行政院國家科學委員會專題研究計畫 成果報告
脂蛋白(a)在動脈硬化危險因子,代謝症候群,次臨床疾病與
心血管疾病發生扮演的角色研究
研究成果報告(精簡版)
計 畫 類 別 : 個別型 計 畫 編 號 : NSC 95-2314-B-002-125- 執 行 期 間 : 95 年 08 月 01 日至 96 年 07 月 31 日 執 行 單 位 : 國立臺灣大學公共衛生學院預防醫學研究所 計 畫 主 持 人 : 簡國龍 共 同 主 持 人 : 許秀卿 計畫參與人員: 專科畢-專任助理:李惠真 處 理 方 式 : 本計畫可公開查詢中 華 民 國 96 年 10 月 26 日
行政院國家科學委員會補助專題研究計畫
■成 果 報 告
□期中進度報告
Role of Lipoprotein(a) in Atherosclerotic Risk Factors,
Metabolic Syndrome, Subclinical Diseases and Incidence of
Cardiovascular Disease
脂蛋白(a)在動脈硬化危險因子,代謝症候群,次臨床疾病與心
血管疾病發生扮演的角色研究
計畫類別:■ 個別型計畫
□ 整合型計畫
計畫編號:NSC
95
-
2314
-
B
-
002
-
125
-
執行期間:95 年 08 月 01 日至 96 年 07 月 31 日
計畫主持人:簡國龍
共同主持人:許秀卿
計畫參與人員:李惠真
成果報告類型(依經費核定清單規定繳交):■精簡報告 □完整報告
本成果報告包括以下應繳交之附件:
□赴國外出差或研習心得報告一份
□赴大陸地區出差或研習心得報告一份
□出席國際學術會議心得報告及發表之論文各一份
□國際合作研究計畫國外研究報告書一份
處理方式:除產學合作研究計畫、提升產業技術及人才培育研究計畫、
列管計畫及下列情形者外,得立即公開查詢
□涉及專利或其他智慧財產權,□一年□二年後可公開查詢
執行單位:台大公衛學院 預防醫學研究所
中
華
民
國
97 年
10 月
22 日
報告內容
Introduction
Lp(a) lipoprotein is a low-density lipoprotein particle in which apolipoprotein B-100 is linked by a single disulfide bridge to apolipoprotein(a) which is structurally similar to plasminogen(1, 2). The dual effects of atherogenicity and thrombogeneis from Lp(a) lipoprotein particles in basic research make it plausible to investigate the role of Lp(a) lipoprotein for atherosclerosis in population science. However, while many cross-sectional studies based on hospital-based population have consistently proven that Lp(a) lipoprotein was related to various vascular
diseases(3-6), many prospective cohort studies have provided disputable arguments. In addition, previous large-scale prospective cohorts were restricted in women(7) or in men(8) or in older population(9, 10). Moreover, most studies specified only one vascular outcome, either coronary heart disease or stroke, whereas some studies combined both outcomes into just one endpoint(9). Furthermore, the risk of Lp(a) on cardiovascular events varied according to different ethnicity(11). Scant prospective studies were conducted to investigate the role of Lp(a) lipoprotein among ethnic Chinese, who had distinct cardiovascular disease patterns from Caucasians and African Americans. Therefore, we investigated prospectively the association between plasma
concentrations of Lp(a) lipoprotein and cardiovascular disease and all-cause death among ethnic Chinese in Taiwan.
Materials and Methods
STUDY DESIGN AND STUDY PARTICIPANTS
The participants were enrolled in the Chin-Shan Community Cardiovascular Study (CCCC), a prospective, community-based study of risk factors and cardiovascular consequences in men and women 35 years of age or older, sponsored by the National Science Council, Taiwan. The study was started in 1990 with an initial cohort of 3602 participants, who were recruited on the basis of official registrations. The institutional review boards of the National Taiwan University
approved the study. These participants were non-institutionalized persons who gave oral informed consent to enter and to remain in the study. Participants were eligible for enrollment regardless of whether or not they had a history of cardiovascular disease. The full details of the recruitment process have been published elsewhere(12, 13). Briefly, information about detailed medical history, a physical examination, laboratory tests and assessment of health status that included any evidence of cardiovascular disease in 1990 and 1991 and the follow-up
periods(14-16). We also collected detailed information about lifestyle factors, including alcohol intake, smoking, and regular exercise as well as socioeconomic status, including marital status, educational level, , and family history of coronary heart disease. We defined the cardiovascular disease at baseline according to history of stroke and coronary heart disease events in the questionnaire. With regards to the schedule of follow-up, we collected the information about the cardiovascular events and death cases by monthly collection of official death certificate documents and by annual questionnaires and house-to-house visits.
