Elsevier Science Publishers B.V., Amsterdam - Printed in The Netherlands
PRE-CONCENTRATION EFFICIENCY OF CHELEX-100 RESIN
FOR HEAVY METALS IN SEAWATER
Part 1. Effects of pH and Salts on the Distribution Ratios of Heavy Metals
SU-CHENG PAI*
Institute of Oceanography, National Taiwan University, Taipei (Taiwan) PAI-YEE WHUNG and RUEI-LUNG LA1
Department of Oceanography, National Taiwan College of Marine Science and Technology, Keelung (Taiwan)
(Received 10th December 198_7)
SUMMARY
The chelating characteristics of Chelex-100 resin were studied for selected heavy metals in seawater medium. The results of batch equilibrium and breakthrough experiments show that the metal-chelating efficiency of the resin is lower in seawater than in freshwater. These differences are caused by the complicated speciation of heavy metals in seawater medium and by the high concentrations of magnesium and calcium present which act as competitors to heavy metal ions. The optimal pH values for column operation are strongly affected by the salt matrix. Careful choice of experimental conditions is necessary to avoid losses of cadmium and manganese from seawater. For better performance, seawater samples should be adjusted to pH 6-7 before loading onto a Chelex-100 column.
Seawater contains a variety of trace metals at concentrations which rarely exceed 1 pg 1-l in open oceans [ 1,2]. To determine elements at such low levels, Slavin [3] has concluded that the most practical way is to pre-concentrate those elements by using extraction with ammonium pyrrolidinedithiocarba- mate into methyl isobutyl ketone, or on a Chelex-100 column, followed by graphite-furnace atomic absorption spectrometry. The Chelex-100 technique, which is easily applied on board ship, has received a great deal of attention by many chemical oceanographers.
Chelex-100 is the commercial name of a polystrene structured resin with iminodiacetate functional groups (Bio-Rad Laboratories). Its high chelating selectivity makes it suitable for separating trace heavy metals from major al- kali and alkaline earth salts. The first application of Chelex-100 in seawater analysis was reported by Riley and Taylor 143 and the method has gained extensive popularity because of its practical simplicity and the low analytical blanks. However, arguments about the quantitative efficiency of the resin for
258
pre-concentrating trace metals from seawater have continued for years and various improvements have been made. Florence and Batley [ 5 ] demonstrated that the column should be used in the ammonium form, rather than the hy- drogen or sodium form, for higher recoveries. Pakalns and Batley [6] found that the hydrolysis of certain trace metals could occur in the column, thus causing incomplete absorption of manganese and iron. Kingston et al. [ 71 sug- gested that the seawater should be adjusted to an optimal pH of 5.0-5.5 before being loaded onto the column and that the column should be washed with ammonium acetate to remove magnesium and calcium, to obtain an almost matrix-free solution for subsequent determination. Bruland et al. [8] applied Chelex-100 to seawater at its natural pH and found incomplete collection of copper. A careful re-examination of the technique was made by Sturgeon et al. [ 91 who showed that at pH 5.4 the Chelex-100 technique indeed provided much lower recoveries of trace metals in seawater than in freshwater. They con- cluded that colloidal or organic species of trace metals are responsible for the analytical losses. Recent work by Paulson [lo] also illustrated that the results obtained by Chelex-100 tend to be underestimates. Nevertheless, the Chelex- 100 technique is still ranked by Boniforti et al. [ 111 as the most appropriate method for preconcentrating Fe, Co, Zn, Cu, and Ni on board ship.
During a review of previous publications, it appeared, surprisingly, that some investigators assumed that the optimal pH range for Chelex-100 column op- eration is 5.0-5.5, and that this assumption was based on earlier tests by Ley- den and Underwood [ 121 in non-seawater media. Other workers chose different pH ranges (6.6-6.8 or 7.8-8.2) [6, 81 but their reason was not specified. As seawaters are far more complicated than freshwaters, so that the optimal op- erating pH ranges for these waters are probably not the same, a comparison of the efficiency of the Chelex-100 technique in the two media was deemed nec- essary. In this paper, the chelating efficiencies of Chelex-100 resin for selected heavy metals in both freshwater and seawater media are re-examined. The main aims were to clarify the salt effect and to establish the optimum pH for seawater analysis. In these experiments, high trace-metal concentrations (O.l- 1 mg 1-l) were used so that problems arising from interferences and contam- ination could be easily controlled.
