Subscriber access provided by NATIONAL TAIWAN UNIV
Industrial & Engineering Chemistry Research is published by the American Chemical Society. 1155 Sixteenth Street N.W., Washington, DC 20036
Article
Effects of Flue Gas Components on the Reaction of Ca(OH)
2with SO
2Chiung-Fang Liu, and Shin-Min Shih
Ind. Eng. Chem. Res., 2006, 45 (26), 8765-8769 • DOI: 10.1021/ie0605534
Downloaded from http://pubs.acs.org on November 24, 2008
More About This Article
Additional resources and features associated with this article are available within the HTML version: • Supporting Information
• Links to the 1 articles that cite this article, as of the time of this article download • Access to high resolution figures
• Links to articles and content related to this article
Effects of Flue Gas Components on the Reaction of Ca(OH)
2with SO
2 Chiung-Fang Liu†and Shin-Min Shih*,‡New Energy Technology DiVision., Energy and EnVironmental Research Laboratory, Industrial Technology Research Institute, Hsinchu, Taiwan, 310, and Department of Chemical Engineering, National Taiwan UniVersity, Taipei, Taiwan 106
A differential fixed-bed reactor was employed to study the effects of the flue gas components, H2O, CO2,
NOX, and O2, on the reaction between Ca(OH)2and SO2under conditions similar to those in the bag filters
of a spray-drying flue gas desulfurization (FGD) system. The presence of CO2with SO2 in the gas phase
enhanced the sulfation of Ca(OH)2only when NOXwas also present. When either NOX(mainly NO) or O2
was present with SO2, the enhancement effect was slight, but became great when both NOX and O2were
present, and was even greater when CO2 was also present. The great enhancement effect exerted by the
presence of NOX/O2resulted from the rise in the NO2concentration, which enhanced the oxidation of HSO3
-and SO32-to SO42-in the water layer adsorbed on Ca(OH)2surface and the formation of deliquescent salts
of calcium nitrite and nitrate. The enhancement effect due to the presence of NOX/O2was more pronounced
when the relative humidity was above that at which the salts deliquesced; the extent of sulfation was more than twice that obtained when SO2alone was present. The presence of H2O, CO2, NOX, and O2in the flue gas
is beneficial to the SO2 capture in the low-temperature dry and semidry FGD processes. The presence of
NOX/O2also enhanced CO2removal when SO2was absent.
Introduction
SO2is one of the major air pollutants emitted from power
stations burning fossil fuels. Hydrated lime (Ca(OH)2) is the
commonly used sorbent for SO2removal in flue gas
desulfur-ization (FGD) processes, such as the dry and semidry FGD processes. In the low-temperature dry process or in the dry stage of the semidry process, SO2is captured by Ca(OH)2particles
through the reaction between them under humid conditions. Besides SO2and H2O, the gaseous species in flue gas, NOX,
CO2, and O2,1 also take part in the reaction of Ca(OH)2.
However, most studies on the sulfation of Ca(OH)2 at low
temperatures have been carried out without the presence of NOX,
CO2, and O2.2,3 Nevertheless, there are some reports on the
effects of these species on the sulfation of Ca(OH)2.
The study of Chu and Rochelle4revealed that the presence
of NOXin the gas phase has a negligible effect on the reaction
of Ca(OH)2with SO2. They reacted Ca(OH)2with a gas mixture
containing 14% H2O, 500 ppm SO2, 7% O2, 10% CO2, and
500 ppm NOXat 66 and 92°C for 1 h. However, when NO2
was used instead of NOX, Nelli and Rochelle5found that the
SO2removal by Ca(OH)2at 70°C and 60% relative humidity
(RH) is enhanced by NO2, but the enhancement effect is reduced
when O2is also present. Ishizuka et al.6pointed that NOXbarely
affects the SO2 removal efficiency in the low-temperature
semidry FGD process, but NOXsignificantly enhances the SO2
removal efficiency in the high-temperature dry FGD process: the SO2removal efficiency is very low without the presence of
NOX. They also reported that the main product of the semidry
FGD is CaSO3 and that of the high-temperature dry FGD is
CaSO4.
