Genetic characteristics of human enterovirus 71 isolates from the patients with encephalitis
Tsai-Hsiu Lin1, Jeng-Dau Tsai2, ¶, Yu-Ching Lan3, Hong-Chang Shih1,4, Ching-Tien Peng1, Mu-Chin Shih1,** and Cheng-Wen Lin 1,5*
1Department of Laboratory Medicine, China Medical University Hospital; 2Department of Pediatrics,Chung Shan Medical University Hospital ,
3Department of Health Risk Management, China Medical University, Taiwan 4Department of Veterinary Medicine, National Chung Hsing University,
5 Department of Medical Laboratory Science and Biotechnology, China Medical
University, Taichung 404, Taiwan
*Corresponding author: Cheng-Wen Lin, PhD, Professor. Department of Medical Laboratory Science and Biotechnology, China Medical University, No. 91, Hsueh-Shih Road, Taichung 404, Taiwan
Fax: 886-4-22057414.
Email: cwlin@mail.cmu.edu.tw
**Additional corresponding author. ¶Co-first author.
Abstract
Introduction: Enterovirus 71 (EV71) was first identifed in 1969 in California and became a common cause of hand, foot, and mouth disease (HFMD) in the world. Some of the children subsquent severe neurologic complications but all seen in partial adult. (Epidemics of enterovirus 71 had caused encephalomyelitis since 1998 that caused large stress on parents and clinical physicans in Taiwan.Clinical presentations in EV 71 CNS infection were HFM dz/herpangina associated myoclonic jerk, ataxia and sympathetic overactivation.Neurological images were characterized of brain stem and cerebellum inflammation.).The central nerve system complications caused by EV71 were also manifestatedas brain stem dysfunction and autonomic dysregulation, such as acute flaccid paralysis, aseptic meningitis and rhombencephalitis; rhombencephalitis is one of the most common severe neurologic symptoms in children. The object of this study is trying to clarify the molecular and clinical epidemiology of enterovirus 71 in the middle of Taiwan, and find a disease marker to to predict the disease progression.
Method: Specimens of paients who were diagnosed of herpangina/HFM disease
with CNS involvement were collected .Specimens were collected in viral transport medium from sites of throat swabs, stool and CSF. Phylogenetic analysis of the VP4 and VP1 genes of EV71 strains investigated what kind of genogroups of the virus
circulating in Taiwan.
Result: In 27 cases of this study, the results of confirmation PCR were segregated into 5 group from A to E. The patients in specimen group A presented the EV71 PCR
positive both in throat tissue and stool; group B only presented PCR negative in CSF; group C presented PCR positive in all specimens, group D only present PCR positive in stool; and group E only presented PCR negative in blood specimen. The further investigate of the genetic relationship among 27 EV 71 isolates in this study by using the 5’-UTR and VP1 regions. Both neighbor-joining trees of 5’-UTR and VP1 regions shown these 27 isolates clustering with isolate SHZH03 in bootstrap number 99% (Fig 2, 3). There were five genogroups, group I to V were deduced in the phylogenetic tree with more than 60% bootstrap number. These 5 genogroup represent the 5 epidemic events among these patients.
Introduction
EV71 history in world and Taiwan
Enterovirus 71 (EV71) was first identifed in 1969 in California and became a common cause of hand- foot- mouth disease (HFMD)/herpangina in the world. Some of the children subsquent neurologic complications but all seen in partial adult (Hamaguchi et al., 2008b). General clinical symptoms of EV71 infections can be diverse, including HFMD, herpangina, pulmonary edema and central nervous system (CNS) complications. EV71 associated HFMD outbreaks with severe CNS complications cases have been increased during recent years (Huang et al., 1999a; Chan et al., 2003; Hamaguchi et al., 2008a). The clinical CNS complications caused by EV71 was also present to many signs, such as acute flaccid paralysis, aseptic meningitis and rhombencephalitis; rhombencephalitis is one of the most common severe neurologic symptoms in children(Huang et al., 1999b). In the most serious EV71-associated HFMD outbreak in Taiwan in 1998, 405 children had severe neurologic complications, pulmonary edema; 78 children died(Ho et al., 1999b).