EXPERIMENTAL
Materials and instrumentation
Chelex-100 resin and column preparation. Preliminary tests on the commer- cial resin (100-200 mesh, sodium form; Bio-Rad Laboratories) showed that the resin as provided contained 21% (w/w) resin (dry weight); 1 g of the com- mercial resin formed a bed volume of ca. 1.3 ml in distilled water. For quanti- tative reasons, the commercial resin was pre-weighed in portions (e.g., 1.00 ? 0.02 g ) and sealed in 50-ml Pyrex beakers.
Disposable flexible columns were made by combining different sizes of tygon and silicone rubber tubings as shown in Fig. 1. For packing the columns, the pre-weighed resin was washed into the column through the funnel and 20 ml of distilled vvater was allowed to drain through. The specified resin holds water under hydrostatic pressure without letting the resin bed go dry. The column was converted to the H+-form by passing > 10 ml of 2 M nitric acid and 10 ml of distilled water. For conversion of the H+-form to the sodium, potassium or ammonium form, the Chelex-100 columns were treated with 10 ml of 1 M NaOH, 1 M KOH or 1 M ammonia solution, respectively, followed by 20 ml of distilled water. For conversion to the magnesium or calcium form, the column in the Na+-form was further treated with 10 ml of 1 M magnesium or calcium chloride. All prepared columns were subsequently washed with at least 5 bed volumes of distilled water.
Solutions. Merck general-reagent-grade chemicals were used except where specified. Concentrated nitric and hydrochloric acids were freshly distilled and stored in Pyrex glass bottles. They were diluted to 2 M as required. Sodium hydroxide solution (1 M), was passed through a Chelex-100 column (2 g, so- dium form) in order to remove some particles and impurities. After the column treatment, the solution still contained some Ni, Cu and Zn, but not at inter- fering levels for the present work. For the preparation of ammonia solutions,
PHI
I --/
20 40 60 80 100 rio 140 160 160 ml
f- distilled water- --*- -seawater- +
Fig. 1. The Chelex-100 columns used: (1) tygon tubing, 1 cm o.dJ0.63 cm i.d.; (2) tygon tubing, 0.6 cm o.dJ0.48 cm i.d.; (3) silicone rubber tubing, 5 mm o.d./3 mm i.d.; (4) 30-ml Pyrex funnel;
(5) Chelex-100 resin (100-200 mesh); (6) glass wool.
Fig. 2. The pH change of the effluent from different forms of Chelex-100; each column contained 2 g (wet weight) of resin.
260
ammonia liquor was distilled and the vapor was bubbled through distilled water until equilibrium was reached.
Ammonium acetate buffer (1 M) was prepared by mixing 57 ml of anhydrous acetic acid’and 75 ml of purified 25% ammonia liquor and diluting to 11. The pH was adjusted to ca. 6.5 with hydrochloric acid and ammonia. Sodium ace- tate solution (1 M) was adjusted to ca. 6.5 similarly. Solutions (1 M) of mag- nesium chloride and calcium chloride were prepared, anhydrous acetic acid (5 ml 1-l) was added to each solution so that the pH could be adjusted to ca. 6.5 with HCl and NaOH.
Purification of buffer and salt solutions. The sodium acetate, magnesium chloride and calcium chloride solutions were passed, through Chelex-100 col- umns (containing 5 g of resin, sodium form) at ca. 2 ml min-‘; the first 25-ml portion of the effluent was discarded and the rest was collected in Pyrex bottles. Ammonium acetate solution was treated in the same manner with an ammo- nium-form Chelex-100 column.
Artificial seawater. Artificial seawater (25 1) was prepared as suggested by Kester et al. [ 131. High impurities of Fe, Mn, Ni, and Zn were found; therefore, the preparation was further treated by passing through a Chelex-100 column
(10 g of resin, sodium form) at a flow rate not exceeding 10 ml min-‘.