Ho and Shih7found that, with the presence of O
2, the SO2
capture of Ca(OH)2increases when the RH is above 60% and
decreases when the RH is lower, but the O2concentration
(1-5.4%) does not affect the reaction. Ho et al.8 found that the
total conversion of Ca(OH)2increases when both SO2and CO2
are present with or without O2. Later, Liu and Shih9reported
that the presence of CO2with SO2increases the total conversion
of Ca(OH)2, but does not affect the yield of CaSO3. However,
Klingspor et al.10and Irabien et al.11reported that CO 2and O2
have no influence on the reaction, and Moyeda et al.12 and
Seeker et al.13reported a decrease in the SO
2capture with the
presence of CO2.
Understanding the effects of the flue gas components on the reaction of Ca(OH)2is important to the design and operation
of the FGD processes. However, the effects reported in the literature are inconsistent. Thus, it is worthwhile to undertake a more thorough study on this subject. In the present study, Ca(OH)2 was reacted with gas mixtures containing different
combinations of the gaseous species SO2, CO2, NOX, and O2in
addition to H2O, in order to elucidate the effects of H2O, CO2,
NOX, and O2 on the reaction of Ca(OH)2 with SO2 at low
temperatures. The major findings of this study are that the presence of CO2/NOX/O2has a positive effect on the sulfation
of Ca(OH)2, especially at high RHs, and that the presence of
NOX/O2enhances CO2removal by Ca(OH)2with SO2absent.
Experimental Section
Sulfation Test. The hydrated lime used was reagent-grade Ca(OH)2 (purity > 95%; Hayashi Pure Chemical Industries,
Ltd.), which had a BET surface area of 10.4 m2/g.
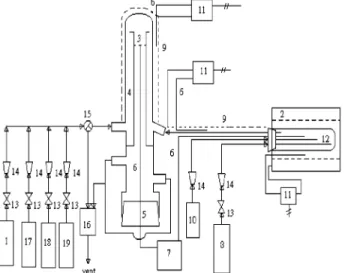
Experiments for the reaction of the sorbent were carried out using a differential fixed-bed reactor made of glass. The experimental setup is shown in Figure 1. About 40 mg of Ca-(OH)2 powder was used for each run. The Ca(OH)2 powder
was dispersed into quartz wool; the wool was then set into the sample pan. The sample pan had dimensions of 10 mm o.d. and 15 mm height and was perforated at the bottom to facilitate the passage of the sweep gas. The sweep gas entered the bottom of the reactor, passed through the 25 mm i.d. and 365 mm length outer tube, and went downward through the sample pan and the 10 mm i.d. and 315 mm length inner tube. The reactor was heated by a heating tape. The gas mixture comprised SO2, CO2,
NOX, O2, H2O, and N2. The H2O vapor was provided by a water
* To whom correspondence should be addressed. Tel.: 886-2-23633974. Fax: 886-2-23623040. E-mail: smshih@ntu.edu.tw.
†Industrial Technology Research Institute. ‡National Taiwan University.
10.1021/ie0605534 CCC: $33.50 © 2006 American Chemical Society Published on Web 11/22/2006
evaporator, and the other gases, with purities >99%, were supplied from cylinders. The NOXgas consisted of NO (>97%)
and NO2. The concentrations of the components in the gas
mixtures were controlled by adjusting their flow rates. As shown in Table 1, the concentrations of SO2, CO2, NOX, and O2were
in the typical concentration ranges in the flue gas evolved in burning medium-sulfur coal.1
Prior to each run, the sample bed was humidified for 30 min with humid N2at a selected relative humidity and temperature
under which the subsequent reaction experiment was performed. After humidification, the reactive gases were admitted into the reactor to start the run. The total gas flow rate was 4 L/min (STP). The reaction time was 1 h. At least two repeated runs were undertaken for each set of experimental conditions.
Chemical and Physical Analyses. After the reaction ended, the sample was vacuum-dried before it was subjected to analysis. The amount of Ca(OH)2 remaining in a reacted sample was
determined by acid/base titration. The amount of sulfite formed was determined by iodimetric titration, the amounts of sulfate, nitrite, and nitrate were determined by ion chromatography, and the amount of Ca was determined by EDTA titration. From the results of the above analyses, the mole fractions of Ca(OH)2
remaining (XHL), calcium sulfite (XS1), calcium sulfate (XS2),
calcium nitrite (XN1), and calcium nitrate (XN2) were calculated,
and the mole fraction of calcium carbonate and/or trace amount of inert substance (XC) was 1 - XHL- XS1- XS2- XN1
-XN2. The detailed procedures of the analyses are described
elsewhere.14The reacted samples were also analyzed by X-ray
diffraction (XRD) and observed by scanning electron micros-copy (SEM).
