Int. J. Electrochem. Sci., 7 (2012) 7206 - 7224
International Journal of
ELECTROCHEMICAL
SCIENCE
www.electrochemsci.orgElectrochemical and Physicochemical Characterizations of
Butylsulfate-Based Ionic Liquids
I-Wen Sun1, Yuan-Chung Lin2, Bor-Kuan Chen3, Chung-Wen Kuo4, Chi-Chang Chen1, Shyh-Gang Su1, Pin-Rong Chen5, Tzi-Yi Wu 5,*
1
Department of Chemistry, National Cheng Kung University, Tainan 70101, Taiwan 2
Institute of Environmental Engineering, National Sun Yat-Sen University, Kaohsiung 804, Taiwan 3
Department of Materials Engineering, Kun Shan University, Tainan 710, Taiwan 4
Department of Chemical and Materials Engineering, National Kaohsiung University of Applied Sciences, Kaohsiung 80778, Taiwan
5
Department of Chemical and Materials Engineering, National Yunlin University of Science and Technology, Yunlin 64002, Taiwan
*
E-mail: wuty@yuntech.edu.tw
Received: 22 June 2012 / Accepted: 14 July 2012 / Published: 1 August 2012
A series of butylsulfate-based ionic liquids (ILs) are prepared using butylsulfate anions with various cations, the influences of ring structural variations of cations on their thermal property, electrochemical window, density, viscosity, and ionic conductivity are investigated. Good electrochemical stability (ca. 4.7 V) of the butylsulfate-based ILs is confirmed by voltammetric measurements, and the incorporation of methyl group to replace acidic C-2 hydrogen atom of imidazolium cation or replace imidazolium unit by pyrrolidinium or tri(n-propyl)ammonium slightly increases the cathodic stability. The deviations between ILs and ideal KCl line in adjust Walden plot decreases significantly than those in general Walden plot owing to the adjusted Walden plot takes the difference of ion size into account.
Keywords: Ionic liquid, molten salt, conductivity, electrochemical window, Walden rule
1. INTRODUCTION
Over the past decade much attention has been given to ionic liquids (ILs), a class of compounds comprised only of ions, with melting points below 100 oC [1]. They are composed of organic cations and organic or inorganic anions and are called “designer solvents” because desired properties can be obtained by combination of their ions [2,3]. These ionic compounds are being explored as potential friendly-environmental solvents due to their negligible vapor pressure in comparison to traditional volatile organic solvents and they are widely used in various electrochemical devices, such as lithium
or electrochemical sensors [4-14], lithium-ion batteries [15-19], electrodeposition [20-24], dye-sensitized solar cells [25-29], fuel cell [30], and electrochemical capacitors [31,32].
Physicochemical properties of bulk ILs are of immense importance for electrochemical and engineering applications [33-43], for instance, viscosity of ILs has fundamental importance, as all the equations expressing the flow of fluids contain this important property and can provide information about various classes of fluids on molecular basis. Viscosity as a transport property has a significant impact on the rate of mass transport and thus is an important factor. Several chemicals and products characteristic can be largely determined by numerical values of viscosity. Temperature dependent viscosity of ILs is more complicated than many molecular solvents because most of them do not follow the typical Arrhenious behaviour. For instance, Okoturo et al. [44] presented a systematic study of viscosity of ILs and showed that the temperature variation of viscosity of ILs follows the Arrhenious equation or Vogel–Tamman–Fulcher (VTF) equation. With the aim of enhancing the understanding of unusual characteristics of RTILs, numerous studies on their thermophysical properties such as viscosity, density, ionic conductivity, molar conductivity, diffusion coefficient etc. have been carried out [45-49]. In the past several years, the most commonly studied ILs contain an imidazolium cation with varying heteroatom functionality. Imidazolium based ILs especially those based on the (PF6−, BF4−, or [(CF3SO2)2N]−) anion are considered historically the most important and commonly investigated. However, the hydrolytical instability of such anions has become obvious, and decomposition of these fluorinated anions leads to the formation of highly toxic and corrosive HF, the use of these ionic liquids will be limited [50]. Accordingly, the synthesis and application of halogen free ionic liquids is necessary. Several types of these ionic liquids are based on alkyl sulfunates, organoborates and alkyl sulfate anions. The most important properties of alkyl sulfate based ionic liquids are easily synthesis, using cheap alkylating agent, solvent free synthesis, high reaction rate, excellent purity, large scale synthesis, safe and nontoxic chemical, low viscosities, and low melting points [51]. In this work, several butylsulfate-based ILs are reported, and their physicochemical and electrochemical properties such as the density, viscosity, thermal property, ionic conductivity, and potential window are studied in detail.
