This content has been downloaded from IOPscience. Please scroll down to see the full text.
Download details:
IP Address: 140.113.38.11
This content was downloaded on 26/04/2014 at 08:08
Please note that terms and conditions apply.
Observation of Growth of Human Fibroblasts on Silver Nanoparticles
View the table of contents for this issue, or go to the journal homepage for more 2007 J. Phys.: Conf. Ser. 61 445
(http://iopscience.iop.org/1742-6596/61/1/089)
Observation of Growth of Human Fibroblasts on
Silver Nanoparticles
Hua-Chiang Wen a, *, Yao-Nan Lin a, Sheng-Rui Jian b, Shih-Chun Tseng a, Ming-Xiang Weng a, Yu-Pin Liu c, Po-Te Lee a, Pai-Yen Chen c, Ray-Quan Hsu a, Wen-Fa Wu c, and Chang-Pin Chou a
a Institute and Department of Mechanical Engineering, National Chiao Tung
University, Hsinchu 300, Taiwan, ROC
bDepartment of Electrophysics, National Chiao Tung University, Hsinchu 300,
Taiwan, ROC
c National Nano Device Laboratories, Hsinchu 300, Taiwan, ROC
Abstract. Silver nanoparticles have drawn extensive attention as biomaterial components.
Human fibroblasts were grown on various concentrations of silver nanoparticles during the period observation. Normal viability (0% silver particles) was increased from 6 to 72 hours,
increasing the amount of human fibroblasts (1.5x104 to 7x106 cells/well) normally.
Nevertheless, at higher concentrations of silver nanoparticles (50%) 1.11x105 cells/well remained after 72 hours. Results indicated that the increase in the concentration of silver nanoparticles reduced the number of fibroblasts and affected their fission. Silver nanoparticles were found under the membranes of fibroblast following dry treatment. The number of tissues declined because the silver nanoparticles interrupted the fission mechanism during their development in vivo.
Keywords: Human Fibroblasts, Sliver, Nanoparticles
1. Introduction
Chemists have, in recent years, devoted considerable efforts to preparing materials that are ordered on length-scale that exceed the molecular, has grown [1]. Examples of mesoscale assembly include the self-organization of amphiphilic molecules [2] or lipophilic nanoparticles [3] into liquid crystals and superlattices, respectively. The latter are generally characterized by low information content due to weak and essentially isotropic forces that act between the nanoparticles and their surface-attached molecules, such that materials with diverse and complex architectures cannot be readily assembled. This limitation can be overcome by increasing the information content of the molecules and nanoparticles employed [4]. Historically, most of the progress in the field of silver compounds and their use has occurred over the past century. Silver in the colloidal state is highly germicidal, quite harmless to humans and absolutely non-toxic. Silver in the colloidal state, rather than in a chemical compound, may be applied in a much more concentrated form, with correspondingly better results. All viruses, fungi, bacteria, streptococci, staphylococci and other pathogenic organisms are killed in three or four minutes upon contact. Even the highest concentrations cause no side effects [5-6]. Silver nanoparticles are preferred as metallic nanoparticles in numerous applications in cells because they markedly enhance the surface [7]. Silver nanoparticles have recently become widely used components of the biomaterials [8].
*Corresponding author: hcwen.me93g@nctu.edu.tw
445 © 2007 IOP Publishing Ltd
The effect of human fibroblasts depends on the silver nanoparticles surface was investigated. Owing to particle interactions with the cytoskeleton may also play a role in particle toxicity. Understanding the interactions between nanomaterials and human fibroblasts is critical to intracellular and the development of micro- and nano-particle delivery in medicinal applications.
2. Experimental details
2.1 Materials
Approximately 10 mL of 10-3 M AgNO
3 solution was added dropwise to 30 mL of 2×10-3 M
NaBH4 solution to produce silver nanoparticles, as the temperature was increased to boiling point. The
silver nanoparticles were converted and citrate-stabilized as extremely stable and water-soluble nanoparticles. All of the other chemicals were reagent-grade, and distilled water was used to make the aqueous solutions. The morphology of the silver nanoparticles was analyzed using a Hitachi S-4000 scanning electron microscope (SEM) and a Digital Instruments DI 5000 atomic force microscope (AFM). The size of the particles was determined from transmission electron micrographs obtained using a JEM-2010F (TEM) and the appropriate software. The aqueous dispersion of the particles was drop-cast onto a carbon coated copper grid and grid was air dried at room temperature before it was loaded into the microscope.