Atomic absorption spectrometry. A Hitachi model Z-8000 polarized Zeeman- effect spectrometer was used throughout this study. The instrument has a strong magnetic field across the burner which provides a double-beam optical correc- tion system based on the Zeeman effect. With this system, trace metals in seawater (0.05-2 mg 1-l ) can be determined by flame atomic absorption spec- trometry (a.a.s.) without suffering from high background caused by the salt matrix.
Metal standard solutions. All metal standard solutions (1 mg ml-’ metal) were prepared from Merck Titrisol standards. A mixed working standard con- taining50 mgl-‘each of Cd(II), Co(II), Cu(II), Mn(II), Ni(II), Pb(I1) and Zn (II) was prepared as needed in dilute nitric acid. The Cr(III), Cr(V1) and Fe (III) standards were prepared separately in 1 M nitric acid because Cr (III) and Fe (III) are readily hydrolyzed at higher pH and Cr (VI) may cause oxi- dation reactions with other elements.
Procedures
Butch equilibrium. The relative affinities of Chelex-100 resin for the various heavy metals were studied by batch equilibration of the resin beads in aqueous media at various pH. When the resin was mixed with seawater, the pH could be adjusted to < 3 with hydrochloric acid; for higher pH, adjustment was some- what difficult because the resin itself acted as a buffer. In this case, external buffer solution was added, (i.e., sodium acetate).
For each experiment, 190 ml of artificial seawater or distilled water, 2 g of Chelex-100 resin, 4 ml of mixed metal standard containing 200 ,ug of each metal,
4 ml of 1 M sodium acetate buffer were added to a 250-ml Pyrex conical flask. The pH was adjusted to the desired value with HCl and NaOH. The final vol- ume was made exactly to 200 ml so that the metal concentrations were at the 1 mg 1-l level. The mixture was stirred for 24 h to reach equilibrium and then the resin was allowed to settle for 4 h, before a lo-ml aliquot of the supernatant solution was carefully withdrawn with a pipette. The metal contents were mea- sured directly by flame a.a.s. and evaluated from calibration curves prepared from standards in artificial seawater. Experiments with Cr (III), Fe (III) and Cr(V1) were done in separate batches in the low pH range only. Thus the removal (R%) of each metal by Chelex-100 resin in this closed system was obtained. The distribution ratio (D) of a given element at a given pH was estimated from the following equation: D= (weight of metal on 2 g of resin)/
(weight of metal in 200 ml of solution) = R%/ (1 -R% ) x 100. D is the gross distribution ratio of a specified metal on the resin, regardless of the species present.
Sorption kinetics. The kinetics of the metal chelating reaction was examined
by mixing the resin with seawater containing metal standards and measuring the residual metals in the aqueous phase. The pH values chosen for this ex- periment were 5.4 and 8.0. The general procedure was similar to that for batch equilibration but the supernatant solution was withdrawn every 10 min after addition of standards. Citrate buffer was tried as well as acetate buffers. Flame a.a.s. was used.
Breakthrough tests. An aliquot (11) of distilled water or artificial seawater
was spiked with 1 mg of Cd, Co, Cu, Mn, Ni, Pb, Zn as well as Mg and Ca. Ammonium acetate buffer (20 ml, 1 M) was added and the pH was adjusted to 5.4. The sample was passed through a Chelex-100 column containing 0.25 g of resin (in the Mg form) at a flow rate of 2.0 ml mine1 controlled by a Phar- macia P-3 peristaltic pump. Each lo-ml portion of the effluent was collected in a test tube by means of a Pharmacia fraction collector. Each collected frac- tion was stirred by a tube mixer to ensure homogeneity before the metal con- tents were determined by flame a.a.s.