2. EXPERIMENTAL
2.1. Materials and measurement
All starting materials were purchased from Aldrich, Lancaster, TCI, and Acros and used as received. The conductivity () of the ILs was systematically measured with a conductivity meter LF 340 and a standard conductivity cell TetraCon 325 (Wissenschaftlich-Technische Werkstätten GmbH, Germany). The cell constant was determined by calibration after each sample measurement using an aqueous 0.01 M KCl solution. The density of the ILs was measured with a dilatometer, which was calibrated by measuring the density of neat glycerin at 30, 40, 50, 60, 70, and 80 °C. To measure the density, IL or binary mixture was placed into the dilatometer up to the mark, sealed the top of capillary tube, which was on the top of the dilatometer, and placed into a temperature bath for 10 min to allow
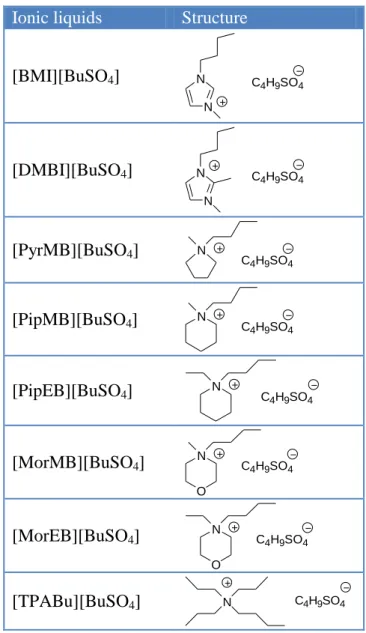
the temperature to equilibrate. The main interval between two marks in capillary tube is 0.01 cm3, and the minor interval between two marks is 0.001 cm3. From the correction coefficient of glycerin in capillary tube at various temperatures, we can calculate the density of neat IL or binary system by the expanded volume of liquid in capillary tube at various temperatures. Each sample was measured at least three times to determine an average value, and the values of the density are ±0.0001 g mL−1. The viscosities (η) of the ILs were measured using a calibrated modified Ostwald viscometer (Cannon-Fenske glass capillary viscometers, CFRU, 9721-A50) with inner diameters of 1.2 ± 2% mm. The viscometer was placed in a thermostatic water bath (TV-4000, TAMSON), in which the temperature was regulated to within ± 0.01 K. The flow time was measured with a stop watch capable of recording to 0.01 s. For each IL, the experimental viscosity was obtained by averaging three to five flow time measurements. The melting point of each IL was analyzed by using a differential scanning calorimeter (DSC, Perkin–Elmer Pyris 1) in the temperature range -140 oC to a predetermined temperature. The sample was sealed in an aluminum pan, and then heated and cooled at a scan rate of 10 oC min-1 under a flow of nitrogen. The thermal data were collected during the second heating–cooling scan. The thermal stabilities were measured with TGA (Perkin–Elmer, 7 series thermal analysis system). The sample was heated at 20 oC min-1 from room temperature to 800 oC under nitrogen. The water content of the dried ILs was detected by a Karl–Fischer moisture titrator (Metrohm 73KF coulometer), and the values were less than 300 ppm. NMR spectra of synthetic ionic liquids were recorded on a BRUKER AV500 spectrometer in D2O and calibrated with tetramethylsilane (TMS) as the internal reference. Cyclic voltammetry was performed at 25oC using an electrochemical workstation (CH instruments Inc., CHI, model 750A). The electrochemical cell consist a glassy carbon working electrode, a Pt wire counter electrode, and a Pt quasi-reference electrode. All electrochemical experiments were performed under a dry argon atmosphere to avoid the presence of oxygen and air humidity. The structures of the ILs studied in the present work are shown in Table 1.