Results and Discussion
Effects of Flue Gas Components on the Reaction of Ca-(OH)2. The mole fractions of reaction products and Ca(OH)2
remaining after Ca(OH)2had reacted with various gas mixtures
at 60°C and 80% RH for 1 h are listed in Table 1. The gas mixtures contained different combinations of SO2, CO2, NOX,
and O2 in addition to water vapor and N2. The 1 h reaction
time was long enough for the sulfation of Ca(OH)2to reach the
ultimate extent.3,14 The absolute errors of the mole fractions
measured were about 0.02; thus a value of mole fraction around
0.02 shown in Table 1 indicates just the presence of the species instead of its true value.
As shown in Table 1, when SO2alone reacted with Ca(OH)2,
calcium sulfite was formed, and XS1was 0.29. When either NOX
or O2was also present, a small amount of calcium sulfate (XS2
) 0.05) was generated, and XS1 slightly increased with the
presence of O2. Similar results have been reported for Ca(OH)2
reacted with SO2alone2,3and with SO2and O2.7
Ca(OH)2is also reactive toward CO2.8,15Table 1 shows that
XCwas 0.34 when CO2alone was present. However, when both
SO2and CO2were present, XCwas small (about 0.06), and XS1
was almost the same as that obtained when SO2 alone was
present. Therefore, CO2nearly did not affect the ultimate SO2
capture of Ca(OH)2. It has been reported that when both SO2
and CO2are present, Ca(OH)2reacts with both gases to form
sulfite and carbonate in the initial stage of reaction, but the carbonate formed reacts further with SO2to form sulfite; thus
the ultimate XCis small.8,9
The gas mixture containing SO2, CO2, and O2was to simulate
the flue gas after the NOX removal unit and before the SO2
removal unit. Under this gas mixture, the total conversion to sulfite and sulfate (about 0.34) was close to that obtained when only SO2and O2were present (about 0.37), but smaller than
that obtained (about 0.40) when the gas mixture contained SO2,
CO2, and NOX.
As seen in Table 1, Ca(OH)2barely reacted with NOXwhen
NOXpresented alone. However, the conversion of Ca(OH)2to
form calcium nitrite and nitrate was appreciable when both NOX
and O2were present; XN1and XN2were about 0.15 and 0.03,
respectively. The reacted sample appeared as yellow mud, indicating an excessive amount of water had been collected by the sample. It is known that calcium nitrite and nitrate are deliquescent salts and the color of calcium nitrite is yellow.16
The presence of NOX and O2 together with SO2markedly
increased the SO2captured by Ca(OH)2. As shown in Table 1,
both XS1and XS2were about 0.28, but nitrite and nitrate were
hardly detected. The sample became a wet white cake with some yellow spots after the reaction. This appearance indicates that the deliquescent species played an important role during the reaction.
When Ca(OH)2reacted with the NOX, O2, and CO2mixture,
the major product was CaCO3. The value of XC, 0.84, was much
larger than that obtained when CO2presented alone. The reacted
sample appeared as white mud. This again indicates that a great quantity of water had been collected by the sample during the reaction when the gas mixture contained both NOXand O2.
The gas mixture containing SO2, NOX, CO2, and O2was to
simulate the typical flue gas before entering the NOXand SO2
removal units. As shown in Table 1, under this gas mixture, XS1and XS2 were 0.34 and 0.32, respectively, XC was small,
and XN1and XN2were nearly zero. Evidently, this gas mixture
greatly enhanced the extent of sulfation of Ca(OH)2, which was
more than twice that obtained when SO2 alone was present,
and was 0.10 greater than that obtained when only SO2, NOX,
and O2were present. Therefore, the presence of NOX, CO2, and
O2together with SO2in the flue gas would be beneficial to the
SO2removal using hydrated lime. The reacted sample in this
case also became a cake similar to that observed when only SO2, NOX, and O2were present.
The effects of the component gas-phase concentrations on the ultimate sulfation extent of Ca(OH)2were also studied by
varying the SO2concentration from 100 to 1000 ppm, NOXfrom
300 to 600 ppm, CO2from 3.2 to 12.6%, and O2from 1.0 to
5.4%, and negligible effects were found.