RESULTS
The Chelex-100 resin
The Chelex-100 resin as dispatched is described by Bio-Rad Laboratories (1983) as the disodium form, which usually contains considerable free hy- droxide in the pore structure and has a buffered pH at ca. 10-11. The resin bed swells and shrinks drastically during conversion of the resin to different forms. Table 1 shows the relative bed volumes and relative flow rates for different resin forms. Generally, resin beds in the potassium, sodium and ammonium forms in the distilled water medium have the highest bed volumes and the slowest flow rates. When seawater is passed through the column the resin bed
262 TABLE 1
Relative bed volume and flow rate for a Chelex-100 column under atmospheric pressure
Resin form
Medium Relative Relative bed volume’ flow rate
Orig. Na+ H+ Na+C K+ NH: Mg*+ Ca*+ Mixed Dist. water 2 M HNOB Dist. water 2 M NaOH Dist. water 1MKOH Dist. water 2M NH,OH Dist. water 1 M MgC12 Dist. water 1 M CaCl, Dist. water Seawater Dist. water 1.00 1.0 0.50 2.3 0.45b 2.6 0.60 0.9 1.05 1.0 0.70 1.2 1.21 1.0 1.05 0.9 1.03 1.0 0.64 1.7 0.59 1.8 0.62 1.9 0.55 2.0 0.63 1.8 0.60 1.9
“Original sodium form resin (from package) as 1.0. bBed volume may slightly change depending on pH. Regenerated sodium form.
rapidly changes to a mixed form. Such changes for columns in the Na+ and K+ forms can be visually identified and are faster than the changes for the H+ or NH,+ forms. The explanation is that the release of hydrogen ions creates low pH in the column and so reduces the affinities for major ions; also the resin has a relatively higher affinity for ammonium ions. A recovery test in which 2 M nitric acid was used as eluent to strip all metals from a Chelex-100 column showed that each 1 g (wet weight) of the original resin contained ca. 500 pmol of sodium (Table 2). When the column was regenerated to the sodium form, the capacity became 660 pmol, which is about 30% more than that of the orig- inal. The capacities of the same column for K, Mg and Ca were found to be approximately 550,330 and 280 pmol, respectively.
It was of interest to establish the final proportions of Na, K, Mg and Ca on the column after a certain quantity of seawater has passed through. It might be casually assumed that calcium is dominant in the final elution, as the affin- ity for Ca2+ ions is the highest among these four metal ions. However, Kings- ton et al. [ 71 found that Na+ and Ca2+ are the major ions eluted from a Chelex- 100 column together with minor amounts of Mg2+ and K+. Bruland et al. [8] showed that the final eluate contained 1800 mg 1-l Ca, 1700 mg 1-l Mg, 150 mg 1-l Na and 20 mg 1-l K. Apparently, the presence of major ions on a Chelex- 100 column is not only affected by the individual affinities but also related to
TABLE 2
Recovery of major ions from Chelex-100 columns after passage of seawater
Resin form
Resin Seawater Final recovery” (pmol) weight passed’ (9) (ml) Na K Mg Ca Na+’ Na+d K+ Mg2+ Ca*+ H+ NH+, 0.5 0 250 n.d. n.d. n.d. 1.0 0 495 n.d. n.d. n.d. 1.0 0 662 n.d. n.d. n.d. 1.0 200 56 1 196 120 1.0 0 n.d. 546 n.d. n.d. 1.0 200 48 1 208 122 1.0 0 n.d. n.d. 334 n.d. 1.0 200 48 n.d. 202 118 1.0 1000 40 n.d. 200 120 1.0 0 n.d. n.d. n.d. 283 1.0 200 45 n.d. 200 128 1.0 0 n.d. n.d. n.d. n.d. 1.0 200 81 1 133 75 1.0 0 n.d. n.d. n.d. n.d. 1.0 200 85 1 136 75 1.0 1000 38 n.d. 198 120
“Seawater contained 10 500 mg 1-l Na, 390 mg 1-l K, 1260 mg 1-l Mg, 405 mg 1-i Ca. The pH was 8.0. bin 10 ml of 2 M nitric acid, n.d., not detectable. ‘Original sodium form from package. dRegenerated sodium form.
the concentrations of ions present in the seawater. In the present work, it was found that after a large amount of seawater had passed through, the resins were equilibrated with all ions and the ratios of major ions on the exchange sites gradually reached constant values no matter what the original form was. The relative mole ratios for Mg, Ca, Na, and K recovered from a typical column operation were found to be ca. 1,0.6,0.2 and 0.005, respectively, which is very close to the data provided by Bruland et al. [ 81. Ammonium ions would also compete with alkali and alkaline earth ions if ammonium acetate were added to the sample.
