Antibacterial TaN-Ag coatings on titanium dental implants
Heng-Li Huang
a, Yin-Yu Chang
b,c,⁎
, Meng-Cheng Lai
b, Cai-Rong Lin
b, Chih-Ho Lai
d, Tzong-Ming Shieh
ea
Oral Biology Research Lab., School of Dentistry, China Medical University and Hospital, Taichung, 404 Taiwan
b
Department of Materials Science and Engineering, Mingdao University, Changhua 52345, Taiwan
cSurface Engineering Research Center, Mingdao University, Changhua 52345, Taiwan d
School of Medicine, China Medical University and Hospital, Taichung, 404 Taiwan
e
Department of Dental Hygiene, China Medical University and Hospital, Taichung, 404 Taiwan
a b s t r a c t
a r t i c l e i n f o
Available online 3 August 2010 Keywords: Sputtering Antibacterial Biocompatibility TaN Silver Coating
Titanium-based materials have been used for dental implants due to their excellent biological compatibility, superior mechanical strength and high corrosion resistance. The osseointegration of titanium dental implants is related to their composition and surface treatment. A better anti-bacterial performance of the abutment seated in the prosthetic crown is beneficial for the osseointegration and for avoiding the infection after implantation surgery. In this study, TaN-Ag coatings with different Ag contents were deposited on a bio-grade pure Ti dental implant material. A twin-gun magnetron sputtering system was used for the deposition of TaN-Ag coatings. The Ag content in the deposited coatings was controlled by the magnetron power ratio of Ag/(Ta + Ag) targets. To verify the susceptibility of implant surface to bacterial adhesion, Staphylococcus aureus, one of the major pathogen frequently found in the implant-associated infections, was chosen for in vitro anti-bacterial analyses. In addition, the biocompatibility of human gingivalfibroblast (HGF) cells on coatings was also evaluated. A composite structure of crystalline TaN and Ag nanoparticles was identified. The TaN-Ag coating with the highest Ag content of 21.4 at.% possessed the lowest bacterial retention and viability of S. aureus. From the MTT assay test, the mean optical density values for the TaN and TaN-Ag coated samples after 72 h of HGF adhesion were greater than the value obtained from the uncoated Ti. The results suggested that the TaN-Ag coatings improve antibacterial performance with compatible biological response.
© 2010 Elsevier B.V. All rights reserved.
1. Introduction
Pure titanium (Ti) is commonly used as artificial joints and implants in both dental and orthopedic clinics because of its biocompatibility and mechanical properties. The excellent biocompatibility of titanium surfaces as an implant materials results from its surface properties. While problems in the osseous healing of implants appear to be resolved, the adsorption of biomolecular pellicles and the subsequent accumulation of bacteria on these surfaces are still the main stimulus for the induction of inflammatory processes. Although the biocompatibility of Ti has been confirmed [1,2], it is still difficult to meet all the requirements, such as antibacterial ability, biocompatibility, osseointe-gration, and mechanical properties. Good biocompatibility and rapid osseointegration are essential factors of prolonged stability of the implant. Especially for dental implants, some of the studies indicated that both the quality and quantity of plaque adhesives on the implant abutment surface, which contact with gingival tissue, influence the
long-term implant success[3,4]. The initial adhesion and the coloniza-tion of bacteria to an implant surface are considered to play a key role in the pathogenesis of infections related biomaterials. Because Ti does not exhibit antimicrobial properties, one approach to achieve better disinfection with biocompatibility is to modify the surface material of the Ti-based implant[5,6].