2.2. Synthetic procedure of butylsulfate-based ILs
2.2.1. 1-Butyl-3-methyl-imidazolium butyl sulfate ([BMI][BuSO4])
Dibutyl sulfate (75.7 g, 360 mmol) was added dropwise to a solution of equal molar amounts of 1-methylimidazole (29.56 g, 360 mmol) in 200 mL toluene, and then cooled in an ice-bath under nitrogen at a rate that maintained the reaction temperature below 313.15 K (highly exothermic reaction). The reaction mixture was stirred at room temperature for 4h. After the reaction stopped, the upper organic phase of the resulting mixture was decanted, and the lower ionic liquid phase was washed with ethyl acetate (4 x 70 mL). After the last washing, the remaining ethyl acetate was removed by rotavapor under reduced pressure. The IL obtained was dried by heating at (343.15 to 353.15) K and stirring under a high vacuum (2 10-1 Pa) for 48 h. In order to reduce the water content to negligible values (lower than 0.03 mass %), a vacuum (2 x 10-1 Pa) and moderate temperature (343.15 K) were applied to the IL for 2 days. The IL was kept in bottles under an inert gas.
Yield: 91 %. 1H NMR (300 MHz, D2O, ppm): 8.63 (s, 1H, imidazole hydrogen), 7.38 (d, 2H, imidazole hydrogen), 4.12 (t, 2H, N-CH2CH2), 3.97 (t, 2H, -CH2SO4), 3.81 (s, 3H, N-CH3), 1.76 (m,
2H, N-CH2CH2-), 1.56 (m, 2H, -CH2CH2SO4), 1.29 (m, 4H, N-CH2CH2CH2- and -CH2CH2CH2SO4), 0.83 (m, 6H, N-CH2CH2CH2CH3 and CH3CH2CH2CH2SO4). Elem. Anal. Calcd. for C12H24N2O4S: C, 49.29 %; H, 8.27 %; N, 9.58 %. Found: C, 49.38 %; H, 8.32 %; N, 9.50 %.
Table 1. The denominations and chemical structures of butylsulfate-based ILs.
Ionic liquids Structure
[BMI][BuSO4] C 4H9SO4 N N [DMBI][BuSO4] C 4H9SO4 N N [PyrMB][BuSO4] N C 4H9SO4 [PipMB][BuSO4] N C4H9SO4 [PipEB][BuSO4] N C4H9SO4 [MorMB][BuSO4] N C4H9SO4 O [MorEB][BuSO4] N C4H9SO4 O [TPABu][BuSO4] N C4H9SO4
2.2.2. 1-Butyl-2,3-dimethyl-imidazolium butyl sulfate [DMBu][BuSO4]
Yield: 88 %. 1H NMR (300 MHz, D2O, ppm): 7.27 (d, 2H, imidazole hydrogen), 4.02 (t, 2H, N-CH2-), 3.96 (t, 2H, -CH2SO4), 3.68 (s, 3H, N-CH3), 2.50 (s, 3H, C-CH3), 1.70 (m, 2H, N-CH2CH2-), 1.56 (m, 2H, -CH2CH2SO4), 1.27 (m, 4H, N-CH2CH2CH2- and -CH2CH2CH2SO4), 0.83 (m, 6H, N-CH2CH2CH2CH3 and CH3CH2CH2CH2SO4). Elem. Anal. Calcd. for C13H26N2O4S: C, 50.96 %; H, 8.55 %; N, 9.14 %. Found: C, 50.88 %; H, 8.48 %; N, 9.02 %.
