugu, Tetraodon and Xenopus). Gene 377:46-55. 21. Cockrell AS, Kafri T. 2007. Gene delivery by lentivirus vectors. Mol Biotechnol
36:184-204.
22. Couto LB, High KA. 2010. Viral vector-mediated RNA interference. Curr Opin Pharmacol 10:534-42.
23. Gallagher PE, Ferrario CM, Tallant EA. 2008. Regulation of ACE2 in cardiac myocytes and fibroblasts. Am J Physiol Heart Circ Physiol 295:H2373-9.
24. Grove D., Zak R., Nair K.G., et al. 1969. Biochemical correlates of cardiac hypertrophy, observations on the cellular organization of growth during myocardial hypertrophy in the rat. Circ Res 2:5473-85.
25. Greenberg B. Cardiac remodeling. In: Walsh RA, editor. 2005. Molecular mechanisms of cardiac hypertrophy and failure, London UK: Taylor and Francis, p.247-64.
26. Porter KE, Turner NA. 2009. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther 123:255-278.
27. Ishiyama Y, Gallagher PE, Averill DB, et al. 2004. Upregulation of
angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension 43:970-976.
11
Fig. 1. Expression of ACE2 mRNA in Ang II-treated human cardiac fibroblast (HCF) cells. Treatment with
Ang II produced a dose-dependent (A) and time-dependent (B) effect on ACE2 mRNA expression. ACE2 expression was normalized against GAPDH, and relative mRNA expression of ACE2 was calculated using the control group as 100%. Values are expressed as mean ± SD (n = 3). *, p < 0.05 vs. control; **, p < 0.01 vs. control.
Fig. 2. Role of the ERK–MAPK signaling pathway of AT1R in Ang II-mediated ACE2 upregulation. The
effect of Ang II on ACE2 mRNA expression was determined in HCF cells pretreated with the AT1R blocker Val and the MEK1/2 inhibitor PD98059 to confirm the ACE2 expression being associated with Ang II treatment (A). The protein expression of phosphorylated MEK1/2 (p-MEK1/2), phosphorylated ERK1/2 (p-ERK1/2) and ACE2 was examined by western blotting (B). p-MEK1/2 (C), p-ERK1/2 (D) and ACE2 (E) expression was normalized using GAPDH expression, and the relative expression of p-MEK1/2, p-ERK1/2 and ACE2 was calculated using the control group as 100%. Values are expressed as mean ± SD (n = 3). *, p < 0.05 vs. control; **, p < 0.01 vs. control; †, p < 0.05 vs. Ang II treatment; ‡, p < 0.01 vs. Ang II treatment.
12
Fig. 3. Distribution and expression of ACE2 in Ang II-treated HCF cells. HCF cells grown on a coverslip
were treated with Ang II with and without prior treatment with the AT1R inhibitor Val for 24 h. Treated cells were washed, fixed, and immunostained for ACE2 (green), and nuclei were counterstained with DAPI (blue). The localization of ACE2 protein was visualized by confocal microscopy (A) and the relative ACE2 fluorescence intensity was calculated using the control group as 100% (B). Values are expressed as mean ± SD (n = 3). **, p < 0.01 vs. control; ‡, p < 0.01 vs. Ang II treatment. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Fig. 4. Expression of ACE2 in HCF cells following Ang 1–7 treatment. The effect of Ang 1–7 on ACE2
mRNA and protein expression was examined by RT-PCR (A) and western blotting (B), respectively.
Upregulation of ACE2 was further confirmed using cells pretreated with the Ang 1–7 Mas receptor blocker A779. ACE2 expression was normalized using GAPDH expression, and the relative expression of ACE2 mRNA was calculated using the control group as 100%. Values are expressed as mean ± SD (n = 3). *, p < 0.05 vs. control; **, p < 0.01 vs. control; †, p < 0.05 vs. Ang 1–7 treatment; ‡, p < 0.01 vs. Ang 1–7 treatment.