Bacterial attachment plays a significant role in determining the outcome and success of a Ti-based implant [7]. Therefore, surface modification of titanium by coating or adding antibacterial properties of metals or alloys to reduce the number of bacteria and microbial adhesion seems an efficient way to increase the benefit of clinical treatment. An in vitro study by B. Groessner-Schreiber et al. [6,8] showed that TiN and ZrN coatings possessed antibacterial performance to the oral microflora and Streptococcus. Ag and Cu are known to be efficient antibacterial agents because of their specific antimicrobial activity and nontoxicity of the active Ag and Cu ions to human cells [9,10]. Recently, it was reported that Ag-doped TaN and Cu-doped TaN with nanoparticles can decrease the multiplication of Escherichia coli bacteria, and showed improved antibacterial effect[11,12]. In addition, Ta alloys are known to have excellent biocompatibility which makes TaN an excellent protective coatings in biomedical applications[13]. Ag, as a doping element, is proved not miscible with TaN, which makes the
⁎ Corresponding author. Department of Materials Science and Engineering, Mingdao University, Changhua 52345, Taiwan. Tel.: +886 4 8876660x8050; fax: +886 4 8879050.
E-mail address:yinyu@mdu.edu.tw(Y.-Y. Chang).
0257-8972/$– see front matter © 2010 Elsevier B.V. All rights reserved. doi:10.1016/j.surfcoat.2010.07.096
Contents lists available atScienceDirect
Surface & Coatings Technology
synthesis of TaN-Ag nanocomposite coatings possible. Even though TaN-Ag has been confirmed that it can improve the antibacterial efficiency of E. coli., no study has been investigated the effects on the bacterial ability when TaN-Ag is used as a coating material for dental or even orthopaedic implants.
In this study, TaN-Ag nanocomposite coatings with different Ag contents were synthesized by a twin-gun reactive magnetron sputter-ing. The objective of this work is to study the effect of Ag contents on the antibacterial performance to bacterial and biocompatibility in dental applications. Staphylococcus aureus, a major pathogen frequently found in the percutaneous and dental implant-associated infections, was chosen as the model for this in vitro study[14,15]. The adherence of S. aureus to the implanted surfaces was observed and quantified. To verify the biocompatibility and cell proliferation activity of the TaN-Ag coatings, the attachment and growth behavior of normal human gingival fibroblast (HGF) cells cultured on the deposited samples were also investigated.
2. Experimental details
TaN and TaN-Ag coatings were deposited on pure Ti plate samples (Surface roughness Ra= 0.1μm, bio-grade 2, Uniti Titanium, Moon Township, PA, USA) using an unbalanced magnetron sputtering with Ta and Ag targets. Each target had a diameter of 50 mm and was tilted by 45° to the substrate. The distance between the target and the substrate was 100 mm. The samples were placed on a rotational substrate holder for the deposition. A base pressure prior to deposition was less than 1 × 10-3Pa. For the deposition of TaN, the cathode power of Ta was
100 W. At aflow rate of Ar fixed at 110 sccm, N2was introduced into the
chamber to maintain the deposition pressure of 1.1 Pa to synthesize stoichiometric TaN. In order to prepare TaN-Ag with different Ag contents, the cathode power of Ag was kept at 20 W while the cathode power of Ta was varied from 150 to 250 W, as shown inTable 1. The cathode power ratios of Ag/(Ta+ Ag) targets were 0.07, 0.09 and 0.12. Substrate bias voltage of -40 V was used. The total thickness of the coatings was 1.4–1.7 μm, which was controlled by a deposition time of 30 min. The temperature of the sample during the deposition was measured by a thermocouple located near the sample to be within the range of 100 ± 20 °C.
The deposited coatings were examined in a Joel JSM-7000F high resolution field emission scanning electron microscope (FESEM). Chemical composition of the deposited coatings was identified by using a high resolution electron probe microanalyzer (FE-EPMA, JEOL JXA-8500F) equipped with a wavelength dispersive X-ray spectrom-eter (WDS). Glancing angle X-ray diffractomspectrom-eter (PANalytical X'pert Pro) with a high resolution ψ goniometer and Cu radiation was employed for phase identification. The diffractometer was operated at 40 kV and 30 mA with a glancing angle of 1°–2°. Hardness and Young's modulus of thefilms were obtained using XP-MTS nanoin-dentation with a Berkovich indenter, under load-unloading condition, and measured as a function of indenter displacement using contin-uous stiffness measurement method.