The pH of the column
The pH value in the column is the most important parameter in the Chelex- 100 technique because this method is strongly pH-dependent. Because the resin contains weakly acidic functional groups, the pH of a column is often affected by the resin itself and may be changed during the sorption processes if an external buffer system is not used. As mentioned earlier, the newly prepared columns in the Na+ or K+ form had very high pH values; extended washing with distilled water decreased the pH only slightly to 11. A newly presented ammonium-form column had a pH of 9-10. If the column was prepared in the
i
2
A
50 Ac
50- 0 n!, 1 2 3 5 6 PH R ‘“2 50. R I’?; c Mn A 8 A l A 50. 0 A l 1 R’EI A” CrMl) AA A A A 50. A R IOO- A” % A A Zn 0 OA $ 50. 0 i ,on&&,
1 2 3 L 5 6 PH : 1 2 3 Fig. 3 (continued).R’!j
on
%
it-
u
Pb A Mg 0 A 0 A 0 A 50- 50. 0 : a0 0 zep CA /r&w 1 2 3 L 5 6 PH 1 2 3 L 5 6 PH R loo- % !iY’fiE~ A FellIB
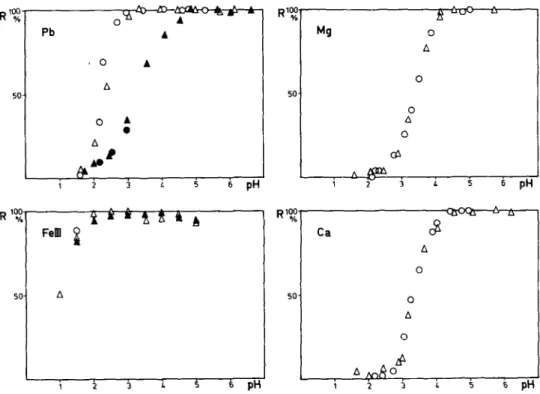
SO- A 1 2 3 L 5 6 PHFig. 3. Removal of 0.2 mg of trace metal from 200 ml of solution by equilibrating with 2 g of Chelex- 100 resin at various pH: (0 ) freshwater; ( A ) freshwater with acetate buffer; ( 0 ) artificial sea- water; (A ) artificial seawater with acetate buffer.
hydrogen form, then its pH would be maintained at pH 3 or slightly higher on extended washing with distilled water. However, when the column was con- verted to the magnesium or calcium form, its buffering ability was lost and the pH remained the same as that of the eluent. Figure 2 shows the pH changes of different columns when seawater was loaded at its natural pH 8.0 and the pH values of lo-ml portions of effluent were measured. It can be seen that large pH changes occurred with columns in the Na+, K+, NH,+ and H+ forms whereas the changes for the Mg2+ and Ca2+ forms were not significant.
In agreement with the findings of Florence and Batley [5], the H+-form column exhibits a low pH (down to 2.5) after seawater has passed through. It is therefore not recommended for either seawater or freshwater analysis. When resins in the Na+, K+ or NH,+ forms are used, it is advisable to apply external buffer systems, usually acetates, to compensate for such pH changes. This can be done either by pre-washing the column with acetate reagent, or by adding acetate buffer directly to the seawater sample, or both. Although the ammo- nium form of the resin has been highly recommended [5, 7,9], the above re- sults indicate that the magnesium form is likely to be the most suitable for seawater analysis, especially when buffer systems are undesirable.
266
6 PH
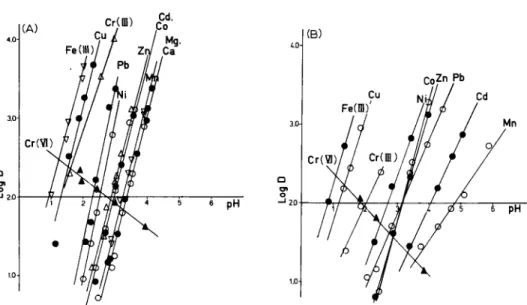
Fig. 4. Log (distribution ratio) vs. pH plots for trace metals with Chelex-100 resin: (A) in fresh- water; (B) in seawater.