13
Fig. 5. Distribution and expression of ACE2 in HCF cells treated with Ang 1–7. HCF cells grown on a
coverslip were treated with Ang 1–7 with or without prior treatment with the Mas receptor blocker A779 for 24 h. Treated cells were washed, fixed, and immunostained for ACE2 (green), and nuclei were counterstained with DAPI (blue). The localization of the ACE2 protein was visualized by confocal microscopy (A) and the relative ACE2 fluorescence intensity was calculated using the control group as 100% (B). Values are expressed as mean ± SD (n = 3). *, p < 0.05 vs. control; †, p < 0.05 vs. Ang 1–7 treatment. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Fig. 6. Effect of blocking the AT1R-mediated signaling pathway on Ang 1–7-affected p-ERK1/2 and ACE2 protein expression in HCF cells. (A) Relative p-ERK1/2 protein expression was determined by western blotting
in HCF cells treated with Ang 1–7 alone or following pretreatment with the Ang 1–7 Mas receptor blocker A779 or the MEK1/2 inhibitor PD98059. Expression of p-ERK1/2 was normalized using GAPDH expression, and the relative expression of p-ERK1/2 was calculated using the control group as 100%. (B) HCF cells were treated with Ang 1–7 with or without prior treatment with the AT1R blocker Val for 24 h. The extracted protein of the cells was analyzed by western blotting. ACE2 expression was normalized using GAPDH expression, and the relative expression of ACE2 was calculated using the control group as 100%. Values are expressed as mean ± SD (n = 3). *, p < 0.05 vs. control; †, p < 0.05 vs. Ang 1–7 treatment.
14
Fig. 7. Promoter activity of the constructs with a series of deleted 5’-flanking region of ace2 gene to drive reporter luciferase expression in HCFs. (A) The diagram indicated the different fragments of 5'-flanking
region of human ace2 gene were fused to the firefly luciferase cDNA in the vector of pGL3-Basic. The position of the promoter fragments relative to transcription start site (+1) was indicated. (B) These constructs with ace2 regulatory region were transfected into HCFs and the luciferase activities were measured 24 h after DNA transfection. Data were presented as relative fold changes of luciferase activity to the pGL3-Basic vector. The diagram integrates the results of three independent experiments and all values are expressed as the mean ± S.D.. ** indicates p < 0.01 compared with the -2069/+20 construct.
Fig. 8. Analyses of the promoter activity of deleted and reversed -516/-481 domain within the 5’-flanking region of ace2 gene. (A) Schematic representation of -516/-481 deleted (-2069~-516/-481~+20) and reversed
domain (-2069~-481/-516~+20) within the -2069/+20 construct. (B) HCFs were transfected with the indicated reporter gene constructs. The cells were lysed 24 h after transfection, and luciferase activities were determined. Data are presented as relative expression of luciferase activity to the (-2069/+20) construct. The diagram integrates the results of three independent experiments. Histograms of all values are expressed as the mean ± S.D.. ** indicates p < 0.01 compared with the -2069/+20 construct.
15
Fig. 9. Sequences identification of the regulatory element within -516/-481 domain. Sequences, -516/-481,
was analyzed for binding element(s) using database TFSEARCH and the sequences of “5’-ATTTGGA-3” was identified as a potential binding element. (A) According to the 5’-ATTTGGA-3 sequences, the constructs with mutant sequences were made by PCR site-directed mutagenesis and mutant sequences were shown in underline blue letters. Seven mutant sequences were designed and the relative element binding score was calculated according to the score in TFSEARCH of -516/-418 domain as 100. (B) HCFs were transfected with the indicated reporter gene constructs. The cells were lysed 24 h after transfection, and luciferase activities were determined. Data are presented as relative expression of luciferase activity to the (-516/+20) construct. Histograms of all values are expressed as the mean ± S.D..
Fig. 10. Mobility shift electrophoresis of the double-stranded oligonucleotides encompassing the -516/-481 domain and its derived sequences. Electrophoretic mobility shift assays with the nuclear extracts from HCFs
were performed. The binding complexes were separated on 6% non-denaturing polyacrylamide gels. (A) HCF extract, nuclear extracts from HCFs; anti-Ikaros antibody, the antibody for Ikaros; labeled (-516/-481), the labeled -516/-481 oligonucleotides only; unlabeled (-516/-481); a 66-fold molar excess of unlabeled -516/-481 oligonucleotides. (B) probe, labeled -516/-481 oligonucleotides only; (-516/-481), labeled -516/-481
oligonucleotides mixed with nuclear extracts from HCFs; M1(-516/-481)~ M7(-516/-481), labeled M1~M7 mutant oligonucleotides (M1~M7 mutant sequences shown in Fig. 3A) mixed with nuclear extracts from HCFs.