A surface roughness profilometer (Surf-Corder SE 1200, Kosaka Lab Ltd., Tokyo, Japan) was used to characterize the surface roughness of the deposited coatings. The static contact angle was measured by
using an instrument for measuring contact angles (FTA-125, First Ten Angstroms, Portsmouth, VA, USA). The obtained images were analyzed to calculate the contact angle of the deionized water of each sample at room temperature. Each reported contact angle is the mean of at least three independent measurements.
The retention of bacteria on the coated samples was determined by a fluorescence staining method employing Syto9 (Molecular Probes, Eugene, OR, USA). Briefly, 500 μl of S. aureus suspension (2×107cfu/ml)
was added onto the sample surface. After 6 h of incubation at 37 °C at a relative humidity of 96% with avoiding light exposure, the sample surface was rinsed three times with PBS, then the bacteria wasfixed with 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) and stained with 10μM of Syto9 at room temperature for 30 min. The adhered bacteria on the coated samples was measured using a fluorescence detected at 488 nm by an ELISA (enzyme-linked immu-nosorbent assay) reader Synergy HT (BioTek Instrument, Winooski, VT, USA), and was determined from three independent experiments performed in duplicate, and quantified in relative fluorescence intensity. The retention of bacteria on the surfaces of coated samples was also examined using scanning electron microscopy (SEM). Each sample was immersed in the aqueous solution of 3 ml of the cultured S. aureus (5 × 108cfu/ml) in LB broth, followed by incubated at 37 °C for 6 h. The tested samples were rinsed three times with PBS and immediatelyfixed in 2.5% glutaraldehyde for 2 h. Prior to SEM, the tested samples were rinsed with PBS again, and were immersed in distilled deionized water for 10 min, and then were dehydrated in an ethanol series (50, 70, 90, 95, and 100%, each for 10 min). The tested samples werefixed and subsequently dried by using critical point drying with CO2, which has its critical point at 31.1 °C and 7.39 MPa,
using a Samdri-PVT-3D apparatus. The chamber is pre-cooled (10 °C) to allow it to be readilyfilled with liquid CO2from a gas cylinder. The
chamber is then heated to just above the critical temperature with subsequent critical pressure being achieved. Immediately after critical point drying, samples were coated with Pt and then observed in secondary electron (SE) detection mode.
The bacterial survival on TaN and TaN-Ag coated samples was also assessed by bacterial viability test[16]. A total of 200 ml LB agar (5 g LB broth and 1.6 g agar plus with 193 ml glass-distilled deionized water) (Difco Laboratories, Detroit, MI, USA) mixed with 0.2 ml of S. aureus (1 × 106cfu/ml) was prepared. The pure Ti plates, and TaN and
TaN-Ag coated samples were placed on sterile plates, subsequently overlayed and immersed of 8 ml LB agar containing S. aureus onto each sample plate. After air drying for 30 min at room temperature, the plates were incubated at 37 °C for 16 ho and visible bacterial colonies on the LB agar plates were counted. The number of the bacteria growths was then determined by colony-forming units (cfu) counts. The adherent number was expressed by the ratio of the total bacteria growths on the measured sample to the area of the measured sample.
The proliferation of human gingival fibroblast (HGF) cells was examined with an MTT test assay (Sigma-Aldrich, St Louis, MO, USA) after the cells were cultured on the Ti plates, and TaN and TaN-Ag coated samples. The substance used for the MTT test was a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium salt, which turns into a purple formazan product due to the viable mitochondria in living cells. Three milliliters of HGF cells was seeded at a density of 2 × 104cells/ml,
and incubated at 37 °C in 5% CO2for 72 h, which complete proliferation
was attributed. The absorbance (optical density, O.D.) of the purple formazan was quantified by measuring at 570 nm by a SpectraMax spectrophotometer (Molecular Devices, Sunnyvale, California) with SoftMax Pro 5.2 241 software (Molecular Devices). Thus, the optical density of formazan reflected the level of cell viability, and higher O.D. values showed more living cells on the sample which presented better biocompatibility.