2.2.3. 1-Butyl-1-methylpyrrolidinium butyl sulfate [PyrMB][BuSO4]
Yield: 91 %. 1H NMR (300 MHz, D2O, ppm): 3.95 (t, 2H, -CH2SO4), 3.38 (m, 4H, N-CH2-), 3.21 (m, 2H, N-CH2-), 2.92 (s, 3H, N-CH3), 2.10 (m, 4H, N-CH2CH2-), 1.67 (m, 2H, -CH2CH2SO4), 1.55 (m, 2H, N-CH2CH2-), 1.28 (m, 4H, N-CH2CH2CH2- and -CH2CH2CH2SO4), 0.82 (m, 6H, N-CH2CH2CH2CH3 and CH3CH2CH2CH2SO4). Elem. Anal. Calcd. for C13H29NO4S: C, 52.85 %; H, 9.89 %; N, 4.74 %. Found: C, 52.68 %; H, 9.79 %; N, 4.65 %.
2.2.4. 1-Butyl-1-methylpiperidinium butyl sulfate [PipMB][BuSO4]
Yield: 86 %. 1H NMR (300 MHz, D2O, ppm): 3.98 (t, 2H, -CH2SO4), 3.22 (m, 6H, N-CH2-), 2.93 (s, 3H, N-CH3), 1.79 (m, 4H, N-CH2CH2- and -CH2CH2SO4), 1.61 (m, 6H, N-CH2CH2- and CH2CH2CH2-), 1.31 (m, 4H, CH2CH2CH2- and -CH2CH2CH2SO4), 0.85 (m, 6H, N-CH2CH2CH2CH3 and CH3CH2CH2CH2SO4). Elem. Anal. Calcd. for C14H31NO4S: C, 54.34 %; H, 10.10 %; N, 4.53 %. Found: C, 54.18 %; H, 10.03 %; N, 4.39 %.
2.2.5. 1-Butyl-1-ethylpiperidinium butyl sulfate [PipEB][BuSO4]
Yield: 82 %. 1H NMR (300 MHz, D2O, ppm): 3.96 (t, 2H, -CH2SO4), 3.28-3.12 (m, 8H, N-CH2-), 1.76 (m, 4H, N-CH2N-CH2-), 1.57 (m, 6H, N-CH2CH2-, N-CH2CH2CH2-, and -CH2CH2SO4), 1.29 (m, 4H, CH2CH2CH2- and -CH2CH2CH2SO4), 1.17 (t, 3H, CH2CH3), 0.84 (m, 6H, N-CH2CH2CH2CH3 and CH3CH2CH2CH2SO4). Elem. Anal. Calcd. for C15H33NO4S: C, 55.69 %; H, 10.28 %; N, 4.33 %. Found: C, 55.46 %; H, 10.21 %; N, 4.21 %.
2.2.6. 4-Butyl-4-methylmorpholinium butyl sulfate [MorMB][BuSO4]
Yield: 89 %. 1H NMR (300 MHz, D2O, ppm): 3.98 (m, 6H, -CH2SO4 and OCH2-), 3.39 (m, 6H, N-CH2-), 3.10 (s, 3H, N-CH3), 1.68 (m, 2H, -CH2CH2SO4), 1.57 (m, 2H, N-CH2CH2-), 1.32 (m, 4H, N-CH2CH2CH2- and -CH2CH2CH2SO4), 0.86 (m, 6H, N-CH2CH2CH2CH3 and CH3CH2CH2CH2SO4). Elem. Anal. Calcd. for C13H29NO5S: C, 50.13 %; H, 9.39 %; N, 4.50 %. Found: C, 50.98 %; H, 9.36 %; N, 4.37 %.
2.2.7. 4-Butyl-4-ethylmorpholinium butyl sulfate [MorEB][BuSO4]
Yield: 80 %. 1H NMR (300 MHz, D2O, ppm): 3.98 (m, 6H, -CH2SO4 and OCH2-), 3.44 (m, 6H, N-CH2-), 3.33 (t, 2H, N-CH2-), 1.60 (m, 4H, N-CH2CH2- and -CH2CH2SO4), 1.35-1.21 (m, 7H, N-CH2CH3, N-CH2CH2CH2-, and -CH2CH2CH2SO4), 0.86 (m, 6H, N-CH2CH2CH2CH3 and CH3CH2CH2CH2SO4). Elem. Anal. Calcd. for C14H31NO5S: C, 51.66 %; H, 9.60 %; N, 4.30 %. Found: C, 51.45 %; H, 9.62 %; N, 4.18 %.