ASCERTAINMENT OF EVENTS
The outcomes studies were stroke, coronary heart disease and all-cause death. Stroke was defined according to the following criteria: a sudden neurological deficit of vascular origin that
lasted longer than 24 hours, with supporting evidence from image study. Transient ischemic attacks were not included in this study. Incident coronary heart disease cases were defined as nonfatal myocardial infarction, fatal coronary heart disease and hospitalization due to
percutaneous coronary intervention and coronary bypass surgery. Fatal coronary heart disease was considered to have occurred if fatal myocardial infarction was confirmed by hospital records or if coronary heart disease was listed as the cause of death on the death certificate was the underlying and most plausible cause of death, or if evidence of previous coronary heart disease was available. Deaths from any cause were identified from official certificate documents and further verified by house-to-house visits.
MEASUREMENT OF BIOCHEMICAL VARIABLES
The procedures of blood sampling were reported elsewhere(15, 17). Briefly, all venous blood samples drawn after a 12-hour overnight fast were immediately refrigerated and transported within 6 hours to the National Taiwan University Hospital. Serum samples were then stored at -70°C before batch assay for levels of total cholesterol, triglyceride, and high density lipoprotein cholesterol (HDL-C). Standard enzymatic tests for serum cholesterol and triglyceride were used (Merck 14354 and 14366, Germany, respectively). HDL-C levels were measured in supernatants after the precipitation of specimens with magnesium chloride phosphotungstate reagents (Merck 14993). LDL-C concentrations were calculated as total cholesterol minus cholesterol in the supernatant by precipitation method (Merck 14992)(18). Apo A1 and Apo B concentrations were measured by turbidimetric immunoassay with commercial kits (Sigma). Non-HDL-C was calculated by subtracting HDL-C from total cholesterol. Lp(a) lipoprotein was determined by enzyme-linked immunosorbent assay (ELISA) (Organon) regardless of isoforms. The
coefficient of variation for measuring Lp(a) lipoprotein was 5%. A total of 3484 participants with complete Lp(a) lipoprotein and free from cardiovascular disease at baseline were included in the study.
STATISTICAL ANALYSIS
Participants were categorized on the basis of quartiles of Lp(a) lipoprotein levels, and continuous variables were presented by mean, standard deviation or median levels; categorical data were presented in contingency table. ANOVA and the chi-square test were used to test the difference across the quartiles. Relationships between baseline Lp(a) lipoprotein and other obesity and lipid markerswereexamined by ageand genderadjusted Spearman’spartialcorrelation coefficients.
Incidence rates of stroke, coronary heart disease and all-cause death were calculated by dividing cases by the person-years of follow-up for each quartiles of Lp(a) lipoprotein. Relative risk of event was calculated by dividing the incidence rate of each quartile by the rate in the first quartile. We used Cox proportional-hazards models to adjust for potential confounding
variables. We specified five models to estimate relative risk of events in the higher Lp(a) lipoprotein compared with the lowest quartile. First, we estimated the univariate relative risk of Lp(a) lipoprotein with the first quartile as reference in Model 1. Second, we adjusted for age groups (35-44, 45-54, 55-64, 65-74, >=75 years old) and gender variables in Model 2. In Model 3, we adjusted for additional body mass index (<18, 18 to 20.9, 21 to 22.9, 23 to 24.9, or >=25
kg/m2) and lifestyle factors, including alcohol intake (nondrinker/current), smoking, (yes/no) and exercise(yes/no), as well as socioeconomic status, including marital status(single, married, or divorced),educationallevel(<9 years,≥9 years),occupation (no work,manualwork,or
professional), and family history of coronary heart disease (yes/no). In addition to the variables in Model 3, we further adjusted for the presence or absence of baseline hypertension and diabetes in Model 4. Moreover, we included continuous variables of HDL cholesterol and LDL
cholesterol in Model 5. In all analyses, we modeled the Lp(a) lipoprotein as quartiles to avoid a linearity assumption and to reduce the effect of outliers. Furthermore, to test for linear trend across quartiles, we used the median Lp(a) lipoprotein levels for the categories. We categorized the data according to the 90th, 95thand 99thpercentile values and performed threshold analysis. We also tested the goodness of fit for the model by using the Hosmer and Lemeshow test(19), and the results of the model fitted the data at an acceptable level.