The statistical correlation of the antibacterial activity and the MTT test between the coated samples and uncoated pure Ti plates was
Table 1
Deposition conditions and coating composition of TaN-Ag coatings. Samples Power of Ta (W) Power of Ag (W) Cathode power ratio of Ag/(Ta+Ag) targets Coating composition (at.%) Ta N Ag TaN 100 0 0 56.4 43.6 0 TaN-Ag14.9% 250 20 0.07 44.8 40.3 14.9 TaN-Ag17.5% 200 20 0.09 40.1 42.4 17.5 TaN-Ag21.4% 150 20 0.12 31.2 47.4 21.4
determined by Student's t-test [17]. Differences were considered significant at pb0.05 level.
3. Results and discussion 3.1. Microstructure analyses
Table 1shows the chemical composition of the deposited TaN and TaN-Ag coatings as measured by WDS. The elemental composition of the TaN coating was 56.4 at.% of Ta and 43.6 at.% of N. Due to the base pressure lower than 1 × 10-3Pa, oxygen was not detected by WDS. When
Ta and Ag were cosputtered during the coating process, a composite TaN-Ag coating was formed. With different ratios of Ag/(Ta+ Ag) cathode power (P[Ag]/(P[Ta]+ P[Ta]) = 0.07, 0.09, and 0.12), The TaN-Ag
coatings were identified as Ag14.9%, Ag17.5%, and TaN-Ag21.4%, respectively. The higher the ratio of Ag/(Ta+ Ag) cathode power, the higher the Ag content in the TaN-Ag coatings was obtained. During the reactive sputtering deposition process in the N2atmosphere,
only Ta reacts with N2to form TaN, and Ag is not miscible with TaN. It
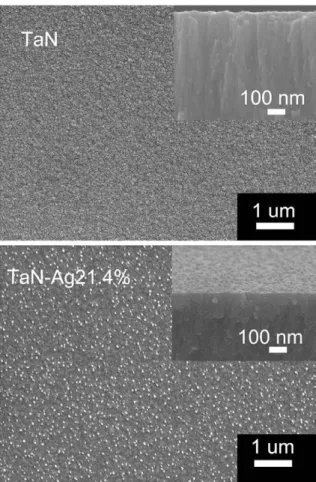
makes the synthesis of TaN-Ag nanocomposite coatings possible. Typical glancing angle X-ray diffraction spectra from the TaN and TaN-Ag nanocomposite coatings are shown in Fig. 1. The result revealed that TaN exhibited a NaCl crystal structure (JCPDFfile No.: #895196). The lattice parameter was 0.432 nm. When Ag was cosputtered with Ta, in addition to TaN, fcc Ag phases were found (JCPDFfile No.: #893722). It showed that a composite structure of TaN and Ag was obtained.Fig. 2shows SEM micrographs of TaN and TaN-Ag21.4% with 21.4 at.% of Ag. The TaN showed a dense columnar structure with smooth surface, as shown in the top micrograph of Fig. 2. With increasing Ag content, a dense and fine grain TaN-Ag without columnar structure was formed. Silver nanoparticles (15– 53 nm) were well distributed in the TaN-Ag21.4% composite coatings. However, the density of Ag particles on the surface of the TaN-Ag14.9% and TaN-Ag17.5% is low. The emergence of Ag particles would influence the surface hydrophilicity and mechanical properties of the coated samples. As shown inFig. 3, the hardness and elastic modulus of the TaN coating were 13 ± 1 and, 195 ± 20 GPa, respectively. The TaN-Ag17.5% and TaN-Ag21.4% possessed lower hardness (7–10 GPa) and elastic modulus (140–180 GPa) due to the disruption offine columnar structure[11]. The TaN-Ag21.4% had the lowest hardness (7 ± 0.5 GPa) and elastic modulus (140 ± 5 GPa). Similar results were also found in the study of TaN-Cu coatings[12]. In order to enhance the bioactivity and control the bacterial infection, Ti implants were subjected to surface modification whereby the properties such as chemical nature and morphology were controlled. The average surface roughness (Ra) of the uncoated Ti was 0.84 ± 0.03μm. The deposited TaN and TaN-Ag coated samples possessed
higher Ra values (1.03–1.07 μm). As shown inFig. 4, the contact angle of the uncoated Ti was 55 ± 1.3°. The dense TaN possessed the highest contact angle of 92 ± 1.2°, and it showed hydrophobic feature. The contact angle of TaN-Ag coated samples were 64–71°. The presence of Ag particles on the surface results in a lower contact angle than TaN. 3.2. Bacterial retention and viability
The antibacterial activity of Ti has been somewhat controversial. While some studies [5–7,18,19]had shown no influence of Ti on various oral bacteria in vitro, others had found some antibacterial activity[20,21]. Ti has also been suggested to have antimicrobial and
Fig. 1. Glancing angle X-ray diffraction spectra of the deposited TaN, Ag14.9%, TaN-Ag17.5%, and TaN-Ag21.4%.