Butch equilibrium
The results of the batch equilibrium experiments are presented in metal removal (R% ) vs. pH diagrams (Fig. 3). The calculated distribution ratios (D) are also plotted as log D vs. pH in Fig. 4. In most cases, there are large differ- ences between the results for freshwater and seawater media, indicating that the chelating affinities for trace metals are indeed affected by the highly saline matrix. For some metals, the Chelex-100 resins behaved in the same way whether or not acetate buffer was used.
In freshwater, the uptake of trace metals increases sharply with increase of pH. The curves (Fig. 3 ) are similar in shape to strong acid-base titration curves, ranging over ca. 2 pH for most cations tested. In contrast, the resin sorbed Cr(V1) at low pH and desorbed it at high pH. At pH 2.5, the resins could completely remove Cu (II), Fe (III) and Cr (III) from the aqueous medium; at pH >4.5, nearly all Cd(II), Co(II), Mn(II), Ni(II), Pb(II), Zn(II), as well as Mg and Ca, were taken up. From the log D vs. pH plot, the relative affinities for those elements were in the following order: Fe (III) > Cu (II) > Cr (III) > Pb(I1) > Ni(I1) > Zn(I1) > Cd(I1) = Co(I1) > Mn(I1) > Ca=Mg. The pH,,, values for uptake in this system are 1.0,1.3,1.4,2.3,2.6,2.8,2.9,3.0,3.3, 3.4 and 3.4, respectively.
In the case of seawater, the results became somewhat erratic for certain ele- ments. The R% curves are shifted to higher pH values for almost all metals (Fig. 3 ) . The curves for Cu, Co, Ni, and Zn shift toward higher pH by up to 0.5 pH, but generally maintain the same shape as in freshwater. The curves for
Stirring time (min)
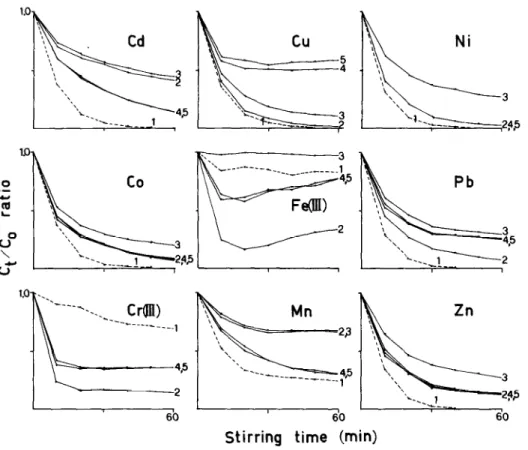
Fig. 5. Kinetics of uptake of trace metals by Chelex-100 resin in different media: (1) in freshwater with acetate buffer, at pH 5.4, (2) in seawater with acetate buffer, at pH 5.4; (3) in seawater with citrate buffer, at pH 5.4; (4) in seawater with acetate buffer, at pH 8.0; (5) in seawater without buffer, at pH 8.0. The resin (0.4 g) was stirred with 200 ml of sample containing 0.2 mg of each element.