【I suggest delet this section, because it means nothing.】CNS was indicated in five types of symptoms. Those patients with aseptic meningitis had headache
level of consciousness. The second type involved encephalitis with altered level of consciousness plus CSF pleocytosis. Cerebellitis was defined as the presence of
cerebellar ataxia and dis-coordination. Poliomyelitislike syndrome was defined as acute limb weakness and decreased reflex and muscle strength. Finally, patients with
encephalomyelitis had the present of both encephalitis and poliomyelitis-like
syndrome(Chang et al., 2007).
In 1998, the largest outbreak of EV71 infection reported in Taiwan had 34 fatalities (Ho et al., 1999a). Brainstem encephalitis is a major cause of deaths by developing cardiopulmonary (Wang et al., 2000). The previous study demonstrated that the EV71 isolated in Taiwan had both dermatotropic and neurotropic characteristics since 1998 (Liu et al., 2000). Because of the serious morbidity and mortality associated with EV71 infection, EV71 cases identification is an important consideration. Early detecting CNS and starting therapy may reduce the mortality. The previous study suggest that EV71 related encephalitis(EVE) severity varies by serotype, confirm the importance of CSF/brain tissue polymerase chain reaction, and demonstrate that serum IgM findings are of little value in diagnosing EVE. (Fowlkes
The Enterovirus genus is belong to family Picornaviridae and it consists of five species which isolated from humans: Human enterovirus A (HEV-A), HEV-B, HEV-C, HEV-D and poliovirus. EV71 is one of the HEV-A. The genome of enteroviruses is a single-stranded positive-sense RNA with approximately 7400 nucleotides. The viral genome contains a 5’- and 3’-untranslated regions (UTRs) which are essential for viral RNA replication. The genome structure translated into a polyprotein which including four capsid proteins, VP1 to VP4, and seven nonstructural proteins, 2A, 2B, 2C, 3A, 3B, 3C, and 3D (Palmenberg, 1990). Nucleotide sequencing and phylogenetic analysis have been used extensively as epidemiological and diagnostic tools for poliovirus, the prototype of the Enterovirus genus (Kew et al., 1995). Phylogenetic analysis of the VP4 and VP1 genes of present EV71 strains indicates that different genogroups of the virus have been circulating in
the Asia-Pacific region since 1997. The first of these outbreaks, described in Sarawak
(Malaysian Borneo) in 1997, was caused by genogroup B3. This outbreak was followed by large outbreaks in Taiwan in 1998, caused by genogroup C2, and in
Perth (Western Australia) in 1999, where viruses belonging to genogroups B3 and C2 cocirculated. Singapore, Taiwan, and Sarawak had HEV71 epidemics in 2000, caused predominantly by viruses belonging to genogroup B4; however, large numbers of fatalities were observed only in Taiwan. HEV71 was identified during an epidemic
of hand, foot and mouth disease in Korea; that epidemic was found to be due to viruses constituting a new genogroup, C3(Cardosa et al., 2003). In pervious phylogenetic study, the viruses have been grouped into different genetic clusters, based upon analysis of sequences at the 5′UTR and VP1 (Huttunen et al., 1996; Huang et al., 2008; Pulli et al., 1995; Poyry et al., 1996; Zell and Stelzner, 1997).
The objectives of this study is trying to understand the molecular and clinical epidemiology of enterovirus 71 in the middle of Taiwan and find a disease marker to to predict the disease progression.
Materials and methods
Specimens collection and processing
We collected 27 specimens from 2005 in China Medical University
Hospital .Specimens from throat swabs, stool were collected in viral transport medium. CSF specimen was directly collected in sterile tubes from inpatients or outpatients suspected of having enteroviral infection..