Fig. 2. SEM micrographs of the deposited TaN(top) and TaN-Ag21.4% coatings. Small figures on the right top of each images showed its cross-sectional morphology.
Fig. 3. Hardness and elastic modulus of the deposited TaN and TaN-Ag (TaN-Ag14.9%, TaN-Ag17.5%, and TaN-Ag21.4%) coatings.
anti-inflammatory effects due to the formation of peroxides at the Ti surface in vitro[22]. A previous study by X. Wang et al. showed that smooth Ti possessed low S. aureus bacterial adherence that resulted in low probability of infection[15]. In this study, S. aureus after 6 h of incubation on the surfaces of pure Ti (Control sample) and coated samples was observed. Fig. 5 shows the quantitative result of the cultured S. aureus bacteria on the samples. The highest relative fluorescence intensity of the bacterial retention was observed on the uncoated Ti surfaces. The adherence of oral bacteria to implants is regarded as a critical step in causing implant failure and leading to soft-tissue inflammation. The adherence of bacteria to a solid surface is generally thought to involve certain physical or chemical processes, such as hydrophobic interactions, van der Waals forces, electrostatic interactions, hydrodynamic forces[23], and roughness[15]. Decreasing surface free energy inhibited biofilm formation and bacterial adherence on dental implants and abutment surfaces[24]. In this study, the TaN coating with hydrophobic surface (contact angle = 92 ± 1.2°) possessed lowerfluorescence intensity, and it showed smaller number of adherent S. aureus than the uncoated Ti. Although the deposited TaN-Ag possessed higher surface roughness Ra values, the TaN and TaN-Ag coated samples had lower retention of S. aureus. The TaN-Ag21.4% with the highest Ag content had the lowestfluorescence intensity, which
meant the fewest adhering bacterial counts, and it showed the most significant short-term antibacterial effect. As shown in the SEM micrographs ofFig. 6, in adhered S. aureus only few bacteria was distributed on the surface of TaN-Ag21.4%, while those on the uncoated Ti were considerably higher. Statistical results confirmed that there was a significant difference between the number of bacteria on the uncoated Ti and coated samples (pb0.01). For the TaN-Ag14.9% and TaN-Ag17.5% coated samples, the antibacterial efficiency to S. aureus is not obvious. It probably resulted from the hydrophilic surface (contact angle were 64– 71°) and the emergence of Ag nanoparticles on the surface is not enough. The results suggest TaN-Ag composite coatings containing Ag nanoparticles potential for improving the antibacterial activities of Ti-based materials. For the results of bacterial viability test (Fig. 7), the TaN-Ag21.4% possessed fewer viable S. aureus than those on the TaN coated and uncoated Ti samples. The TaN-Ag coating shows a remarkable antibacterial property. In this study, the higher Ra values of the deposited TaN-Ag did not deteriorate the antibacterial performance. The use of a surface coating containing Ag can provide antibacterial actions to suppress microbial proliferation and thereby reduce bacterial counts. It may show a lower probability of implant-related infections [25]. Chen et al.[26]had cosputtered Ag and hydroxyapatite (HA) to make an antibacterial-bioactive coating, which inhibited bacterial attachment without cytotoxic effects. A recent study by B. S. Necula et al. also confirmed the in vitro antibacterial activity of TiO2-Ag coatings to
S. aureus[27]. The antibacterial action of Ag may be explained that Ag nanoparticles on the surface of TaN-Ag coatings attached to the bacteria and resulted in bacterial wall pitting. The inhibitory effect of Ag ions, released from Ag nanoparticles under a complex physiological condi-tion, is believed to be due to its sorption to the negatively charged bacterial cell wall, deactivating cellular enzymes, disrupting membrane permeability, andfinally leading to bacteria lysis and death[28–31].
