Date 2013/December/19
Type of manuscript: Original article
Title: Hearing loss may be a non-motor feature of Parkinson’s disease in
older people in Taiwan
Running head: hearing loss and Parkinson’s disease Authors' full names:
Shih-Wei Lai 1,2, Kuan-Fu Liao3,4, Cheng-Li Lin5,6, Cheng-Chieh Lin1,2,7 Fung-Chang Sung 5,6
1School of Medicine, China Medical University and 2 Department of Family Medicine, China Medical University Hospital, Taichung, Taiwan
3Graduate Institute of Integrated Medicine, China Medical University and 4Department of Internal Medicine, Taichung Tzu Chi General Hospital, Taichung, Taiwan
5Department of Public Health, China Medical University and 6Management Office for Health Data, China Medical University Hospital, Taichung, Taiwan
7Department of Health Care Administration, College of Health Science, Asia University, Taichung, Taiwan
(The first 2 authors contributed equally to this study.)
Corresponding author:
Fung-Chang Sung, PhD, MPH Professor
China Medical University Department of Public Health No. 91, Hsueh-Shih Road, Taichung 404, Taiwan Phone: 886-4-2206-2295
Fax: 886-4-2201-9901
E-mail: fcsung1008@yahoo.com
ABSTRACT
Background. The aim of this study was to explore whether hearing loss is associated with the risk of Parkinson’s disease in the elderly in Taiwan. Methods. Using claims data of the Taiwan National Health Insurance Program, we identified 4976 patients (aged 65 years or older) with newly diagnosed hearing loss from 2000 to 2010 and we randomly selected 19904 subjects without hearing loss as comparisons, frequency matched by sex, age and index year of diagnosing hearing loss. The incidence of Parkinson’s disease by the end of 2010 and the associated risk factors were
investigated. Results. The incidence of Parkinson’s disease in the hearing loss group was 1.77-fold higher than that in the non-hearing loss group (3.11 vs. 1.76 per 1000 person-years). After controlling for confounding factors, the adjusted HR of
Parkinson’s disease was 1.53 (95% CI 1.17, 1.99) for hearing loss group, compared to the non-hearing loss group. Male (HR=1.33, 95% CI=1.02, 1.74), age (every one year, HR=1.06, 95% CI=1.04, 1.09), hypertension (HR= 1.70, 95% CI= 1.26, 2.30) and cerebrovascular disease (HR= 1.78, 95% CI=1.37, 2.32) were also significantly associated with the risk of Parkinson’s disease. Conclusions. Hearing loss correlates with the increased risk of Parkinson’s disease in the elderly. Further studies are needed to confirm whether hearing loss could be a non-motor feature of Parkinson’s disease.
Keywords: hearing loss; Parkinson's disease; retrospective cohort study; the elderly; universal insurance
INTRODUCTION
Parkinson's disease is a progressive and neurodegenerative disease commonly seen in older people. The prevalence of Parkinson's disease increases with age. A systematic review by Muangpaisan et al. revealed that the prevalence of the disease varies worldwide, from 57 to 230 per 100000 . Parkinson's disease typically demonstrates cardinal motor disorders, including resting tremor, cogwheel rigidity and
bradykinesia. A growing body of evidence shows that non-motor features occur early in Parkinson's disease and some may precede motor impairment but poorly
recognized or under diagnosed in the clinical practice . These non-motor features include neuropsychiatric symptoms, sleep disturbances, autonomic symptoms, gastrointestinal symptoms and olfactory dysfunction . Few studies have explored auditory function in PD patients by means of both pure tone audiometry (PTA) and brainstem auditory evoked potentials with findings varying among studies . Recently, Vitale et al. first systematically evaluated hearing loss in PD patients and found high-frequency age-dependent unilateral or bilateral hearing impairment, compared with both controls and normative values . Since then no further studies have explored this issue.