Cd, Pb and Mn become notably spread out (over > 3 pH), indicating that speciation changes are involved, the formation of stable chloride complex ions in seawater could be one of the reasons. In this experiment, the concentrations were at the 1 mg 1-l level, and some conversion of Mn(I1) to Mn(IV) at pH
> 4 might account for the incomplete absorption. The Chelex-100 resin did not completely remove Cr (III) from seawater at pH < 3; some hydrolysis occurred at higher pH. Leyden and Underwood [ 121 studied the distributions of metal ions on a iminodiacetate resin (Dowex Al) in 0.1 M NaCl and showed that uptakes were maximal at pH 4-5. In the present work with seawaters, the optimal pH ranges are higher, which is understandable; although the resin has low affinity for sodium ions, their presence in high concentration will affect the distribution of heavy metals. Magnesium and calcium are much more easily sorbed than sodium and their high concentrations in seawater provides further
268
cd Mn C$ (8)
Mn
Cd
Ni
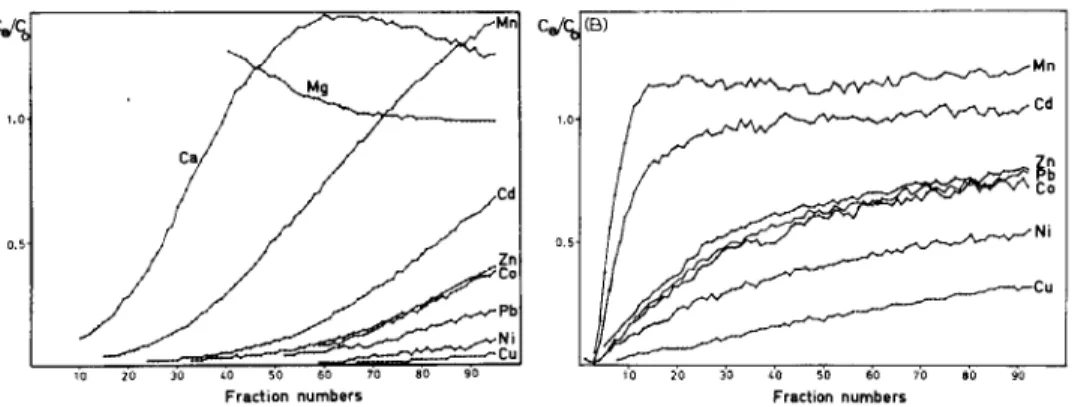
Fig. 6. Breakthrough curves for samples of freshwater (A) and seawater (B) samples containing 1 mg 1-l each of the trace metals and buffered at pH 5.4. The column contained 0.25 g of resin in the magnesium form; flow rate was 2 ml min-’ and lo-ml portions of the effluent were collected. The C,/C, ratio is the ratio of the metal concentrations in the effluent and influent solutions.
competition for the reaction of heavy metal ions on the available exchange sites.
Effects of salt matrix and buffers on the sorption kinetics
The results for batch experiments are given in Fig. 5. It is easily seen that the resin can sorb all the divalent heavy metals more quickly in freshwater than in seawater. The complete reaction in seawater usually requires several hours, depending on the amounts of heavy metals and resin in the closed sys- tem, as well as on the pH applied. The removal was incomplete for Cr (III) and Fe (III) in freshwater because they were partly precipitated at this pH. Never- theless, it can be concluded that the salt matrix effect not only decreases the chelating affinities of the resin but also prolongs the equilibration time. Adding acetate buffers did not cause extra delay because the formation of heavy metal/ acetate complexes is weaker than the chelating mechanism between heavy metals and the resin. Citrate ions generally form stronger complexes with metal ions and so significantly affect the uptake kinetics for Co, Cu, Ni, Pb, and Zn; they also mask iron completely. The results also indicate that the removal of Cu and Pb is favorable at pH 5.4 whereas the removal of Cd and Mn is much more efficient at pH 8.0
Breakthrough curves
The results of multi-element breakthrough experiments are shown in Fig. 6. For the freshwater solution, the resin absorbed trace metals rapidly and a dark color layer formed at the top of the resin bed. The spiked metal concentrations were high so that the column was soon saturated. The breakthrough appear- ance order was Mg, Ca, Mn, Cd, Zn, Co, Pb, Ni, with Cu last. This sequence agrees well with the affinity order as shown in Fig. 4, except for lead and nickel.
It seemed that the resin has a higher affinity for lead than for nickel, but the reaction rate is slower so that lead appeared ahead of nickel.
For the seawater solution, the breakthrough curves showed quite different patterns. The curves for Mn and Cd rise sharply. As shown in former experi- ments, the salt effect decreases the chelating affinities and delays the reac- tions; thus the apparent column capacity is much smaller than that for freshwater. With manganese, for instance, the CJC,, ratio rapidly reached 1.0 at fraction 10 in the seawater effluent but at fraction 70 in the freshwater effluent. Similar effects were found for other elements. The appearance of lead in the seawater effluent coincided with those of zinc and cobalt, instead of afterwards as in freshwater, possibly because of the formation of chloride com- plexes of lead.