Extraction of EV71 RNA
EV71 was grown in Vero cells and the infected cells were scraped and pelleted by centrifugation when 75% cytopathic effect was seen. The viral RNA was extracted by QlAagen® Viral RNA mini kit (QIAGEN Inc,CA,USA). Purified viral RNA was resuspended in 60µl AVE buffer.The viral RNA was stored at -70 .℃
Reverse transcription and PCR
The primer sequences EV-1F (sense): 5’-GGCCCACTGGGCGCTAGCA-3’ (nt 34–53); EV-1R (antisense): 5’-TGTCCCAATGACATACTCT-3’ (nt 1337–1356)) amplified a 1323-bp cDNA which included the 5’ NCR and VP4 of the enterovirus genome. The primer sequences
EV71-1 (sense): 5’-TGGCAGATGTGATTGAGAGTT-3’; EV71-2 (antisense): 5’-TGAACAGCTCCACCTTTC-3’;EV71-3 (sense):
5’-AGTGATGAGAGTATGATTGA-3’; EV71-4 (antisense):
5’-TTATGTCTATGTCCCAGTT-3’amplified VP1 of the enterovirus genome. The cDNA was synthesized and amplified by Omniscript Reverse transcription
kit(QIAGEN Inc,CA,USA). Briefly, a 20-ml reaction mixture was which contained 1X buffer RT, 0.5 mM of each deoxynucleoside triphosphates, 20 pmol of EV1337R primer,10units of Rnase inhibitor,4units Omniscript reverse transcriptase, and 5 mg of purified RNA. After incubation at 37°C for 1hr. The PCR mixture contained 50 mM KCl, 10 mM Tris–HCl (pH 8.9), 3.6 mM MgCl2, 0.2 mM (each) deoxynucleoside triphosphates, 100 mg of bovine serum albumin per ml, 20 pmol of primers, and 1 U of Taq DNA polymerase was added after denaturing the cDNA at 94°C for 5 min. DNA amplification was performed with 40 cycles consisting of denaturation for 1 min at 94°C, primer annealing for 1 min at 56°C, extension for 2 min at 72°C, and
then72 for 10 min. The PCR products were analyzed by electrophoresis in 2% ℃ agarose gels.
Sequencing of PCR products
using four EV71 specific primers: EV-1F (sense), EV-1R (antisense), EV-2F (sense): 5’-GTCCCAATGACATACTCT-3’ (nt 583–602), and EV-2R (antisense):
5’-CTAGCTCAATAGACTCTTCGCA-3’ (nt 418–440),EV71-1(sense), EV71-2(antisense), EV71-3(sense), EV71-4(antisense) according to the
manufacture’s instructions (PE Applied Biosystems). After completion of the cycle- sequencing reactions, products were electrophoresed in an automated DNA sequencer (model 373A; DNA Sequencer, Applied Biosystems).Each PCR product was
sequenced in both directions to resolve possible ambiguous nucleotides.
Data analysis
We used ABI PRISM BigDye Terminator Cycle Sequencing Ready Reaction Kit, V3.1 (Applied Biosystem,CA,USA)for DNA sequence. Sequence data generated by the automated sequencer ABI 3730 XL DNA Analyzer(Applied Biosystem,CA,USA). The nucleotide sequence data were inspected and prepared with the Bioedit (Version 5.0)sequence analysis program. Multiplesequence alignments were performed with the ClustalW program.Phylogenetic analysis was performed using the neighbor-joining method with PHYLIP(version 3.6),and the reliability was evaluated by bootstrap analysis with 1,000 data sets. Cladograms were drawn with the TREEVIEW program.
Phylogenetic analyses of the 5′-UTR and VP4 regions
EV71 genome products were aligned sequences by using BioEdit program. Phylogenetic trees were constructed by the following steps in MEGA program, version 3. The Kimura two-parameter method was used to construct a distance matrix for neighbor-joining analysis. Maximum Composite Likelihood also used to confirm the topology of the phylogenetic trees. The statistical significance of phylogenies was estimated by bootstrap analysis with 1000 replicates.
Result and discussion
Clinical phase
Table 1
Specimen group PCR
positive site
A T(+),S(+),B(-),C(-)
B T(+),S(+),B(+),C(-)
C T(+),S(+),B(+),C(+)
D T(-),S(+),B(-),C(-)
E T(+),S(+),B(-),C(+)
T: Throat S: Stool B:Blood C:CSF
In 27 cases of this study, the specimens collection from throat tissue, stool, blood and cerebrospinal fluid (CSF) were collected. The result of confirmation PCR was segregated into 5 group from A to E which shown in Table 1. The patients in specimen group A presented the EV71 PCR positive both in throat tissue and stool; group B only presented PCR negative in CSF; group C presented PCR positive in all specimens, group D only present PCR positive in stool; and group E only presented PCR negative in blood specimen.