2.2 In vitro accumulation in cultured cells
Primary immortal murine NIH3T3 cells (Mouse NIH/Swiss embryo, also called human fibroblasts) were seeded with 1.5x104 cells per disk in 1ml of complete medium. The medium used
was 90% Dulbeccos Modified Eagles Medium (DMEM) (Sigma, UK) with 4mM L-glutamine adjusted to contain 1.5g/L sodium bicarbonate, 4.5g/L glucose and 10% calf serum (CS). The cells were incubated at 37
°C
in a 5% CO2 atmosphere. Three silver nanoparticle concentrations (10, 30 and50%) were inoculated into as-grown human fibroblasts (1.5x104cells/well) and the cell population was
determined by optical microscopy (OM) at 6, 12, 24, 48 and 72 hours. The morphology of the silver nanoparticles on cellular membranes was elucidated by SEM before and after they were dried.
3. Results
3.1 Observation of silver nanoparticles
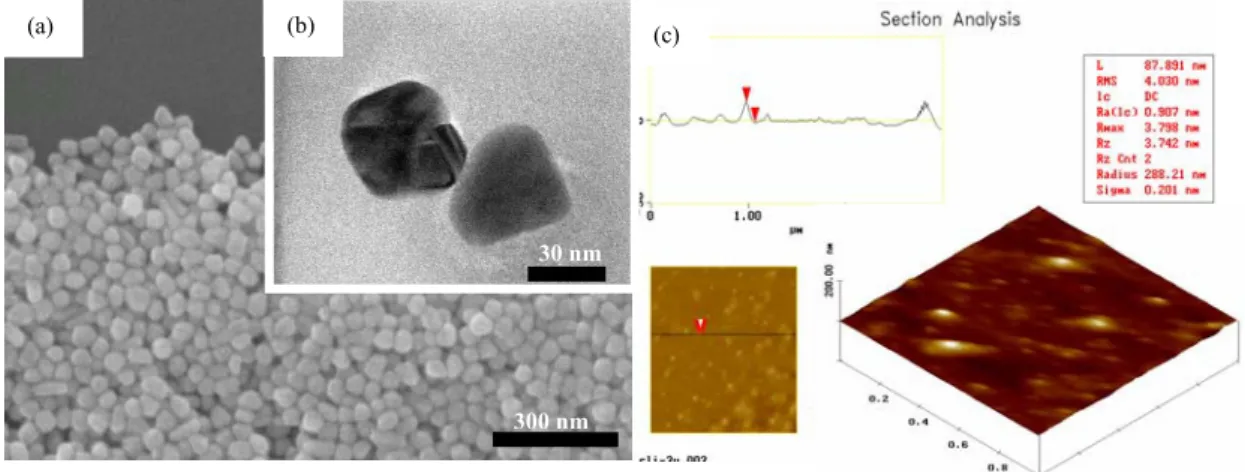
Fig.1(a) displays the SEM images of silver nanoparticles on the silicon substrate. The silver nanoparticles were synthesized by the chemical method into water-soluble nanoparticles with uniform size. The distribution of nanoparticles can be controlled during the synthesis process. The size of the nanoparticles was determined to be below 60 nm, from the TEM cross-section, as presented in Fig. 1(b). The silver nanoparticles were analyzed by AFM and their diameter determined (87.891 nm), as shown in Fig. 1(c). The above analysis revealed that silver particles are nano-sized, and can be used as the target on the cell membrane.
3.2 Effects of incubation time and the percentage silver nanoparticles on cellular accumulation
Fig.2 displays an optical microscopic photograph of 0 and 50% silver nanoparticles added to as-grown human fibroblasts (1.5x104cells/well). The viability assay of the tissue culture cells after the
silver nanoparticles were mixed was examined. The number of cells did not vary much with the addition of silver nanoparticles when they were attached to tissue culture flasks or suspended for 6 hour. However, silver nanoparticles not only reduced the number of fibroblasts but also interrupted fission on the highly concentrated (30, 50 %) silver nanoparticles from 6 to 24 hours. However, normal viability increased from 24 to 72 hours, suggesting that the high concentration of silver nanoparticles, inhibiting the viability of fission of human fibroblasts without causing internal damage. Tab. 1 plots the resulting distribution. Silver nanoparticles may be important on the surface of membranes because they are highly germicidal, quite harmless to humans and absolutely non-toxic.
Clearly, varying the percentage of silver nanoparticles can alter the viability of human fibroblasts in
vivo.
300 nm
(b)
(a) (c)
Fig. 1 Fig.1 (a) SEM image; (b) TEM image and (c) AFM morphology image for Ag nanoparticles on the substrate.
30 nm
(a) (b) (c) (d)
(e) (f) (g) (h)
Fig. 2 The optical microscopy images (magnification 200x) of 0 and 50 % of concentration silver nanoparticles on as-grew human fibroblasts (1.5x104cells/well) (a) 0 %, 12h; (b) 0 %, 24 h; (c) 0 %,
48 h; (d) 0 %, 72 h; (e) 50 %, 12 h; (f) 50 %, 24 h; (g) 50 %, 48 h; (h) 50 %, 72 h.