DISCUSSION
When the experimental results for freshwater and seawater media are com- pared, it can be seen that the metal pre-concentration efficiency of Chelex-100 is strongly affected by the salt matrix. The effect appears in two ways. First, the major cations, which have low distribution ratios but are present at high concentrations, compete with heavy metals on the exchange sites and so reduce the exchange efficiency for heavy metals; the presence of anions which form complexes with heavy metals also reduces the affinity of the resin for heavy metals, as was proved by the decreased distribution ratios (D) in the batch equilibrium experiments. Secondly, the salt matrix significantly retards the chelating reactions, which generally require several hours for complete sorp- tion from seawater. This effect is very important in column operations, in which the seawater contacts the resin beads only for a short time as it passes through. The effective column capacity in seawater was generally estimated as one order of magnitude less than that in freshwater at the same pH and identical flow rate.
The low efficiency of Chelex-100 columns for seawater samples can be im- proved by adjusting the sample to a pH range higher than that normally used for freshwaters, but not so high as to cause formation of hydroxide complexes. Although the best pH ranges for individual elements may differ, it is still pos- sible to use a designated optimal pH range to cover most elements to an ac- ceptable >98% recovery. Working at non-optimal pH frequently produces incomplete recoveries. For manganese, for instance, previous spiking studies
[ 9,111 showed poor recoveries from seawater at pH 5.0-5.5, whereas the pres- ent study suggests that the recovery would be much improved at pH 6.5.
Apart from the operating pH value, other parameters such as column size, flow rate and buffer ligands should also be carefully considered. Increasing the column dimensions and slowing the flow rate will enhance the chelating effi- ciency, as has been proved by Paulson [lo]. However, such treatments also
270
increase the difficulties in actual practice. In addition, temperature effects are important for on-board operation, because when seawater samples are col- lected from the ocean at different depths, the cold water may lead to deviations in column efficiency. These aspects will be further discussed in Part 2.
This project was sponsored by the National Science Council of Taiwan. The authors thank Prof. J.P. Riley of Liverpool University for his kind suggestions on the preparation of the manuscript; Prof. S.H. Jeng and Prof. H.W. Li of the National College of Marine Science and Technology, Keelung, Prof. K.K. Liu of the Academia Sinica, Taipei, and Prof. C.Y. Liu of the National Taiwan University for their support and criticism, and Mr. L.S. Wen, Mr. T.H. Fang and Mr. C.C. Yang for their valued assistance.
REFERENCES 1 2 8 9 10 11 12 13
C.J. Jones and J.W. Murray, Limnol. Oceanogr., 29 (1984) 711.
S. Kajiura (Ed.), Ocean Characteristics and their Changes, Koseisha Koseikaku, Tokyo, 1985, p. 531 (in Japanese).
W. Slavin, At. Spectrosc., 1 (3) (1980) 66.
J.P. Riley and D. Taylor, Anal. Chim. Acta, 40 (1968) 479. T.M. Florence and G.E. Batley, Talanta, 22 (1975) 201. P. Pakalns and G.E. Batley, Anal. Chim. Acta, 99 (1978) 333.
H.M. Kingston, I.L. Barnes, T.J. Brady, T.C. Rains and M.A. Champ, Anal. Chem., 50 (1978) 2064.
K.W. Bruland, R.P. Franks, G.A. Knauer and J.H. Martin, Anal. Chim. Acta, 105 (1979) 233.
R.E. Sturgeon, S.S. Berman, A. Desaulniers and D.S. Russell, Talanta, 27 (1980) 85. A.J. Paulson, Anal. Chem., 58 (1986) 183.
B. Boniforti, R. Ferraroli, P. Frigieri, D. Heltai and G. Queirazza, Anal. Chim. Acta, 162 (1984) 33.
D.E. Leyden and A.L. Underwood, J. Phys. Chem., 68 (1964) 2093.
D.R. Kester, I.W. Duedall, D.N. Conner and R.M. Pytkowicz, Limnol. Oceanogr., 12 (1967) 176.