Phylogenetic analyses of the 5′-UTR and VP1 regions
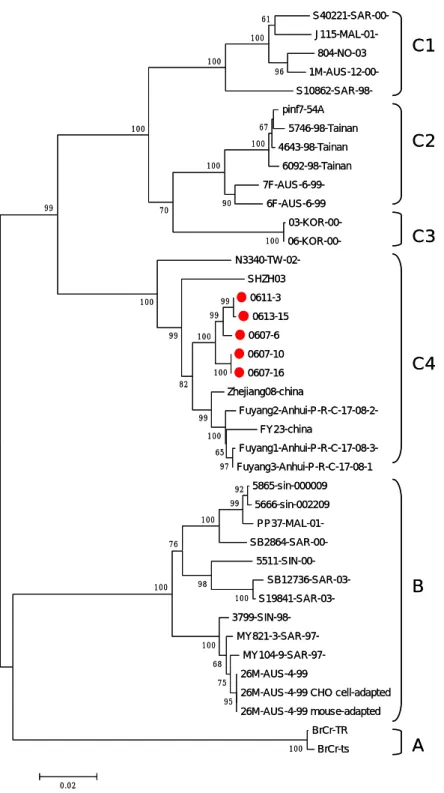
The EV71 viral subgenogroup identified by phylogenetic analysis for 40 EV71 strains worldwide was based on BrCr-ts strains sequence (gi60099451 nucleotide
position 34-1352). Bootstrap values(%) in 1,000 replicates were indicated at the branch nodes. This phylogenetic evidence proved the virus isolates in this study belong to subgenogroup C4 which became the predominant virus strain from 2004 (Lin et al., 2006) in Taiwan (Fig.1). The Taiwan C4 subgenogroup was clustered together with 100% bootstrap which different with the virus in China.
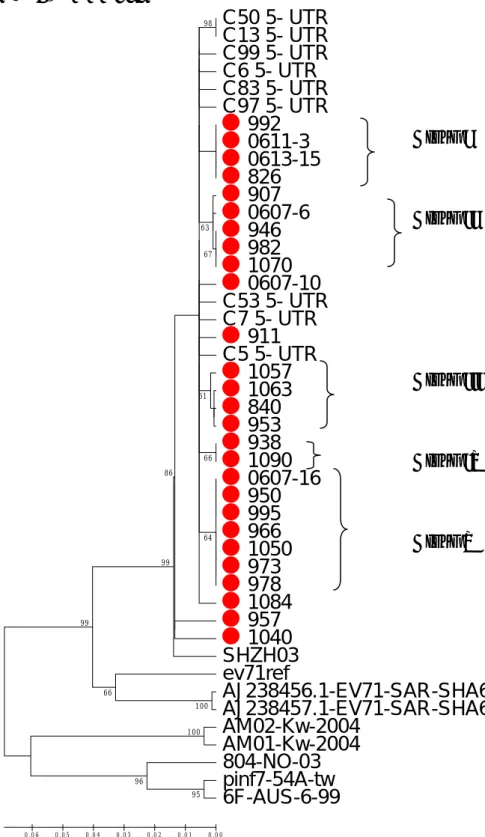
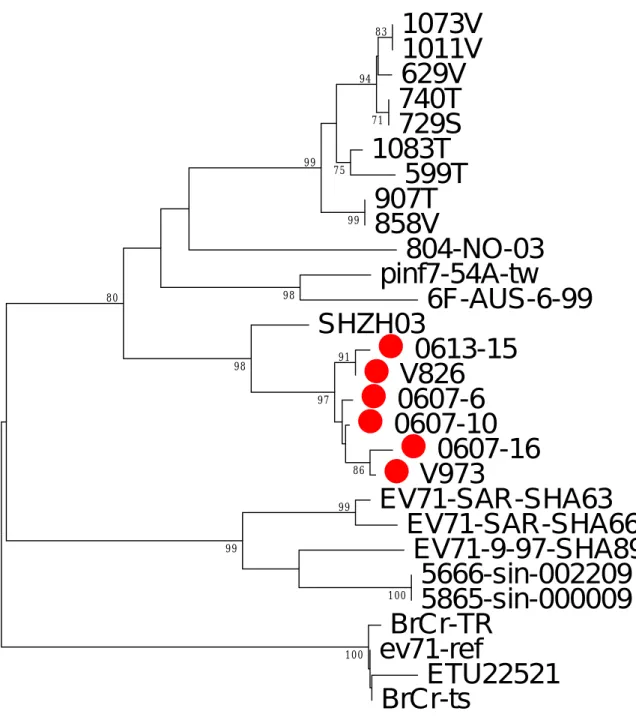
The further investigate of the genetic relationship among 27 EV 71 isolates in this study by using the 5’-UTR and VP1 regions. Both neighbor-joining trees of 5’-UTR and VP1 regions shown these 27 isolates clustering with isolate SHZH03 in bootstrap number 99% (Fig 2, 3). There were five genogroups, group I to V were deduced in the phylogenetic tree with more than 60% bootstrap number. In VP1 region, the isolates 0613-15 and 826 were clustered together with bootstrap number 91%. And, the isolates 0607-16 and V973 were clustered together in another genogroup with bootstrap number 87%. Furthermore, genogroup I to V were shown in phylogenetic tree of 