Hearing loss is a relatively prevalent disorder in the elderly. The prevalence increases with age, and varies among ethnic groups due to different diagnostic criteria and the populations studied. The prevalence rates of hearing loss among the elderly are around 25%-40% for those aged 65-74 years, 40%-66% for those aged 75-84 years, and more than 80% for those aged 85 years or older in Western countries . In Taiwan, the prevalence of hearing loss ranges from 47% to 65% in population aged 60-89 years .
The above referred studies indicate that hearing loss and Parkinson’s disease are prevalent disorders in the elderly and both conditions have the involvement of central
nervous system. If hearing loss is an early manifestation of Parkinson’s disease, interventions could be performed for this non-motor symptom. To date, the
population aged 65 years or older in Taiwan increased from 7.0% in 1993 to 11.15% in 2012 , but little attention has been paid to the relationship between hearing loss and Parkinson's disease in the elderly. Furthermore, few studies have ever used a
population-based design to investigate this relationship. Therefore, we used the insurance claims data of the Taiwan National Health Insurance Program to perform a retrospective cohort study and we derived from a different methodological approach to explore whether the elderly with hearing loss are at risk of developing Parkinson’s disease.
MATERIALS AND METHODS Data sources
We used the health insurance database of one million entries randomly selected from the all 23 million insured population to perform this study. Established in March 1995, 99% of all people in Taiwan have enrolled in this national universal health insurance program. The details of insurance program have been described in previous published studies .
Criteria and definition
The criteria of diseases were defined according to the International Classification of Diseases (ICD) 9th Revision. The hearing loss group consisted of 4976 patients aged 65 to 84 years with newly diagnosed hearing loss, identified from the 1-million insured population database in 2000-2010. For each patient with hearing loss, 4 comparison persons without the diagnosis of hearing loss were also randomly selected
from the same database into the non-hearing loss group (N = 19904), frequency matched by sex, age (within 5 years) and index year of diagnosing hearing loss. The index date was defined as the diagnosed date of hearing loss. Both groups were followed up until subjects received a diagnosis of Parkinson's disease, withdrawal from the insurance program, loss to follow-up, death or December 31, 2010. To increase unbiased results, subjects who had been diagnosed with Parkinson’s disease before the diagnosed date of hearing loss were excluded from the study. Similarly, subjects who had a diagnosis of secondary Parkinsonism, noise-induced hearing loss, sudden hearing loss, other types of hearing loss, major psychiatric diseases or mental retardation before the diagnosed date of hearing loss and during the follow-up period were excluded from the study.
Comorbidities at baseline were identified, which included head injury, hypertension, diabetes mellitus, hyperlipidemia, dementia, cerebrovascular disease, depression and chronic kidney disease. In order to increase the accuracy of diagnosis, hearing loss, Parkinson’s disease and comorbidities were recorded for three or more visits in the ambulatory care. All exclusion and inclusion criteria of disorders were based on the diagnoses with ICD-9 codes.
Statistical analysis
The Chi-square test and t-test were used to compare the differences between the hearing loss group and the non-hearing loss group regarding demographic status and comorbidities. The incidence of Parkinson’s disease was calculated as the number of Parkinson’s disease patients identified during the follow-up, divided by the total follow-up person-years for each group. Poisson regression model was used to estimate the hearing loss group to the non-hearing loss group incidence rate ratio (IRR) and 95% confidence interval (CI) of Parkinson’s disease by sex, age and follow-up year. Cox proportional hazards regression analysis was used to estimate the corresponding hazard ratios (HR) and 95% CI of Parkinson’s disease in association with hearing
loss, sex, age and comorbidities. We also estimated the joint effects between hearing loss and comorbidities on risk of Parkinson’s disease using Cox model. All statistical analyses were performed using the SAS software version 9.1 (SAS Institute Inc., Cary, NC). The tests of significance were two-sided at p < 0.05.