16
Fig. 11. Effect of Ang II stimulation on the ACE2 expression in HCFs. (A) In the experiments of ace2
promoter activity affected by Ang II treatment, HCFs were transfected with the -516/+20 and -481/-516/+20 (reversed -516/-481 sequences) constructs and then treated with 0, 0.1, 1 and 10 µM of Ang II. The transfected HCFs were lysed after Ang II treatment for 24 h, the report luciferase activities were determined. Data are presented as relative expression of luciferase activity in the HCFs without Ang II treatment. The diagram integrates results of three independent experiments. (B) The signaling pathway of Ang II-induced ACE2 expression in HCFs was also investigated. HCFs were transfected with the -516/+20 construct, and the cells pre-treated with 1 µg/mL of Valsartan (AT1R inhibitor) or PD98059 (MEK inhibitor) for 1 h and then treated with 1 µg/mL of Ang II. The HCFs were lysed after the Ang II treatments for 24 h, the report luciferase activities were determined. Data are presented as relative expression of luciferase activity to the treatment without
Valsartan or PD98059 co-treatment. ** indicates p < 0.01 as compared with the group without Ang II treatment; † indicates p < 0.05 as compared with the group with Ang II treatment.
Fig. 12. ACE2 and MMP-2 activity of the HCFs infected with ACE2 lentivirus. HCFs infected with ACE2
lentivirus, TLC-ACE2, at 1, 5, 10 and 20 MOI to obtain HCFs/ACE2 cells. The cells were lysed 24 h after infection at different MOI and the ACE2 (A) and MMP-2 (B) activity assay were measured, respectively. ACE2 activity was determined by the ability to cleave the fluorescent substrate at 37°C for 1 h with a specific ACE2 inhibitor. All values are expressed as the mean ± SD from three independent experiments; * and ** indicate p < 0.05 and p < 0.01, respectively, compared to the non-infected HCFs.
17
Fig. 13. MMP-2 activities of the HCFs/ACE2 cells treated with Ang II and Ang 1-7. HCFs/ACE2 cells
pre-treated with 1 μM of valsartan (AT1R inhibitor) and A779 (Mas receptor inhibitor) for 1 h, and then treated with 1 μM of Ang II and Ang 1-7, respectively. HCFs/ACE2 cells also co-infected with TRCN-46697 at 5 MOI and treated with Ang II and Ang 1-7. The cells were lysed 24 h after Ang II and Ang 1-7 treated and then carried out the MMP-2 activity assay. All values are expressed as the mean ± SD from three independent experiments; * and ** indicate p < 0.05 and p < 0.01, respectively, compared to the non-treated HCFs/ACE2 cells.
Fig. 14. The ACE2 expression of ACE2 knockout mice. Wild type and ACE2 knockout mice
(B6;129S5-Ace2tm1Lex/Mmcd; hetero, hemi and homo) were sacrificed and the protein extract of hearts was prepared by homogenizer. The ACE2 expression of heart extract was detected by real-time RT-PCR (A) and western blot (B). ACE2 expression was normalized using by GAPDH expression, and the relative expression of ACE2 was calculated using the control group as 100%. All values are expressed as the mean ± SD from three independent experiments; * and ** indicate p < 0.05 and p < 0.01, respectively, compared to the wild type mice.
18
Fig. 15. The ACE2 and MMP-2 activity of ACE2 knockout mice. Wild type and ACE2 knockout mice
(B6;129S5-Ace2tm1Lex/Mmcd; hetero, hemi and homo) were sacrificed and the protein extract of heart was prepared by homogenizer. The ACE2 (A) and MMP-2 (B) activity of the heart extracts were detected by fluorescence ACE2 activity assay and gelatin zymography assay, respectively. ACE2 activity of medium was detected by the ability to cleave the fluorescent substrate at 37°C for 1 h with a specific ACE2 inhibitor. All values are expressed as the mean ± SD from three independent experiments; * and ** indicate p < 0.05 and p < 0.01, respectively, compared to the wild type mice.
Fig. 16. Transfection and expression of human ACE2 in rat neonatal cardiomyocytes H9c2. H9c2 cells were
transfected with CMV-ACE2 construct and analyses for gene expression were performed after DNA transfection for 48 h. (A) RT-PCR for the gene expression of exogenic human ACE2, endogenous rat ACE2, and rat GAPDH were detected. (B) Western blot detection was performed for the protein expression of human ACE2. We want to note the antibody also cross react with rat ACE2 protein significantly due to sequence homology. (C) The ACE2 enzymatic activity of transfected cells was shown. The results show that the functional ACE2 protein was synthesized in the cardiac cells.