2.2.8. N,N,N-tripropylbutan-1-aminium butyl sulfate [TPABu][BuSO4]
Yield: 88 %. 1H NMR (300 MHz, D2O, ppm): 3.96 (t, 2H, -CH2SO4), 3.06 (m, 8H, N-CH2CH2 -), 1.58 (m, 10H, N-CH2CH2- and -CH2CH2SO4-), 1.28 (m, 4H, N-CH2CH2CH2- and -CH2CH2CH2SO4-), 0.85 (m, 15H, N-CH2CH2CH3, N-CH2CH2CH2CH3, and CH3CH2CH2CH2SO4). Elem. Anal. Calcd. for C17H39NO4S: C, 57.75 %; H, 11.12 %; N, 3.96 %. Found: C, 57.56 %; H, 11.03 %; N, 3.87 %.
3. RESULTS AND DISCUSSION 3.1. Thermal Property
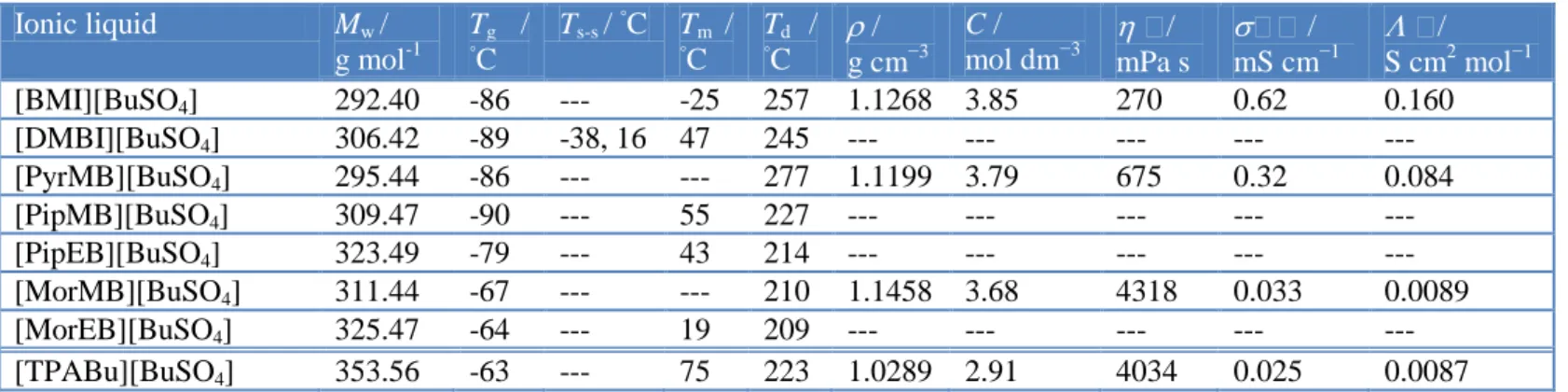
Table 2 summarizes the melting points and glass phase transitions along with the thermal decomposition onset temperatures and physicochemical quantities of density (), viscosity (), conductivity (σ), and concentration (C) at 30 oC. The thermal properties of the eight ILs were investigated using thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC), and their TGA traces are shown in Fig. 1. As shown in Fig. 1, the eight ILs show one stage decomposition behavior, all of the thermal decomposition onset temperatures (5 % weight loss) are above 209 oC, with the highest being found for [PyrMB][BuSO4] (277 oC). The Td of these ILs has some relationship with the cations, the thermal stability of cations is pyrrolidium > imidazolium > piperidinium and morpholinium. These ionic liquids have potential usage as alternative to conventional organic solvents due to their thermal stability. The glass temperature (Tg) and melting temperature (Tm) can be obtained from the DSC thermograms during the programmed reheating step. From the DSC, there are three endothermic peaks for [DMBI][BuSO4], which indicated that it probably has the transition from solid to solid at -38 and 16 oC and melting at 47 oC. The Tm of a compound is associated with the strength of its crystal lattice, which is controlled by intermolecular forces, molecular symmetry, and degrees of the freedom of the molecule.