Figure 1. Schematic diagram of experimental apparatus: 1, SO2cylinder; 2, furnace; 3, Pyrex sample pan; 4, reactor; 5, rubber stopper; 6, thermocouple; 7, temperature recorder; 8, N2cylinder; 9, heating tape; 10, syringe pump; 11, temperature controller; 12, evaporator; 13, needle valve; 14, rotameter; 15, three-way valve; 16, SO2absorber; 17, O2cylinder; 18, CO2cylinder; 19, NOXcylinder.
Effect of RH on the Reaction of Ca(OH)2. The effect of RH or water vapor on the reaction of Ca(OH)2was studied with
two gas mixtures: one containing SO2alone and one simulating
the typical flue gas composition. The results for 1 h reaction at 60°C and 30-80% RH are also listed in Table 1.
As seen in Table 1, under either gas mixture the conversion of Ca(OH)2, 1 - XHL, increased as RH increased, but the
conversion under the synthesized flue gas was much greater at each RH, especially when the RH was high (g70%). Both XS1
and XS2increased with increasing RH, but there were no marked
changes in XS1and XS2when RH increased from 70 to 80%.
XS2 was smaller than 0.09 when RH e 50%, but increased
drastically to 0.32 when RH increased to 70%. The CaSO3/
CaSO4molar ratio was about 1 at 70 and 80% RH, but was
about 2 at 30 and 50% RH. XCwas about 0.12 when RH was
low (e50%), but was very small when RH was high, indicating that high RH favored the sulfation of Ca(OH)2instead of the
carbonation. The mole fractions of Ca(NO2)2and Ca(NO3)2were
very small (e0.01) at each RH. Nevertheless, these two species were helpful to the conversion of Ca(OH)2.
SEM Observations. The unreacted Ca(OH)2 sample is a
white powder. When NOX and O2were not present
simulta-neously in the gas phase, the sulfated samples were still white powders. The SEM micrographs shown in Figure 2 reveal that the sample particles after reacting with SO2(Figure 2a), CO2
(Figure 2b), and SO2/CO2/NOX (Figure 2d) are similar in
morphology: the product grains densely cover the particle surface. However, the particles that reacted with SO2/CO2have
a quite different appearance (Figure 2c): rods and plates of calcium sulfite appear on the particle surface.
The samples after reacting with SO2/NOX/O2 or SO2/CO2/
NOX/O2at RH g 70% became a wet white cake with some
yellow spots of calcium nitrite. The samples were vacuum-dried at 60°C and ground to fine particles. As shown in Figure 2e,f, the particles seem to have larger grains and pores.
Effects of the Presence of both NOX and O2. At low temperatures, the reaction between Ca(OH)2and SO2requires
the presence of water vapor; the higher the RH, the greater the extent of reaction of Ca(OH)2. Under humid circumstances,
water molecules are adsorbed on the surface of Ca(OH)2,
forming a thin water layer, e.g., 2.3 monolayers thick at 70% RH.2SO
2molecules in the gas phase are adsorbed onto the water
layer and combine with water to form SO2‚H2O. SO2‚H2O may
then dissociate to H+, HSO3-, and SO32-, and these ions
subsequently react with Ca(OH)2to form CaSO3.2,3When O2
or NOX(mainly NO) is also present in the gas phase, some O2
or NOXmolecules are also present in the water layer and oxidize
HSO3-and SO32-to SO42-; however, the extent of oxidation
is low due to the low solubility of O2or NOXin water. When
both O2and NOXare present in the gas phase, the tendency of
approach to the equilibrium of the O2/NO/NO2system raises
the concentration of NO2in the gas phase to a higher value.17
As NO2is a much stronger oxidant and more soluble in water
than NO and O2,17,18there are more NO2molecules present in
the water layer, and the oxidation of HSO3-and SO32-to SO4
2-is enhanced, which induces more SO2molecules to be captured
into the water layer. The reactions that take place in the water layer involving NO2 can be represented by the following
stoichiometric equations:17,18
As seen in the above equations, H+, NO2-, and NO3-are
formed when NO and NO2react with HSO3-, SO32-, and water.
These ions react with Ca(OH)2to form Ca(NO2)2and Ca(NO3)2.