All statistical tests were two-tailed with a type I error of 0.05, and P values < 0.05 were considered statistically significant. Analyses were performed with SAS version 9.1 (SAS Institute, Cary, NC) and Stata version 9.1 (Stata Corporation, College Station, Texas).
Results
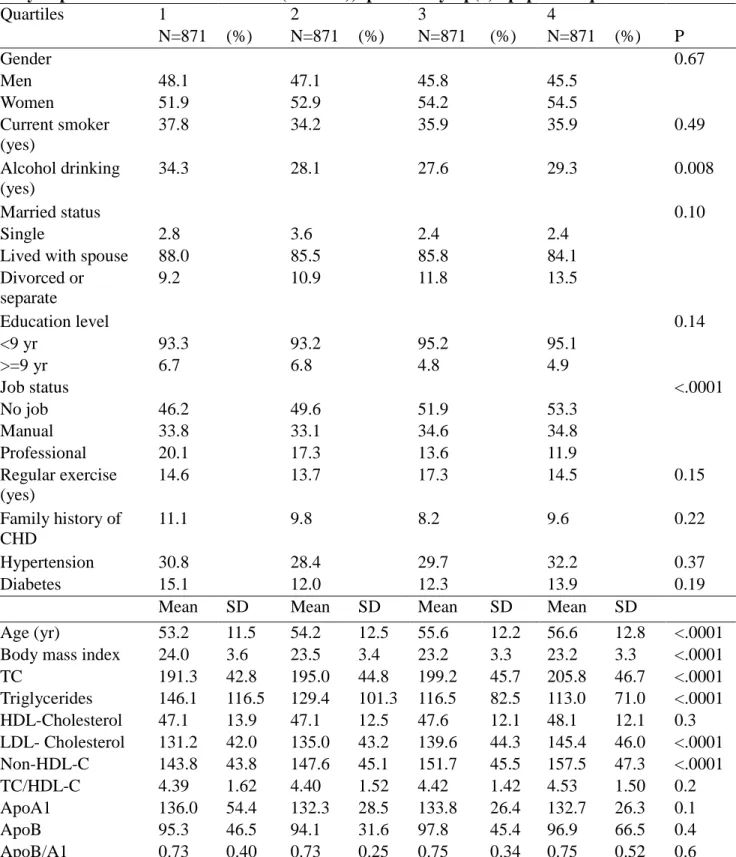
The participants in the highest Lp(a) lipoprotein quartile were less likely to be drinkers and in less professional jobs, compared with those in the lower quartiles (Table 1). Participants had similar distributions of female gender, the presence of smoking, regular exercise, marital status and education levels across quartiles. In addition, the participants had similar rates of the baseline hypertension, diabetes, and family history of coronary heart disease across different quartiles. A higher Lp(a) lipoprotein was associated with higher age, total cholesterol, LDL cholesterol, and non-HDL cholesterol, but had lower body-mass index and triglycerides, compared with lower Lp(a) lipoprotein levels. We did not find significantly different mean values of HDL-cholesterol, apolipoprotein A1 and apolipoprotein B across different quartiles of Lp(a) concentrations.