Table 2. Physical and thermal properties of butylsulfate-based ILs. Ionic liquid Mw / g mol-1 Tg / ◦C Ts-s / ◦ C Tm / ◦C T◦C d / / g cm−3 C / mol dm−3 / mPa s / mS cm−1 / S cm2 mol−1 [BMI][BuSO4] 292.40 -86 --- -25 257 1.1268 3.85 270 0.62 0.160 [DMBI][BuSO4] 306.42 -89 -38, 16 47 245 --- --- --- --- --- [PyrMB][BuSO4] 295.44 -86 --- --- 277 1.1199 3.79 675 0.32 0.084 [PipMB][BuSO4] 309.47 -90 --- 55 227 --- --- --- --- --- [PipEB][BuSO4] 323.49 -79 --- 43 214 --- --- --- --- --- [MorMB][BuSO4] 311.44 -67 --- --- 210 1.1458 3.68 4318 0.033 0.0089 [MorEB][BuSO4] 325.47 -64 --- 19 209 --- --- --- --- --- [TPABu][BuSO4] 353.56 -63 --- 75 223 1.0289 2.91 4034 0.025 0.0087 a
Density (), concentration (C), viscosity (), conductivity () and molar conductivity () are measured at 30 ◦C.
b
ACKNOWLEDGEMENTS
The authors would like to thank the National Science Council of the Republic of China for financially supporting this project.
References
1. R.D. Rogers, K.R. Seddon, Ionic Liquids: Industrial Applications for Green Chemistry, American Chemical Society, Washington, DC, 2003.
2. T. Torimoto, T. Tsuda, K. Okazaki, S. Kuwabata, Adv. Mater., 22 (2010) 1196.
3. T.Y. Wu, S.G. Su, K.F. Lin, Y.C. Lin, H.P. Wang, M.W. Lin, S.T. Gung, I.W. Sun, Electrochim. Acta, 56 (2011) 7278.
4. M.R. Ganjali, H. Ganjali, M. Hosseini, P. Norouzi, Int. J. Electrochem. Sci., 5 (2010) 967.
5. M.R. Ganjali, S. Aghabalazadeh, M. Rezapour, M. Hosseini, P. Norouzi, Int. J. Electrochem. Sci., 5 (2010) 1743.
6. M. Pandurangachar, B.E.K. Swamy, B.N. Chandrashekar, O. Gilbert, S. Reddy, B.S. Sherigara, Int. J. Electrochem. Sci., 5 (2010) 1187.
7. P. Norouzi, Z. Rafiei-Sarmazdeh, F. Faridbod, M. Adibi, M.R. Ganjali, Int. J. Electrochem. Sci., 5 (2010) 367.
8. F. Faridbod, M.R. Ganjali, M. Pirali-Hamedani, P. Norouzi, Int. J. Electrochem. Sci., 5 (2010) 1103.
9. M.R. Ganjali, M.H. Eshraghi, S. Ghadimi, S.M. Moosavi, M. Hosseini, H. Haji-Hashemi, P. Norouzi, Int. J. Electrochem. Sci., 6 (2011) 739.
10. M.R. Ganjali, T. Poursaberi, M. Khoobi, A. Shafiee, M. Adibi, M. Pirali-Hamedani, P. Norouzi, Int. J. Electrochem. Sci., 6 (2011) 717.
11. P. Norouzi, M. Hosseini, M.R. Ganjali, M. Rezapour, M. Adibi, Int. J. Electrochem. Sci., 6 (2011) 2012.
12. M.R. Ganjali, M.R. Moghaddam, M. Hosseini, P. Norouzi, Int. J. Electrochem. Sci., 6 (2011) 1981. 13. M.R. Ganjali, M. Hosseini, M. Pirali-Hamedani, H.A. Zamani, Int. J. Electrochem. Sci., 6 (2011)
2808.