Since Ca(NO2)2 and Ca(NO3)2 are deliquescent salts, their
deliquescence would collect a great quantity of water on the sample surface, and thus greatly enhance the reaction of Ca-(OH)2 with the reactive gases. This is thought to be another
reason for the increase in SO2capture by Ca(OH)2with NOX/
O2present, besides the oxidation of bisulfite and sulfite to sulfate
mentioned previously. The high extent of carbonation when Ca-(OH)2reacted with the CO2/NOX/O2mixture is believed to be
also caused by the deliquescence of these salts.
The marked difference in the conversion of Ca(OH)2between
50 and 70% RH as shown in Table 1 indicates that the deliquescence RH, i.e., the RH at which a salt starts to deliquesce,18of Ca(NO
2)2or Ca(NO3)2is in this RH range. This
was also confirmed by that the samples reacted at RH g 70% became a white cake, whereas those reacted at RH e 50% were Table 1. Mole Fractions of Calcium Hydroxide (XHL), Sulfite (XS1), Sulfate (XS2), Nitrite (XN1), Nitrate (XN2), and Carbonate (XC) in Reacted
Samples after Ca(OH)2Samples Reacted with Different Gas Mixtures at 60°C for 1 h
RH, % SO2, ppm NOX, ppm CO2, % O2, % XHL XS1 XS2 XN1 XN2 XC 80 1000 0 0 0 0.68 0.29 0 0 0 0.03 80 1000 0 12.6 0 0.65 0.29 0 0 0 0.06 80 1000 600 0 0 0.63 0.29 0.05 0.01 0 0.02 80 1000 0 0 5 0.60 0.32 0.05 0 0 0.03 80 1000 600 0 5 0.41 0.28 0.28 0 0 0.03 80 1000 600 12.6 0 0.53 0.34 0.06 0.01 0 0.07 80 1000 0 12.6 5 0.61 0.27 0.07 0 0.01 0.04 80 0 0 12.6 0 0.66 0 0 0 0 0.34 80 0 600 0 0 0.95 0 0 0.01 0.01 0.03 80 0 600 0 5 0.77 0 0 0.15 0.03 0.05 80 0 600 12.6 5 0.14 0 0 0.01 0.01 0.84 80 1000 600 12.6 5 0.29 0.34 0.32 0 0.01 0.04 70 1000 600 12.6 5 0.35 0.30 0.32 0 0.01 0.02 50 1000 600 12.6 5 0.58 0.20 0.09 0 0.01 0.12 30 1000 600 12.6 5 0.73 0.11 0.05 0 0 0.11 70 1000 0 0 0 0.76 0.21 0 0 0 0.03 50 1000 0 0 0 0.80 0.17 0 0 0 0.03 30 1000 0 0 0 0.87 0.10 0 0 0 0.03 2NO2+ HSO3-+ H2O f SO4 2-+ 3H++ 2NO 2 -(1) 2NO2+ SO32-+ H2O f SO4 2-+ 2H++ 2NO 2 -(2) 2NO2+ O2+ 3HSO3-+ H2O f 3SO4
2-+ 5H++ 2NO 2
-(3) 2NO2+ O2+ 3SO32-+ H2O f 3SO4
2-+ 2H++ 2NO 2 -(4) NO + NO2+ H2O a 2NO2 -+ 2H+ (5) 2NO2+ H2O a NO2 -+NO3-+ 2H+ (6)
still in powder form. The deliquescence RH of Ca(NO3)2‚4H2O
has been reported to be 50.8% at 25°C;19 its deliquescence
RH at 60 °C would be higher according to the prediction equation (9.72) given in ref 18 because its heat of solution is negative.20
This study has shown that the presence of NOX/O2in the gas
phase can enhance the reaction of Ca(OH)2 with SO2. This
finding is different from that reported by Chu and Rochelle,4
Nelli and Rochelle, 5 and Ishizuka et al.6 This discrepancy
probably is due to the differences in the relative humidity and the reaction temperature used. Chu and Rochelle carried out the reaction experiments at 54% RH (66°C) and 19% RH (92 °C), and Nelli and Rochelle at 60% RH (70°C). Their relative humidity at each temperature may be lower than the deliques-cence relative humidity of Ca(NO2)2 or Ca(NO3)2 at that
Figure 2. SEM micrographs of Ca(OH)2reacted at 60°C, 80% RH, and different gas compositions for 1 h: (a) 1000 ppm SO2; (b) 12.6% CO2; (c) 12.6% CO2and 1000 ppm SO2; (d) 12.6% CO2, 600 ppm NOX, and 1000 ppm SO2; (e) 5% O2, 600 ppm NOX, and 1000 ppm SO2; (f) 12.6% CO2, 600 ppm NOX,
5% O2, and 1000 ppm SO2.
temperature; thus the enhancement effect due to the presence of NOX/O2was absent.