In the experiments performed herein, silver nanoparticles were employed in solution, in contact with the fibroblasts of the surface. The interruption is due mainly the nano-size of the silver nanoparticles, and the particles’ slowing of the fission mechanism during the period of growth. In particular, the possible effect of the feeble poisoning of the cell cycle may explain the low number of human fibroblasts in the presence of (10, 30%) silver nanoparticles. Silver coatings on the surface reduced the viability of the fibroblasts. The cell behaviour, and particularly the number of cells evidently changed, revealing the importance of understanding nanoparticle–cell interactions prior to their use in vivo.
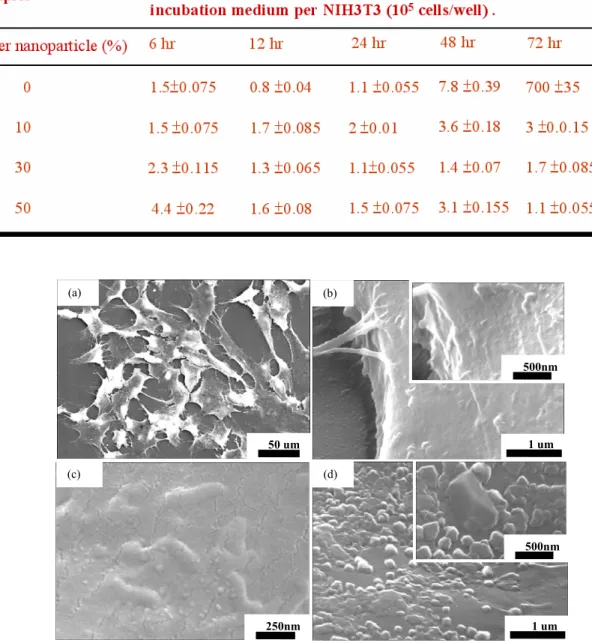
Many fibroblasts exhibited growth in the cell population and the control cell tissue (without silver nanoparticles), as displayed as Fig. 3(a). A few nanoparticles are present on the membrane surface of the tissue, as displayed as Fig. 3(b). The various percentages of silver colloid yielded further information on the response of the cell morphology to particle incubation. Additionally, cells were
spread and stimulated the formation of several lamellapodia and filopodia, projecting from the cell membranes over the surface. The effect of incubating cells with nanoparticles, was determined by comparison with control cells. Furthermore, the cells exposed to silver nanoparticles appeared to cause mitosis of the cell surface membrane at high percentages of silver colloid (50 %). Silver nanoparticles remained temporarily in the cell membranes, as shown as Fig. 3(c). Fig.3(d) displays the breaking of the surface of cellular membranes during dry treatment. Clearly, particle-like entities were found under the membranes. The number of tissues declined because the silver nanoparticles interrupted fission during the development in vivo.
Tab. 1 The fibroblasts incubated depend on various concentration of the silver nanoparticle test at 6 to 72h growth, the values are expressed as % of respective control and correspond to mean valuessSD.
(d) 1 um 500nm (b) 1 um 500nm (a) 50 um 250nm (c)
Fig. 3 Silver nanoparticles and cell membranes surface (a) the control cells tissue, (b) there is no nanoparticles on the membrane surface, (c) silver nanoparticles are to stay temporarily in cell membranes surface, (d) cell membranes are broke after dry treatment.
4. Conclusions
The capacity of the interaction between the silver nanoparticles to induce aggregation of fibroblasts was evaluated. The results of this study elucidate non-toxicity of the interaction of nanonmeter-scale silver nanoparticles and the membrane surface. Experiments must be performed in the future on the surface chemical binding of cells and nanoparticles. Understanding the interactions between nanomaterials and human fibroblasts is essential to intracellular ability, and the development of the clinical delivery of micro- and nano-particles.
Acknowledgments
This research was supported in part by the National Science Council in Taiwan under contract NSC 94-2216-E-009-027. Technical support from the National Nano Device Laboratories contract NDL-95S-C-067 is also acknowledged.
References
[1] Sauvage, J P Lehn, and J M 1999 Transition Metals in Supramolecular Chemistry 5 420 [2] S Förster, M Antonietti, 1998 Adv. Mater. 10195
[3] C B Murray, C R Kagan, M. Bawendi 1995 Science 270 1335
[4] L Cusack, R Rizza, A Gorelov, D. Fitzmaurice, Angew. Chem. Int.Ed. Engl. 36, 848, 1997 [5] Searle, A B 1913 The British Medical Journal. 83
[6] Becker, Robert O, M D, Spardaro, Joseph A. 1978 The Journal of Bone and Joint Surgery, 60(A) 871-881
[7] Omid C Farokhzad 2005 Anal. Chem. 77 5453-5459