5’-UTR region. The sequence variations among group were 0 in group I, IV and V. Group II and III were 0.002 and 0.004 respectively in VP1 region. These 5 genogroup represent the 5 epidemic events among these patients.
804-NO-03 pinf7-54A 5746-98-Tainan 4643-98-Tainan 6092-98-Tainan 6F-AUS-6-99 SHZH03 0611-3 0613-15 0607-6 0607-10 0607-16 Zhejiang08-china FY23-china Fuyang3-Anhui-P-R-C-17-08-1 5865-sin-000009 5666-sin-002209 26M-AUS-4-99
26M-AUS-4-99 CHO cell-adapted 26M-AUS-4-99 mouse-adapted BrCr-TR BrCr-ts 100 96 100 61 100 67 100 90 100 100 70 100 100 92 99 100 95 75 68 100 98 76 100 99 100 99 100 99 100 99 82 99 97 100 65 0.02 C1 C2 C3 C4 B A 804-NO-03 pinf7-54A 5746-98-Tainan 4643-98-Tainan 6092-98-Tainan 6F-AUS-6-99 SHZH03 0611-3 0613-15 0607-6 0607-10 0607-16 Zhejiang08-china FY23-china Fuyang3-Anhui-P-R-C-17-08-1 5865-sin-000009 5666-sin-002209 26M-AUS-4-99
26M-AUS-4-99 CHO cell-adapted 26M-AUS-4-99 mouse-adapted BrCr-TR BrCr-ts 100 96 100 61 100 67 100 90 100 100 70 100 100 92 99 100 95 75 68 100 98 76 100 99 100 99 100 99 100 99 82 99 97 100 65 0.02 C1 C2 C3 C4 B A
Fig 1 Phylogenetic analysis of 40 EV71 strains worldwide based on
BrCr-ts strains sequence (gi60099451 nucleotide position 34-1352). The
nucleotide sequence of the prototype BrCr strains were used as an
outgroup. Bootstrap values(%) in 1,000 replicates were indicated at the
branch nodes.