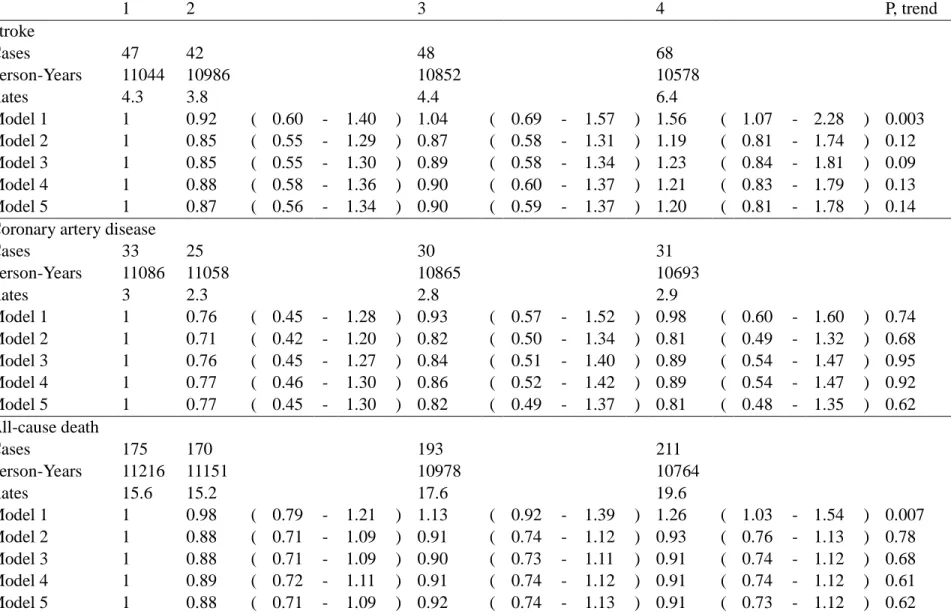
Over a median of 13.8-years’(inter-quartile range: 13.5-14.6 years) follow up among the 3,484 participants, we documented 210 cases of stroke (including 184 nonhemorrhagic stroke and 26 hemorrhagic stroke), 122 cases of coronary heart disease, and 781 cases of death (including 165 cardiovascular deaths). The incidence rates for each event increased appreciably for stroke and all-cause death as the Lp(a) lipoprotein quartiles increased, and the rates were approximately the same for coronary heart disease across quartiles (Table 2). The relative risks (RRs)
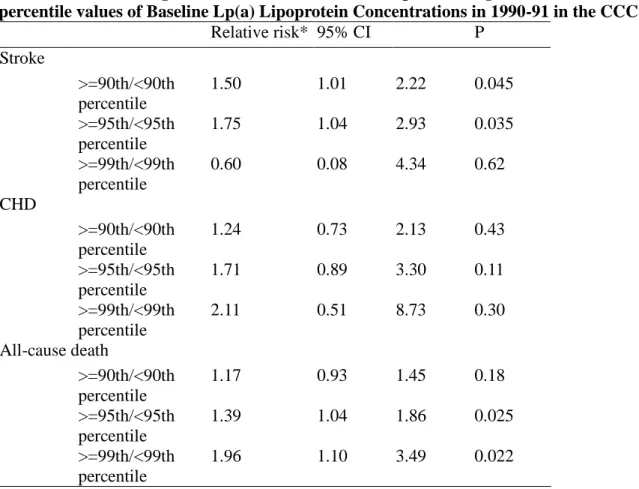
comparing individuals in the highest quartile with those in the lowest quartile of Lp(a) lipoprotein were 1.56 (95% confidence interval [CI], 1.07-2.28; P for trend=0.003) for stroke and 1.26 (95% CI, 1.03-1.54; P for trend=0.007) for all-cause death. In the multivariate analyses with adjusting for potential confounders, baseline Lp(a) lipoprotein by quartile values were not significantly associated with stroke, all-cause death, and coronary heart disease. However, we evaluated the risks of Lp(a) lipoprotein levels greater than or equal to the 90th, 95thand 99thpercentiles (the cutoff levels of the study participant were 34.43 mg/dL, 47.08 mg/dL and 69.30 mg/dL, respectively) and we found the multivariate RRs increased with the cutoff selected and was significant for stroke at the 90thand 95thpercentiles, and for total death at the 95thand 99th percentile (Table 3). However, the RRs for CHD showed a non-significantly increased trend,
with little power.
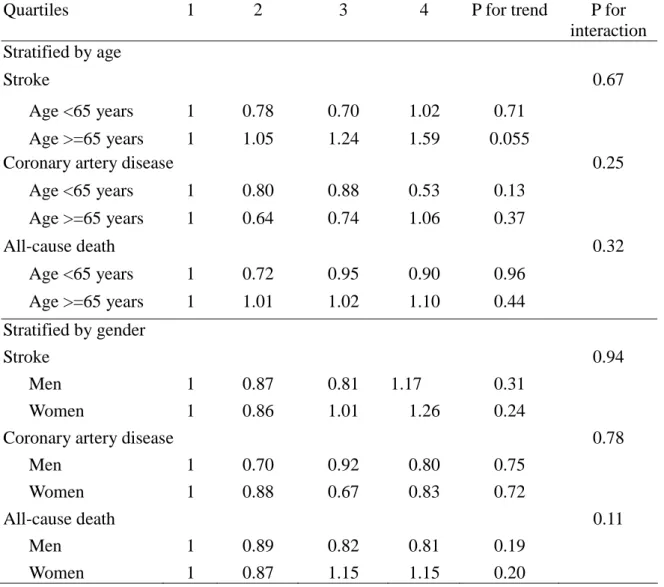
To address the possibility that risk of Lp(a) lipoprotein may have varied by different gender and age groups, we performed stratified analyses according to gender and age. The relationship between Lp(a) lipoprotein and stroke were not modified as a result of age and genders (all interaction P values >0.1)(Table 4). Among participants with age more than 65 years, the association between Lp(a) lipoprotein and stroke remained marginally significant after adjusting for multivariate risk factors, showing increasing risks of each quartile of Lp(a)
lipoprotein, from the second (RR, 1.05), third (RR, 1.24) and fourth quartile (RR, 1.59, P for trend=0.055). However, we cannot find these patterns among participants younger than 65 years old.