Fig. 4. Contact angle values of the uncoated (Control), TaN and TaN-Ag (TaN-Ag14.9%, TaN-Ag17.5%, and TaN-Ag21.4%) coated Ti plates.
Fig. 5. The relativefluorescence intensity of adhered S. aureus bacterial colonies on the uncoated (Control), TaN and TaN-Ag (TaN-Ag14.9%, TaN-Ag17.5%, and TaN-Ag21.4%) coated Ti surfaces and incubated for 6 h. (*) A value of pb0.05 was considered that the mean arb. units of two group of samples are significant different.
Fig. 6. SEM micrographs of S. aureus incubated on the uncoated Ti surface (top) and TaN-Ag21.4% coatings. Smallfigures on the top right of the TaN-Ag21.4% image showed its surface morphology before S. aureus incubation.
3.3. Cell proliferation
In previous studies on the responses of soft tissue to the surfaces of oral implants, it has been shown that the surface treatment of the implant materials significantly influences the attachment of oral fibroblasts. By modifying the surface texture of the implant materials, the tissue-implant attachment can be enhanced, resulting in a material that should be at least as good as normal titanium[32,33]. In addition to antibacterial assessment of the TaN and TaN-Ag coated Ti, it is necessary to examine the HGF cell viability for the application
of dental implants. In this study, an MTT assay test was used for evaluation. The optical density of the formazan produced by HGF cells was measured after 72 h, as shown inFig. 8. The optical density value of the uncoated (Control sample) Ti was 0.134 ± 0.003. Previous studies showed that smooth Ti favors human oral fibroblast attachment and soft tissue growth[34–36]. The biocompatibility of Ti is attributed to surface oxide spontaneously forming in air and/or other surface treatments (e.g. thermal oxidation or anodic oxidation). It is believed that the cellular behavior including proliferation, adhesion, and spreading is greatly influenced by this oxide layer of Ti[13]. In this study, all of the TaN and TaN-Ag coated Ti possessed higher optical density value, and showed better HGF cell viability and proliferation than the uncoated sample. Statistical results proved significant differences among the investigated samples. TaN and TaN-Ag coatings had a comparable biocompatibility to Ti because they allowed more cell attachment and proliferation. In the dental use of Ti implants, where the implant components, such as abutments, contact not only bone but also the gingiva, and are partially exposed to the oral cavity that includes bacteria, it is especially important to fabricate a coating material with both antimicrobial capacity and biocompat-ibility so as to increase the likelihood of implant success. In this study, TaN-Ag nanocomposite coatings possessed good HGF cell viability and improved the antibacterial effect to S. aureus for dental implant applications.