RESULTS
Baseline characteristics of the study cohort
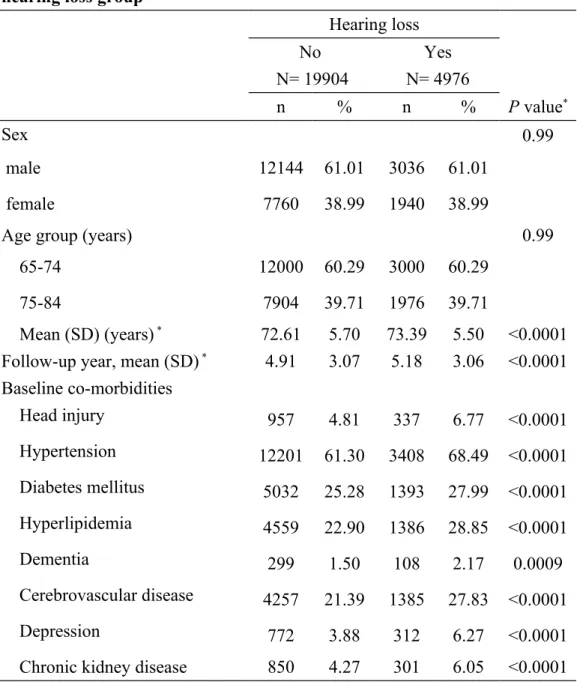
There were more male subjects (61.01%) in both groups with a mean age of approximately 73 years old. The hearing loss group had a longer mean duration of follow-up than did the non-hearing loss group (5.18 vs. 4.91 years, P < 0.0001; Table 1). Comorbidities were more prevalent in hearing loss group than in the non-hearing loss group.
Incidence of Parkinson’s disease by subjects characteristics
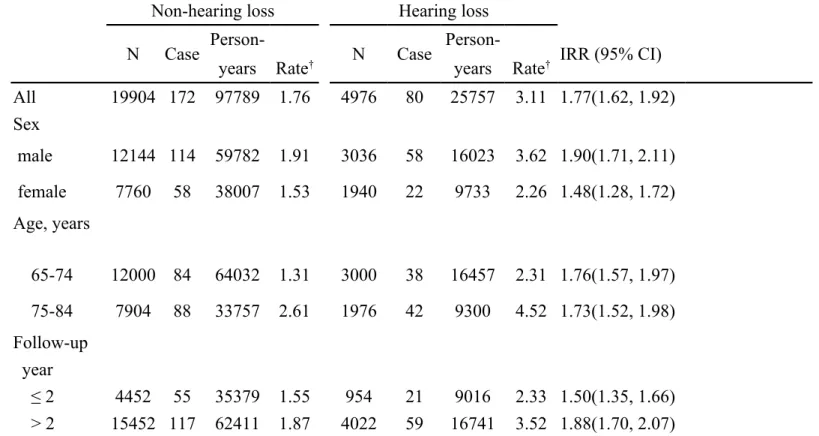
Figure 1 shows that the cumulative incidence of Parkinson’s disease was higher in the hearing loss group than in the non-hearing loss group for 1.48% (3.36% vs. 1.88%; P < 0.0001) by the end of follow-up. Table 2 shows the corresponding overall incidence rates were 3.11 vs. 1.76 per 1000 person-years, with a hearing loss group to non-hearing loss group incidence rate ratio (IRR) of 1.77 (95% CI = 1.62, 1.92). The incidence rates of Parkinson’s disease, as stratified by sex, age and follow-up year, were all higher in hearing loss group than in the non-hearing loss group. The
incidence of Parkinson’s disease increased with age in both groups, the highest in the hearing loss group aged 75-84 years. The stratified analysis by follow-up year showed that the incidence of Parkinson’s disease increased with the follow-up time.