During the reaction of Ca(OH)2, most of the sulfite, sulfate,
and carbonate ions in the water layer on the Ca(OH)2surface
precipitate, once in contact with calcium ions, to form a product layer of calcium salts, due to the relative insolubility of these salts. The buildup of the product layer impedes the reaction between hydrogen ions and hydroxide ions or Ca(OH)2and thus
slows the overall reaction rate. Meanwhile, the water layer is gradually acidified. When the product layer becomes impervious, the reaction ceases, leaving part of Ca(OH)2unreacted.3,15This
is the reason for the incomplete conversion of Ca(OH)2 at
reaction time as long as 1 h.
As pointed out by Ho et al.8and Liu and Shih,9when CO 2is
present with SO2, CaCO3 crystals form in the early stage of
reaction and react again to form sulfite in the latter stage, leaving less carbonate at higher RHs. The mole fractions of CaCO3
measured for the reacted samples in this study are in agreement with the previous results.
The additional SO2capture when CO2 was added together
with NOX or NOX/O2 may be due to NOX enhancing the
formation of CaCO3 and the re-reaction of CaCO3 to form
CaSO3and CaSO4causing the impervious product layer to form
at higher extents of reaction.
Although calcium nitrite and nitrate played an important role in collecting water when samples reacted with gas mixtures containing NOXand O2, only small mole fractions of calcium
nitrite and nitrate were measured for the reacted samples, except for the case when Ca(OH)2reacted with the NOX/O2mixture.
This outcome is thought to be due to their high solubilities. Thus, as the water layer was acidified by the absorption of SO2
or CO2in the latter stage of reaction, the high concentrations
of nitrite and nitrate ions would cause them to form HNO2and
HNO3or to react in the reverse direction of reactions 5 and 6,
resulting in low concentrations of these ions. Furthermore, the reacted samples were vacuum-dried before being subjected to analysis. During vacuum-drying of a wet reacted sample, the nitrite and nitrate ions might transform to NO, NO2, HNO2,
and HNO3, which subsequently evaporate. The above processes,
therefore, would result in small amounts of calcium nitrite and nitrate contained in a dried sample which had reacted with SO2
or CO2with the presence of NOXand O2.
Conclusion
The presence of CO2with SO2 in the gas phase enhanced
the sulfation of Ca(OH)2 only when NOX was also present.
When either NOX(mainly NO) or O2 was present with SO2,
the enhancement effect was slight, but became great when both NOXand O2were present, and was even greater when CO2was
also present. The great enhancement effect exerted by the presence of NOX/O2resulted from the rise in the NO2
concen-tration, which enhanced the oxidation of HSO3-and SO32-to
SO42-in the water layer adsorbed on Ca(OH)2surface and the
formation of deliquescent salts of calcium nitrite and nitrate. The enhancement effect due to the presence of NOX/O2 was
more pronounced when the relative humidity was above that at which the salts deliquesced. The presence of H2O, CO2, NOX,
and O2in the flue gas is beneficial to the SO2capture in the
low-temperature dry and semidry FGD processes. The presence of NOX/O2also enhanced CO2removal by Ca(OH)2when SO2
was absent. Acknowledgment
This research was supported by the National Science Council of the Republic of China (Taiwan).
Notation
RH ) relative humidity
XC ) molar fraction of CaCO3 and/or trace amount of inert
substance
XHL) molar fraction of Ca(OH)2
XN1) molar fraction of Ca(NO2)2‚H2O
XN2) molar fraction of Ca(NO3)2‚4H2O
XS1) molar fraction of CaSO3‚0.5H2O
XS2) molar fraction of CaSO4‚0.5H2O
Literature Cited
(1) Slack, A. V.; Hollinden, G. A. Sulfur Dioxide RemoVal from Waste
Gases; Noyes Data Corp.: Park Ridge, NJ, 1975.