Fig 2 5’-UTR region
C50 5- UTR C13 5- UTR C99 5- UTR C6 5- UTR C83 5- UTR C97 5- UTR 992 0611-3 0613-15 826 907 0607-6 946 982 1070 0607-10 C53 5- UTR C7 5- UTR 911 C5 5- UTR 1057 1063 840 953 938 1090 0607-16 950 995 966 1050 973 978 1084 957 1040 SHZH03 ev71ref AJ238456.1-EV71-SAR-SHA63 AJ238457.1-EV71-SAR-SHA66 AM02-Kw-2004 AM01-Kw-2004 804-NO-03 pinf7-54A-tw 6F-AUS-6-99 100 95 96 100 66 99 98 99 67 66 63 61 86 64 0 .0 0 0 .0 1 0 .0 2 0 .0 3 0 .0 4 0 .0 5 0 . 0 6 Group I Group II Group III Group IV Group VFig 3 VP1 region
1073V
1011V
629V
740T
729S
1083T
599T
907T
858V
804-NO-03
pinf7-54A-tw
6F-AUS-6-99
SHZH03
0613-15
V826
0607-6
0607-10
0607-16
V973
EV71-SAR-SHA63
EV71-SAR-SHA66
EV71-9-97-SHA89
5666-sin-002209
5865-sin-000009
BrCr-TR
ev71-ref
ETU22521
BrCr-ts
100 100 99 99 98 86 91 97 98 80 99 99 75 83 71 94 0.02Phylogeny and clinical correlation
Correlations between the phylogenetic cluster (genetic group) of EV 71 virus isolates from patients and thestages of EV71 encephalomyelitis were shown in Table 1.
The clinical severity of the EV 71 neurological illness was classified according to the level of severity: group 1, complicated EV71 ilness with CNS involvement ; group 2, EV 71 encephalomyelitis with autonomic dysfunction; and group 3, EV71 EV 71 encephalomyelitis complicated with cardiopulmonary failure . Patients who were assigned to group 1 were defined as encephalomyelitis with evidence of cerebrospinal fluid (CSF) pleocytosis (>5×106 leukocytes per liter) withsubtle neurological signs,includede drowsiness,ataxia and myoclonic jerks. Patients who were assigned to group 2 defined as encephalomyelitis with autonomic dysfunction, included hypertension, cold sweating, hyperglycemia andfrequent myoclonic jerks. Patients who were assigned to group 3 had cardiopulmonary failure with hypotension, complicated pulmonary edema.These children all required the use of inotropic
agents,endotracheal intubation, and ventilator support.
EV71 disease progression was split to clinical disease grade from 1-4. Furthermore, we separated the patients’ specimens which EV71 viral PCR results positive site into 5 groups from A-E part (Table 2). Genetic group I and II got more
Group IV and V both had patient in grade 2-4. In PCR specimen positive site classification, Group I and II spread from A to C. Group III and IV had patients located in the A, C and E. Besides A, the patients in genetic group V were including B to E. But, the correlation between the genetic group and two kinds of disease progression markers was no statistical significant.
Furthermore, specimen positive site group A, B and C was belong to Grade 2 and 3. Specimen D with 2 patients both belong to grade 4. And, the relationship between disease progression and PCR specimen was confirmed with the statistically significant (Table 3). This data present EV71 positive specimen group might used as a marker to predict the disease progression grade.
Table2 The correlation of phylogenetic cluster and EV71 illness progression. I II III IV V P value Group 0.533 1 0 (0.0%) 0 (0.0%) 2 (%) 2 (28.6%) 3 (42.9%) 2 2 (50.0%) 3 (60.0%) 2 (%) 4 (57.1%) 2 (28.6%) 3 2 (50.0%) 2 (40.0%) 0 (%) 1 (14.3%) 2 (28.6%) PCR_specimen 0.899 A 1 (25.0%) 2 (40.0%) 2 (50.0%) 2 (28.6%) 0 (0.0%) B 1 (40.0%) 1 (20.0%) 0 (0.0%) 0 (0.0%) 2 (28.6%) C 2 (50.0%) 2 (40.0%) 1 (25.0%) 4 (57.1%) 3 (42.9%) D 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 1 (14.3%) E 0 (0.0%) 0 (0.0%) 1 (25.0%) 1 (14.3%) 1 (14.3%)
Group 1,2 Group3 P value
PCR_specimen 0.039
A、B、C 10 (100.0%) 1 (33.3%)
D、E 0 (0.0%) 2 (66.7%)
DISCUSSION
The results of EV71 viral subgenogroup was shown the majors strains in Taiwan was C4 which prevalent since 2004 (Lin et al., 2006) in Taiwan (Fig.1). The subgenogroup C4 of EV71 has been identified in Japan and emerged in the surrounding countries, such as China and Taiwan. The genotypic evidence in this study recognized the C4 the spreading and variation among this genotype during 3 years (Fig2). Five existed phylogenetic groups and other sporadic strains presented more than 5 strains circulating in Taiwan now.