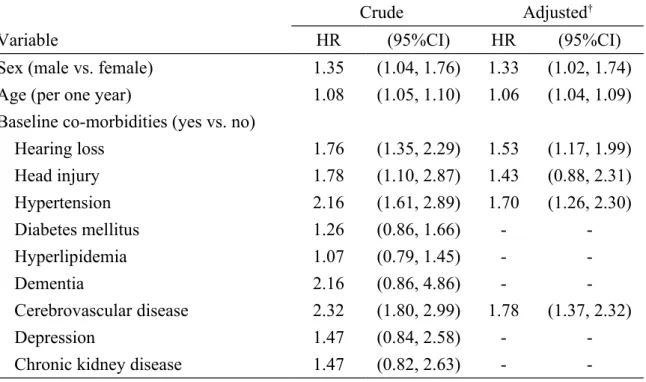
The multivariate Cox proportional hazards regression analysis showed that the adjusted HR of Parkinson’s disease was 1.53 (95% CI 1.17, 1.99) for the hearing loss group, compared to the non-hearing loss group (Table 3). Male subjects had an adjusted HR of 1.33 for Parkinson’s disease compared with female subjects (95% CI=1.02, 1.74), and the HR increased with age (every one year, HR=1.06, 95% CI=1.04, 1.09). Comorbidities, including hypertension (HR= 1.70, 95% CI= 1.26, 2.30) and cerebrovascular disease (HR= 1.78, 95% CI=1.37, 2.32), were also significantly associated with the risk of Parkinson’s disease. In further analysis, we found there was a joint effect between hearing loss and hypertension or
cerebrovascular disease on risk of Parkinson’s disease, but not statistically significant (data not shown).
DISCUSSION
Our study on hearing loss in association with risk of Parkinson’s disease yielded an incidence rate ratio of 1.77 for hearing loss subjects versus non-loss subjects with the incidence of 3.11 and 1.76 per 1000 person-years. In order to confirm our findings, we further conducted an additional data analysis using another 1-million population-based data file with similar design. The corresponding IRR is 1.44 with incidence of 3.17 versus 2.20 per 1000 person-years, respectively.
The risk of Parkinson’s disease varies among populations. In a 2-stage survey conducted among residents aged 40 years and older in a rural county, Taiwan, Chen et al. found a prevalence of 3.58 per 1000 . They followed up subjects free of
was 0.30 per 1000. For those aged 60-79 years, the incidence was 3.19 per 1000. Our further data analysis showed that the annual incidence of Parkinson’s disease for the elderly from 1997 to 2010 in Taiwan ranged from 1.57 to 2.23 per 1000 (data not shown). Despite different methods of collecting Parkinson’s disease data can lead to different estimates of Parkinson’s disease, that is why it would be difficult to compare our results to those of Chen et al. In addition, the Neurological Disorders in Central Spain Study found that the incidence of Parkinsonism and Parkinson’s disease was 5.97 per 1000 person-years .
Extensive evidence has demonstrated that hearing loss in the elderly is a multi-factorial disorder. The potential causes might include that age-related hearing loss, noise exposure, ototoxic medications, obstruction of external auditory canal, tympanic membrane dysfunction, middle ear diseases, genetic factor, and family history . Recent literature shows that natural age-related changes and/or neurodegenerative-related changes in the auditory pathway and in the brain could be also involved in hearing loss . Both hearing loss and neurodegenerative diseases may involve common biological mechanisms that lead to Parkinson's disease. In the Vitale et al. study, high-frequency hearing loss is relatively more evident in patients with Parkinson's disease .
To reduce the estimation bias, the present study excluded subjects with noise-induced hearing loss or sudden hearing loss identified at the baseline and during the follow-up period. We found an overall of 53% excess risk of Parkinson's disease among the hearing loss group. Although subjects with hearing loss preceded the clinical diagnosis of Parkinson's disease, the lag time may really exist between the diagnosis of hearing loss and the onset of Parkinson's disease. Hence, whether hearing loss is one of non-motor features for Parkinson's disease mediated by
this study. Since this is an observational study, we are unable to differentiate whether hearing loss is caused by other otologic disorders or intrinsic to neurodegenerative changes for Parkinson's disease. Limited studies have investigated the association between hearing loss and Parkinson's disease . No previous study has ever
investigated the temporary association between patients with hearing loss and the risk of developing Parkinson’s disease later. Chaudhuri et al’s reviews demonstrated that non-motor features of Parkinson's disease are commonly seen clinically and are also a key role associated with the quality of life, but remain recognized and under-treated by clinicians . Further prospective studies are needed to investigate the association between hearing loss and Parkinson's disease.