4. Conclusions
TaN-Ag nanocomposite coatings with different Ag contents were synthesized by a twin-gun reactive magnetron sputtering. All the TaN and TaN-Ag coatings exhibited the B1-NaCl crystal structure. The TaN showed a dense columnar structure with smooth surface and possessed the highest contact angle of 92 ± 1.2°, and it showed hydrophobic feature. For the TaN-Ag coatings, the higher the ratio of Ag/(Ta + Ag) cathode power, the higher the Ag content in the TaN-Ag coatings was obtained. Silver nanoparticles (15–53 nm) were well distributed in the TaN-Ag composite coatings with 21.4% of Ag. The contact angle of TaN-Ag coated samples was 64–71°. The presence of Ag particles on the surface results in a lower contact angle than TaN. The effect of Ag contents of TaN-Ag composite coatings on the antibacterial performance to S. aureus and HGF cell proliferation activity was investigated. The TaN-Ag composite coatings with the highest Ag content (21.4 at.%) had the lowestfluorescence intensity, which meant the fewest bacterial retention, and it showed the most significant short-term antibacterial effect. HGF cells showed
Fig. 7. The result of bacterial viability test on the uncoated, TaN and TaN-Ag21.4% coated Ti sample plates. The visualized colonies on the agar plates indicated the growth of S. aureus. The results of bacterial growths on each sample are 44 cfu/cm2
on Ti, 37 cfu/cm2
on TaN, and 3 cfu/cm2
on TaN-Ag 21.4%, respectively.
Fig. 8. Cell viability evaluation using a MTT assay of human gingivalfibroblast (HGF) and incubated at 37 °C for 72 h on the uncoated (Control), TaN and TaN-Ag (TaN-Ag14.9%, TaN-Ag17.5%, and TaN-Ag21.4%) coated Ti surfaces. The data are expressed as the mean values ± standard deviation of the independent experiments. (*) A value of pb0.05 was considered that the mean O.D. values of two group of samples are significant different.
comparable biological responses to both TaN and TaN-Ag coated titanium during 72 h culture period. In this study, the application of TaN-Ag nanocomposite coatings on Ti dental materials not only improved the antibacterial effect to S. aureus but also met the re-quirement of HGF cell viability.
Acknowledgement
The authors wish to thank the Mingdao University for providing XRD and FESEM analyses, and the China Medical University for providing antibacterial analyses and HGF cell viability assessment. The funding in part from the National Science Council of Taiwan under the contract NSC 99-2622-E-451-004-CC3 and NSC 98-2221-E-451-006 is sincerely appreciated.
References
[1] W. Zhou, X. Zhong, X. Wu, L. Yuan, Z. Zhao, H. Wang, Y. Xia, Y. Feng, J. He, W. Chen, Surf. Coat. Technol. 6155 (6160) (2006) 200.
[2] C.Y. Chiang, S.H. Chiou, W.E. Yang, M.L. Hsu, M.C. Yung, M.L. Tsai, L.K. Chen, H.H. Huang, Dent. Mater. 1022 (1029) (2009) 25.
[3] J. Lindhe, T. Berglundh, I. Ericsson, B. Liljenberg, C. Marinello, Clin. Oral Implan. Res. 9 (66) (1992) 3.
[4] E.S. Ong, H.N. Newman, M. Wilson, J.S. Bulman, J. Periodontol. 200 (205) (1992) 63.
[5] Leonhardt, G. Dahlen, Eur. J. Oral Sci. 382/387 (1995) 103.
[6] B. Groessner-Schreiber, M. Hannig, A. Dück, M. Griepentrog, D.F. Wenderoth, Eur. J. Oral Sci. 112 (2004) 516.
[7] K. Heydenrijk, H.J. Meijer, W.A. van der Reijden, G.M. Raghoebar, A. Vissink, B. Stegenga, Int. J. Oral Max. Impl. 829 (838) (2002) 17.
[8] B. Groessner-Schreiber, M. Griepentrog, I. Haustein, W.D. Muller, K.P. Lange, H. Briedigkeit, U.B. Gobel, Clin. Oral Impl. Res. 12 (2001) 543.
[9] W. Zhang, P.K. Chu, Surf. Coat. Technol. 203 (2008) 909.