In previous reports in clinical study, laboratory confirmation of enterovirus associated neurologic disease is typically perduced by PCR of CSF samples because of the better sensitivity and rapid turnaround time, compared with culture procedure[13, 21]. Several previous studies have demonstrated a rather low virus isolation in CNS cpecimens compared with that in other clinical samples, such as throat swab, and stool samples from EV71-associated cases with HFMD, encephalitis,
Reference List
Cardosa,M.J., Perera,D., Brown,B.A., Cheon,D., Chan,H.M., Chan,K.P., Cho,H., and McMinn,P. (2003). Molecular epidemiology of human enterovirus 71 strains and recent outbreaks in the Asia-Pacific region: comparative analysis of the VP1 and VP4 genes. Emerg. Infect. Dis. 9, 461-468.
Chan,K.P., Goh,K.T., Chong,C.Y., Teo,E.S., Lau,G., and Ling,A.E. (2003). Epidemic hand, foot and mouth disease caused by human enterovirus 71, Singapore. Emerg. Infect. Dis. 9, 78-85.
Chang,L.Y., Lee,C.Y., Kao,C.L., Fang,T.Y., Lu,C.Y., Lee,P.I., and Huang,L.M. (2007). Hand, foot and mouth disease complicated with central nervous system involvement in Taiwan in 1980-1981. J. Formos. Med. Assoc. 106, 173-176.
Fowlkes,A.L., Honarmand,S., Glaser,C., Yagi,S., Schnurr,D., Oberste,M.S., Anderson,L., Pallansch,M.A., and Khetsuriani,N. (2008). Enterovirus-Associated Encephalitis in the California Encephalitis Project, 1998-2005. The Journal of Infectious Diseases 198, 1685-1691.
Hamaguchi,T., Fujisawa,H., Sakai,K., Okino,S., Kurosaki,N., Nishimura,Y., Shimizu,H., and Yamada,M. (2008a). Acute encephalitis caused by intrafamilial transmission of enterovirus 71 in adult. Emerg. Infect. Dis. 14, 828-830.
Hamaguchi,T., Fujisawa,H., Sakai,K., Okino,S., Kurosaki,N., Nishimura,Y., Shimizu,H., and Yamada,M. (2008b). Acute encephalitis caused by intrafamilial transmission of enterovirus 71 in adult. Emerg. Infect. Dis. 14, 828-830.
Ho,M., Chen,E.R., Hsu,K.H., Twu,S.J., Chen,K.T., Tsai,S.F., Wang,J.R., and Shih,S.R. (1999a). An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N. Engl. J. Med. 341, 929-935.
Ho,M., Chen,E.R., Hsu,K.H., Twu,S.J., Chen,K.T., Tsai,S.F., Wang,J.R., and Shih,S.R. (1999b). An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N. Engl. J. Med. 341, 929-935.
Huang,C.C., Liu,C.C., Chang,Y.C., Chen,C.Y., Wang,S.T., and Yeh,T.F. (1999b). Neurologic complications in children with enterovirus 71 infection. N. Engl. J. Med.
341, 936-942.
Huang,C.C., Liu,C.C., Chang,Y.C., Chen,C.Y., Wang,S.T., and Yeh,T.F. (1999a). Neurologic complications in children with enterovirus 71 infection. N. Engl. J. Med.
341, 936-942.
Huang,S.C., Hsu,Y.W., Wang,H.C., Huang,S.W., Kiang,D., Tsai,H.P., Wang,S.M., Liu,C.C., Lin,K.H., Su,I.J., and Wang,J.R. (2008). Appearance of intratypic
recombination of enterovirus 71 in Taiwan from 2002 to 2005. Virus Research 131, 250-259.