The risk of Parkinson's disease may vary during the follow-up period, which is relatively important in clinical practice. The incidence rate of Parkinson's disease was higher within 2 years of follow-up in the hearing loss group than in the non-hearing loss group and the IRR increased continuously after 2 years of follow-up. Therefore, clinicians should be cautious of the potential diagnosis of Parkinson's disease even after 2 years of diagnosing hearing loss.
There are limitations in this present study. First, the insurance data provided no information on physiological and laboratory examinations. Therefore, the data of pure tone audiometry was not available to validate hearing loss. Similarly, the severity and pattern of hearing loss based on unilateral versus bilateral hearing loss could not be addressed in this study, because the diagnosis of hearing loss was based on ICD-9 codes. We were unable to quantify the dose-response relationship between degree of hearing loss and Parkinson's disease. We were also unable to show a common
neurological basis for hearing loss and Parkinson’s disease. Second, hearing test is not routinely performed in primary care in Taiwan. So there could be a substantial
number of persons with hearing loss who were not detected. The prevalence of hearing loss might be underestimated; those who were included in this study might have severer hearing impairments. Third, some risk factors of Parkinson's disease, such as obesity, alcohol consumption and cigarette smoking, were not recorded in detail in this database. Despite we tried to define obesity, alcoholism and tobacco use by using ICD-9 codes, the case number is too small to be adjusted for potentially confounding variables (data not shown). Forth, we could not find whether other non-motor features of Parkinson's disease existed before hearing loss. Fifth, persons with hearing loss might use the insurance program more often than persons without hearing loss. The diagnosis of Parkinson’s disease might thus be delayed in those without hearing loss relative to those with hearing loss, leading to under-ascertainment in those without hearing loss. Last, the aim of this study was to focus on the elderly. Young-onset or early-onset of Parkinson's disease was not included. Inclusion of young-onset Parkinson's disease in future study will strengthen the argument. Besides these limitations, the strengths of the present study are its use of different methodological approach by the retrospective cohort study design and its use of apparently nationwide, population-based health insurance records, and its use of restriction and proportional hazards regression to adjust for the potentially
confounding effects of sex, age and baseline comorbidities. It also indicates future research directions.
We conclude that hearing loss is associated with increased risk of Parkinson's disease in the elderly in Taiwan. Further studies are needed to confirm whether hearing loss could be one of non-motor features of Parkinson's disease. If yes, clinicians should alert these patients about the potential risk of developing Parkinson's disease.
Funding
This study was supported in part by Taiwan Department of Health Clinical Trial and Research Center of Excellence (Grant number DOH102-TD-B-111-004) and China Medical University Hospital (Grant number 1MS1).The funding agency did not influence the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgements
The authors thank the National Health Research Institutes in Taiwan for providing the insurance claims data.
Specific author contributions:
Shih-Wei Lai: (1) substantial contributions to the conception of this article; (2) planned and conducted the study; (3) initiated the draft of the article and critically revised the article.
Kuan-Fu Liao and Cheng-Chieh Lin: (1) planned and conducted the study; (2) participated in data interpretation; (3) critically revised the article.
Cheng-Li Lin: (1) conducted data analysis; (2) critically revised the article.
Fung-Chang Sung: (1) conducted study design and data analysis, and participated in data interpretation and (2) critically revised the article.
Conflict of Interest Statement
References
[1]. Muangpaisan W, Mathews A, Hori H, Seidel D. A systematic review of the worldwide prevalence and incidence of Parkinson's disease. J Med Assoc Thai. 2011 94: 749-755.