(2) Ho, C. S.; Shih, S. M. Factor Influencing the Reaction of Ca(OH)2 with SO2. J. Chin. Inst. Chem. Eng. 1993, 24, 187-195.
(3) Ho, C. S.; Shih, S. M.; Liu, C. F.; Chu, H. M.; Lee, C. D. Kinetics of the Sulfation of Ca(OH)2at Low Temperatures. Ind. Eng. Chem. Res.
2002, 41 (14), 3357-3364.
(4) Chu, P.; Rochelle, G. T. Removal of SO2and NOXfrom Stack Gas by Reaction with Calcium Hydroxide Solids. JAPCA 1989, 39 (2), 175-179.
(5) Nelli, C. H.; Rochelle, G. T. Simultaneous Sulfur Dioxide and Nitrogen Dioxide Removal by Calcium Hydroxide and Calcium Silicate Solids. J. Air Waste Manage. Assoc. 1998, 48, 819-828.
(6) Ishizuka, T.; Kabashima, H.; Hajime, Y.; Tsutomu, T.; Tanabe, K.; Hattori, H. Initial Step of Flue Gas DesulfurizationsAn IR Study of the Reaction of SO2with NOXon CaO. EnViron. Sci. Technol. 2000, 34, 2799-2803.
(7) Ho, C. S.; Shih, S. M. Effect of O2on the Reaction of Ca(OH)2 with SO2. J. Chin. Inst. Chem. Eng. 1992, 24, 405-411.
(8) Ho, C. S.; Shih, S. M.; Lee, C. D. Influence of CO2and O2on the Reaction of Ca(OH)2 under Spraying-Drying Flue Gas Desulfurization Conditions. Ind. Eng. Chem. Res. 1996, 35 (11), 3915-3919.
(9) Liu, C. F.; Shih, S. M. Study on the Absorption of CO2from Flue Gas by Hydrated Lime. Proceedings Symposium on Transport Phenomena
and Applications, Taipei, Taiwan, 2000; pp 627-630.
(10) Klingspor, J.; Stromberg, A.; Karlsson, H. T.; Bjerle, I. Similarities between Lime and Limestone in Wet-dry Scrubbing. Chem. Eng. Process.
1984, 18, 239-247.
(11) Irabien, A.; Cortabitarte, F.; Viguri, J.; Ortiz, M. I. Kinetic model for Desulfurization at Low-Temperature Using Calcium Hydroxide. Chem.
Eng. Sci. 1990, 45, 3427-3433.
(12) Moyeda, D. K.; Newton, G. H.; La Fond, J. F.; Payne, R.; Kramlich, J. C. EPA/600/2.88/0.47, Order PB88-2459615; U.S. Government Printing Office: Washington, DC, 1988.
(13) Seeker, W. R.; Chen, S. L.; Kramlich, J. C.; Greene, S. B.; Overmoe, B. J. Proceedings of the Joint Symposium on Dry SO2and Simultaneous
SO2/NOXControl Technology, Raleigh, NC, 1986.
(14) Liu, C. F. Study on Iron Blast Furnace Slag/Ca(OH)2Sorbents for SO2Removal from the Flue Gas. Ph.D. Thesis, National Taiwan University, Taipei, Taiwan, 2004.
(15) Shih, S. M.; Ho, C. S.; Song, Y. S.; Lin, J. P. Kinetics of the Reaction of Ca(OH)2with CO2at Low Temperature. Ind. Eng. Chem. Res.
1999, 38, 1316-1322.
(16) Lide, D. R. CRC Handbook of Chemistry and Physics, 81st ed.; CRC Press: Boca Raton, FL, 2000.
(17) Littlejohn, D.; Wang, Y.; Chang, S. G. Oxidation of Aqueous Sulfite Ion by Nitrogen Dioxide. EnViron. Sci. Technol. 1993, 27, 2162-2167.
(18) Seinfeld, J. H.; Pandis, S. N. Atmospheric Chemistry and Physics; Wiley: New York, 1998.
(19) Young, J. F. Humidity Control in the Laboratory Using Salts Solution. J. Appl. Chem. 1967, 17, 241.
(20) Perry, R. H.; Chilton, C. H. Chemical Engineers’ Handbook, 5th ed.; McGraw-Hill: New York, 1973.
ReceiVed for reView May 1, 2006 ReVised manuscript receiVed August 22, 2006 Accepted October 3, 2006 IE0605534