14. M.R. Ganjali, M. Rezapour, S.K. Torkestani, H. Rashedi, P. Norouzi, Int. J. Electrochem. Sci., 6 (2011) 2323.
15. M.T. Montañés, R. Sánchez-Tovar, J. García-Antón, V. Pérez-Herranz, Int. J. Electrochem. Sci., 5 (2010) 1934.
16. V.S.R. Channu, R. Holze, E.H. Walker, S.A. Wicker, R.R. Kalluru, Q.L. Williams, W. Walters, Int. J. Electrochem. Sci., 5 (2010) 1355.
17. Z.H. Li, Q.L. Xia, L.L. Liu, G.T. Lei, Q.Z. Xiao, D.S. Gao, X.D. Zhou, Electrochim. Acta, 56 (2010) 804.
18. A.Lewandowski, A. Świderska-Mocek, I. Acznik, Electrochim. Acta, 55 (2010) 1990.
19. G.H. Lane, A.S. Best, D.R. MacFarlane, M. Forsyth, P.M. Bayley, A.F. Hollenkamp, Electrochim. Acta, 55 (2010) 8947.
20. V. Lair, J. Sirieix-Plenet, L. Gaillon, C. Rizzi, A. Ringuede, Electrochim. Acta, 56 (2010) 784. 21. Y.L. Zhu, Y. Katayama, T. Miura, Electrochim. Acta, 55 (2010) 9019.
22. M.C. Tsai, D.X. Zhuang, P.Y. Chen, Electrochim. Acta, 55 (2010) 1019.
23. S. Zein El Abedin, P. Giridhar, P. Schwab, F. Endres, Electrochem. Commun., 12 (2010) 1084. 24. A.Lisenkov, M.L. Zheludkevich, M.G.S. Ferreira, Electrochem. Commun., 12 (2010) 729.
25. T.Y. Wu, M.H. Tsao, F.L. Chen, S.G. Su, C.W. Chang, H.P. Wang, Y.C. Lin, W.C. Ou-Yang, I.W. Sun, Int. J. Mol. Sci., 11 (2010) 329.
26. T.Y. Wu, M.H. Tsao, F.L. Chen, S.G. Su, C.W. Chang, H.P. Wang, Y.C. Lin, I.W. Sun, J. Iran Chem. Soc., 7 (2010) 707.
27. M.H. Tsao, T.Y. Wu, H.P. Wang, I.W. Sun, S.G. Su, Y.C. Lin, C.W. Chang, Mater. Lett., 65 (2011) 583.
28. S.H. Jeon, A.R.S. Priya, E.J. Kang, K.J. Kim, Electrochim. Acta, 55 (2010) 5652.
29. T.Y. Wu, M.H. Tsao, S.G. Su, H.P. Wang, Y.C. Lin, F.L. Chen, C.W. Chang, I.W. Sun, J. Braz. Chem. Soc., 22 (2011) 780.
30. G. Lakshminarayana, M. Nogami, Electrochim. Acta, 55 (2010) 1160.
31. K.K. Denshchikov, M.Y. Izmaylova, A.Z. Zhuk, Y.S. Vygodskii, V.T. Novikov, A.F. Gerasimov, Electrochim. Acta, 55 (2010) 7506.
32. R. Mysyk, E. Raymundo-Piñero, M. Anouti, D. Lemordant, F. Béguin. Electrochem. Commun., 12 (2010) 414.
33. T.Y. Wu, L. Hao, C.W. Kuo, Y.C. Lin, S.G. Su, P.L. Kuo, I.W. Sun, Int. J. Electrochem. Sci., 7 (2012) 2047.
34. T.Y. Wu, B.K. Chen, L. Hao, C.W. Kuo, I.W. Sun, J. Taiwan Inst. Chem. Eng., 43 (2012) 313. 35. T.Y. Wu, I.W. Sun, S.T. Gung, B.K. Chen, H.P. Wang, S.G. Su, J. Taiwan Inst. Chem. Eng., 43
(2012) 58.