Huttunen,P., Santti,J., Pulli,T., and Hyypia,T. (1996). The major echovirus group is genetically coherent and related to coxsackie B viruses. J Gen Virol 77, 715-725. Kew,O.M., Mulders,M.N., Lipskaya,G.Y., da Silva,E.E., and Patlansch,M.A. (1995). Molecular epidemiology of polioviruses. Seminars in Virology 6, 401-414.
Lin,K.H., Hwang,K.P., Ke,G.M., Wang,C.F., Ke,L.Y., Hsu,Y.T., Tung,Y.C., Chu,P.Y., Chen,B.H., Chen,H.L., Kao,C.L., Wang,J.R., Eng,H.L., Wang,S.Y., Hsu,L.C., and Chen,H.Y. (2006). Evolution of EV71 genogroup in Taiwan from 1998 to 2005: an emerging of subgenogroup C4 of EV71. J. Med. Virol. 78, 254-262.
Liu,C.C., Tseng,H.W., Wang,S.M., Wang,J.R., and Su,I.J. (2000). An outbreak of enterovirus 71 infection in Taiwan, 1998: epidemiologic and clinical manifestations. J. Clin. Virol. 17, 23-30.
Palmenberg,A.C. (1990). Proteolytic Processing of Picornaviral Polyprotein. Annual Review of Microbiology 44, 603-623.
Perez-Velez,C.M., Anderson,M.S., Robinson,C.C., McFarland,E.J., Nix,W.A., Pallansch,M.A., Oberste,M.S., and Glode,M.P. (2007). Outbreak of Neurologic
Enterovirus Type 71 Disease: A Diagnostic Challenge. Clinical Infectious Diseases 45, 950-957.
Poyry,T., Kinnunen,L., Hyypia,T., Brown,B., Horsnell,C., Hovi,T., and Stanway,G. (1996). Genetic and phylogenetic clustering of enteroviruses. J Gen Virol 77, 1699-1717.
Pulli,T., Koskimies,P., and HyypiΣ,T. (1995). Molecular Comparison of Coxsackie A Virus Serotypes. Virology 212, 30-38.
Su,I.J. (2000). An outbreak of enterovirus 71 infection in Taiwan, 1998. II. Laboratory diagnosis and genetic analysis. J. Clin. Virol. 17, 91-99.
Zell,R. and Stelzner,A. (1997). Application of genome sequence information to the classification of bovine enteroviruses: the importance of 5'- and 3'-nontranslated regions. Virus Research 51, 213-229.
• 1. Ho M, Chen ER, Hsu KH, Twu SJ, Chen KT, Tsai SF, et al. An epidemic of enterovirus 71 infection in Taiwan.N Engl J Med. 1999;341:929–35.
• 2. Huang CC, Liu CC, Chang YC, Chen CY, Wang ST, Yeh TF. Neurologic complications in children with enterovirus 71 infection. N Engl J Med. 1999;341:936–42.
• 3. Chan KP, Goh KT, Chong CY, Teo ES, Lau G, Ling AE. Epidemic hand, foot, and mouth disease caused by human enterovirus 71, Singapore. Emerg Infect Dis.
2003;9:78–85.
12. Yan J,Wang J, Liu C, Yang H, Su I. An outbreak of enterovirus 71 infection in Taiwan 1998: a comprehensive pathological, virological, and molecular study on a case of fulminant encephalitis. J Clin Virol 2000; 17:13–22.
Hamaguchi T, Fujisawa H, Sakai K, Okino S, Kurosaki N, Nishimura Y, et al. Acute encephalitis caused by intrafamilial transmission of enterovirus 71 in adult. Emerg Infect Dis [serial on the Internet] 2008 May [date cited]. Available from
http://www.cdc.gov/eid/content/14/5/828.htm
Clinical Infectious Diseases 2007; 45:950–7.
http://www.journals.uchicago.edu/doi/pdf/10.1086/521895(Perez-Velez
et al., 2007; Chang et al., 2007)
Journal of the Formosan Medical Association
http://ajws.elsevier.com/ajws3/switch.asp?journal_issn=0929-6646 Volume 106, Issue 2, February 2007, Pages 173 - 176