[2]. Chaudhuri KR, Yates L, Martinez-Martin P. The non-motor symptom complex of Parkinson's disease: a comprehensive assessment is essential. Curr Neurol
Neurosci Rep. 2005 5: 275-283.
[3]. Mehndiratta M, Garg RK, Pandey S. Nonmotor symptom complex of Parkinson's disease--an under-recognized entity. J Assoc Physicians India. 2011 59: 302-308, 313.
[4]. Weintraub D, Moberg PJ, Duda JE, Katz IR, Stern MB. Effect of psychiatric and other nonmotor symptoms on disability in Parkinson's disease. J Am Geriatr Soc. 2004 52: 784-788.
[5]. Gawel MJ, Das P, Vincent S, Rose FC. Visual and auditory evoked responses in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1981 44: 227-232. [6]. Al-Bunyan MA. Parkinson's disease. Clinical and electrophysiological
evaluation. Saudi Med J. 2000 21: 72-75.
[7]. Yylmaz S, Karaly E, Tokmak A, Guclu E, Kocer A, Ozturk O. Auditory evaluation in Parkinsonian patients. Eur Arch Otorhinolaryngol. 2009 266: 669-671. [8]. Vitale C, Marcelli V, Allocca R, et al. Hearing impairment in Parkinson's disease: expanding the nonmotor phenotype. Mov Disord. 2012 27: 1530-1535. [9]. Yueh B, Shapiro N, MacLean CH, Shekelle PG. Screening and management of adult hearing loss in primary care: scientific review. JAMA. 2003 289: 1976-1985. [10].Chou R, Dana T, Bougatsos C, Fleming C, Beil T. Screening adults aged 50 years or older for hearing loss: a review of the evidence for the U.S. preventive services task force. Ann Intern Med. 2011 154: 347-355.
[11].Lin FR, Thorpe R, Gordon-Salant S, Ferrucci L. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011 66: 582-590.
[12].Lin CY, Yang YC, Guo YL, Wu CH, Chang CJ, Wu JL. Prevalence of hearing impairment in an adult population in Southern Taiwan. Int J Audiol. 2007 46: 732-737.
[13].Ministry of the Interior inTaiwan. Resident Population by 5-Year, 10-Year Age Group. http://sowf.moi.gov.tw/stat/month/m1-06.xls (accessed 23/January/2014).
[14].Liao KF, Lai SW, Li CI, Chen WC. Diabetes mellitus correlates with increased risk of pancreatic cancer: a population-based cohort study in Taiwan. J Gastroenterol
[15].Lai SW, Liao KF, Liao CC, Muo CH, Liu CS, Sung FC. Polypharmacy
correlates with increased risk for hip fracture in the elderly: a population-based study.
Medicine (Baltimore). 2010 89: 295-299.
[16].Lai SW, Muo CH, Liao KF, Sung FC, Chen PC. Risk of acute pancreatitis in type 2 diabetes and risk reduction on anti-diabetic drugs: a population-based cohort study in Taiwan. Am J Gastroenterol. 2011 106: 1697-1704.
[17].Lai SW, Liao KF, Chen PC, Tsai PY, Hsieh DP, Chen CC. Antidiabetes drugs correlate with decreased risk of lung cancer: a population-based observation in taiwan. Clin Lung Cancer. 2012 13: 143-148.
[18].Chen RC, Chang SF, Su CL, et al. Prevalence, incidence, and mortality of PD: a door-to-door survey in Ilan county, Taiwan. Neurology. 2001 57: 1679-1686.
[19].Benito-Leon J, Bermejo-Pareja F, Morales-Gonzalez JM, et al. Incidence of Parkinson disease and parkinsonism in three elderly populations of central Spain.
Neurology. 2004 62: 734-741.
[20].Gates GA, Mills JH. Presbycusis. Lancet. 2005 366: 1111-1120.
[21].Bovo R, Ciorba A, Martini A. Environmental and genetic factors in age-related hearing impairment. Aging Clin Exp Res. 2011 23: 3-10.