[10] M. Shirkhanzadeh, M. Azadegan, G.Q. Liu, Mater. Lett. 7/12 (1995) 24. [11] J.H. Hsieh, C.C. Tseng, Y.K. Chang, S.Y. Chang, W. Wu, Surf. Coat. Technol. 202
(2008) 5586.
[12] P.C. Liu, J.H. Hsieh, C. Li, Y.K. Chang, C.C. Yang, Thin Solid Films 517 (2009) 4956.
[13] B.D. Ratner, A.S. Hoffman, F.J. Schoen, J.E. Lemons, Biomaterials Science, 2nd Ed, Elsevier Academic Press, 2004.
[14] L.G. Harris, S. Tosatti, M. Wieland, M. Textor, R.G. Richards, Biomaterials 25 (2004) 4135.
[15] X. Wang, G. Wang, J. Liang, J. Cheng, W. Ma, Y. Zhao, Surf. Coat. Technol. 203 (2009) 3454.
[16] P.J. Kelly, H. Li, K.A. Whitehead, J. Verran, R.D. Arnell, I. Iordanova, Surf. Coat. Technol. 204 (2009) 1137.
[17] M.S. Srivastava, Methods of Multivariate Statistics, Wiley-Interscience, New York, 2004, p. 109.
[18] R.I. Joshi, A. Eley, J. Med. Microbiol. 27 (1988) 105.
[19] K. Elagli, C. Neut, C. Romond, H.F. Hildebrand, Biomaterials 13 (1992) 25. [20] K.J. Bundy, M.F. Butler, R.F. Hochman, J. Biomed. Mater. Res. 14 (1980) 653. [21] C.W. Berry, T.J. Moore, J.A. Safar, C.A. Henry, M.J. Wagner, Implant Dent. 1 (1992)
59.
[22] P. Tengvall, E.G. Hornsten, H. Elwing, I. Lundstrom, J. Biomed. Mater. Res. 24 (1990) 319.
[23] T.Y. Liu, H.C. Liao, C.C. Lin, S.H. Hu, S.Y. Chen, Langmuir 5804/5809 (2006) 22. [24] K. Subramani, R.E. Jung, A. Molenberg, C.H.F. Hammerle, Int. J. Oral Max. Impl. 24
(2009) 616.
[25] D. Boyd, H. Li, D.A. Tanner, M.R. Towler, J.G. Wall, J. Mater. Sci. Mater. Med. 489/494 (2006) 17.
[26] W. Chen, Y. Liu, H.S. Courtney, M. Bettenga, C.M. Agrawal, J.D. Bumgardner, J.L. Ong, Biomaterials 27 (2006) 5512.
[27] B.S. Necula, L.E. Fratila-Apachitei, S.A.J. Zaat, I. Apachitei, J. Duszczyk, Acta Biomater. 5 (2009) 3573.
[28] G. Colon, B.C. Ward, T.J. Webster, J. Biomed. Mater. Res. A 595/604 (2006) 78. [29] Y. Osamu, K. Miyako, S. Jun, N. Zenbee, J. Mater. Sci. Mater. Med. 847 (851) (2004)
15.
[30] X. Zhu, J. Chen, L. Scheideler, R. Reich, J. Geis-Gerstorfer. 4087 (4103) (2004) 25. [31] O. Choi, K.K. Deng, N.J. Kim, L. Ross Jr., R.Y. Surampalli, Z. Hu, Water Res. 42 (2008)
3066.
[32] M. Hormia, M. Kononen, J. Kivilahti, I. Virtanen, J. Periodontol. Res. 26 (1991) 491. [33] M. Kononen, M. Hormia, J. Kivilahti, J. Hautaniemi, I. Thesleff, J. Biomed. Mater.
Res. 26 (1992) 1325.
[34] D.L. Cochran, J. Simpson, H.P. Weber, D. Buser, Int. J. Oral Maxillofac. Implants 3 (1988) 21.
[35] S.C. Guy, M.J. McQuade, M.J. Scheidt, J.C. McPherson, J.A. Rossmann, T.E.V. Dyke, J. Periodontol. 64 (1993) 542 T.