36. S.Y. Ku, S.Y. Lu, Int. J. Electrochem. Sci., 6 (2011) 5219.
37. T.Y. Wu, B.K. Chen, L. Hao, Y.C. Lin, H.P. Wang, C.W. Kuo, I.W. Sun, Int. J. Mol. Sci., 12 (2011) 8750.
38. T.Y. Wu, B.K. Chen, L. Hao, Y.C. Peng, I.W. Sun, Int. J. Mol. Sci., 12 (2011) 2598.
39. T.Y. Wu, I.W. Sun, S.T. Gung, B.K. Chen, H.P. Wang, S.G. Su, J. Taiwan Inst. Chem. Eng., 42 (2011) 874.
40. T.Y. Wu, S.G. Su, S.T. Gung, M.W. Lin, Y.C. Lin, W.C. Ou-Yang, I.W. Sun, C.A. Lai, J. Iran. Chem. Soc., 8 (2011) 149.
41. T.Y. Wu, S.G. Su, H.P. Wang, Y.C. Lin, S.T. Gung, M.W. Lin, I.W. Sun, Electrochim. Acta, 56 (2011) 3209.
42. T.Y. Wu, B.K. Chen, L. Hao, K.F. Lin, I.W. Sun, J. Taiwan Inst. Chem. Eng., 42 (2011) 914. 43. T.Y. Wu, I.W. Sun, S.T. Gung, M.W. Lin, B.K. Chen, H.P. Wang, S.G. Su, J. Taiwan Inst. Chem.
Eng., 42 (2011) 513.
44. O.O. Okoturo, T.J. Vander Noot, J. Electroanal. Chem., 568 (2004) 167.
45. T.Y. Wu, H.C. Wang, S.G. Su, S.T. Gung, M.W. Lin, C.B. Lin, J. Taiwan Inst. Chem. Eng., 41 (2010) 315.
46. K. Liu, Y.X. Zhou, H.B. Han, S.S. Zhou, W.F. Feng, J. Nie, H. Li, X.J. Huang, M. Armand, Z.B. Zhou, Electrochim. Acta, 55 (2010) 7145.
47. T.Y. Wu, H.C. Wang, S.G. Su, S.T. Gung, M.W. Lin, C.B. Lin, J. Chin. Chem. Soc., 57 (2010) 44. 48. H.B. Han, K. Liu, S.W. Feng, S.S. Zhou, W.F. Feng, J. Nie, H. Li, X.J. Huang, H. Matsumoto, M.
Armand, Z.B. Zhou, Electrochim. Acta, 55 (2010) 7134.
49. T.Y. Wu, S.G. Su, Y.C. Lin, H.P. Wang, M.W. Lin, S.T. Gung, I.W. Sun, Electrochim. Acta, 56 (2010) 853.
50. A.B. Pereiro, A. Rodríguez, J. Chem. Eng. Data, 52 (2007) 600.
51. S. Himmler, S. Hörmann, R. Hal, P.S. Schulz, P. Wasserscheid. Green Chem., 8 (2006) 887. 52. Z.-B. Zhou, H. Matsumoto, K. Tatsumi, Chem. Eur. J., 11 (2005) 752.
53. J. Jacquemin, P. Husson, A.A.H. Padua, V. Majer, Green Chem., 8 (2006) 172.
54. K.R. Seddon, A.S. Starck, M.J. Torres, ACS Symposium Series 901, Washington, DC, 2004. 55. P. Bonhote, A.P. Dias, N. Papageorgiou, K. Kalyanasundaram, M. Grätzel, Inorg. Chem., 35
(1996) 1168.
56. W. Xu, E.I. Cooper, C.A. Angell, J. Phys. Chem. B, 107 (2003) 6170.
57. D.R. MacFarlane, M. Forsyth, E.I. Izgorodina, A.P. Abbott, G. Annat, K. Fraser, Phys. Chem. Chem. Phys., 11 (2009) 4962.
58. N. Senda, Winmostar, version 3.78f (URL: http://winmostar.com/).
Zhou, Electrochim. Acta, 55 (2010) 7145.
60. T.Y. Wu, S.G. Su, H.P. Wang, I.W. Sun, Electrochem. Commun., 13 (2011) 237.