[22].Walling AD, Dickson GM. Hearing loss in older adults. Am Fam Physician. 2012 85: 1150-1156.
[23].Humes LE, Dubno JR, Gordon-Salant S, et al. Central presbycusis: a review and evaluation of the evidence. J Am Acad Audiol. 2012 23: 635-666.
[24].Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009 8: 464-474. [25].Chaudhuri KR, Odin P, Antonini A, Martinez-Martin P. Parkinson's disease: the non-motor issues. Parkinsonism Relat Disord. 2011 17: 717-723.
Table 1. Baseline characteristics between hearing loss group and non-hearing loss group
Hearing loss No N= 19904 Yes N= 4976 n % n % P value* Sex 0.99 male 12144 61.01 3036 61.01 female 7760 38.99 1940 38.99
Age group (years) 0.99
65-74 12000 60.29 3000 60.29
75-84 7904 39.71 1976 39.71
Mean (SD) (years) * 72.61 5.70 73.39 5.50 <0.0001 Follow-up year, mean (SD) * 4.91 3.07 5.18 3.06 <0.0001 Baseline co-morbidities Head injury 957 4.81 337 6.77 <0.0001 Hypertension 12201 61.30 3408 68.49 <0.0001 Diabetes mellitus 5032 25.28 1393 27.99 <0.0001 Hyperlipidemia 4559 22.90 1386 28.85 <0.0001 Dementia 299 1.50 108 2.17 0.0009 Cerebrovascular disease 4257 21.39 1385 27.83 <0.0001 Depression 772 3.88 312 6.27 <0.0001
Chronic kidney disease 850 4.27 301 6.05 <0.0001 Chi-square test and *t-test comparing subjects with hearing loss and non- hearing loss
Table 2. Incidence density of Parkinson's disease and hearing loss group to non-hearing loss group rate ratio
Non-hearing loss Hearing loss
N Case Person- N Case Person- IRR (95% CI)
years Rate† years Rate†
All 19904 172 97789 1.76 4976 80 25757 3.11 1.77(1.62, 1.92) Sex male 12144 114 59782 1.91 3036 58 16023 3.62 1.90(1.71, 2.11) female 7760 58 38007 1.53 1940 22 9733 2.26 1.48(1.28, 1.72) Age, years 65-74 12000 84 64032 1.31 3000 38 16457 2.31 1.76(1.57, 1.97) 75-84 7904 88 33757 2.61 1976 42 9300 4.52 1.73(1.52, 1.98) Follow-up year ≤ 2 4452 55 35379 1.55 954 21 9016 2.33 1.50(1.35, 1.66) > 2 15452 117 62411 1.87 4022 59 16741 3.52 1.88(1.70, 2.07)
† Incidence rate: per 1000 person-years
Table 3. Cox model measured hazard ratio and 95% confidence interval of Parkinson's disease associated with hearing loss and comorbidities
Crude Adjusted†
Variable HR (95%CI) HR (95%CI)
Sex (male vs. female) 1.35 (1.04, 1.76) 1.33 (1.02, 1.74) Age (per one year) 1.08 (1.05, 1.10) 1.06 (1.04, 1.09) Baseline co-morbidities (yes vs. no)
Hearing loss 1.76 (1.35, 2.29) 1.53 (1.17, 1.99) Head injury 1.78 (1.10, 2.87) 1.43 (0.88, 2.31) Hypertension 2.16 (1.61, 2.89) 1.70 (1.26, 2.30) Diabetes mellitus 1.26 (0.86, 1.66) - -Hyperlipidemia 1.07 (0.79, 1.45) - -Dementia 2.16 (0.86, 4.86) - -Cerebrovascular disease 2.32 (1.80, 2.99) 1.78 (1.37, 2.32) Depression 1.47 (0.84, 2.58) -
-Chronic kidney disease 1.47 (0.82, 2.63) -
Figure 1. Cumulative incidence of Parkinson's disease for people with and without hearing loss