Discussion
We have previously reported on an association between cardiovascular risk factors and Lp(a) lipoprotein in the same participants cross-sectionally, and showed that Lp(a) lipoprotein was positively associated with low density lipoprotein and inversely associated with triglyceride and insulin resistance(13). In the present longitudinal study, Our data suggested that a threshold relationship with little gradient of risk across Lp(a) lipoprotein values for stroke and all-cause death among Chinese. We found marginally significant association between Lp(a) lipoprotein and stroke only in men of age 65 years and older. Moreover, the strength of association of Lp(a) and stroke was attributable to the ischemic subtype of stroke.
Different observational studies have provided the controversial evidence about the role of Lp(a) lipoprotein on stroke. Rigal and colleagues conducted a case-control study based on 100 cases of ischemic stroke and 100 matched controls on young adults (18-55 years old) and found that the relationship of Lp(a) lipoprotein to stroke remained significant only in men (odds ratio, 3.55, 95% CI, 1.33-9.48 for comparison of the highest and lowest tertile), but not in women (odds ratio, 0.42, 95% CI, 0.14-1.26)(20). In another case-control study based on Japanese, a high Lp(a) was related to ischemic stroke(21). However, the weakness of these studies due to small sample sizes and little confounding adjustments may invalidate the results. Nested case-control studies, measuring retrospectively stored Lp(a) lipoprotein concentrations which may be unstable, still did not prove significant association for further stroke(8, 22). Among the 198 incident strokeand 198 controlsfrom a7.5 years’follow-up cohort in the nearly 15000 healthy male physicians, Ridker and colleagues found no association between Lp(a) lipoprotein and incidence of stroke(8).
Results based on prospective cohort studies are still inconsistent about the risk of Lp(a) lipoprotein on cardiovascular events. Ariyo and colleagues traced the cardiovascular and death events of 3972 older adults, who were 65 years of age and older and Caucasian,for7.4 years’ follow up and found that the relationship of Lp(a) lipoprotein remained a significant predictor for stroke in men, but not for coronary heart disease(9). Also, they did not find significant
association between Lp(a) lipoprotein and cardiovascular outcomes among older women. In addition,using a3.2 years’follow-up cohort based on 5732 elder Caucasians who had received statin treatment, Gaw and colleagues did not find a statistical association between
elderly men and women(10). Short durations of follow up may contribute to the cause of insignificantresults. Among 27791 healthy women in theWomen’sHealth Study who were most Caucasian and were followed for 10 years, a clear threshold effect by Lp(a) lipoprotein was seen(7): the association of Lp(a) lipoprotein and total cardiovascular events remained significant only in the extremely high levels of Lp(a) lipoprotein (the relative risk, 1.66, 95% CI, 1.38-1.99, comparing the participants above the 90th percentile with those below the percentile). Nearly no risk gradient at the lower Lp(a) lipoprotein quintiles was seen among these women.
Inclusionsofboth coronary heartdiseaseand strokein theWomen’sHealth Study may limitthe biological plausibility for Lp(a) lipoprotein pathogenesis. Ohira and colleagues examined the association between Lp(a) lipoprotein and ischemic stroke among a biracial population of 14221 middle-age adults for 13.5-year follow-up period(11), and they found appreciably association only in African Americans and Caucasian women, but not in Caucasian men. Our data suggested a threshold effect for stroke: the risks above the 90thand 95thpercentiles of Lp(a) lipoprotein had significant risk on stroke, implying that atherosclerotic burdens increased appreciably among individuals with extreme high Lp(a) levels.
Our negative results on Lp(a) lipoprotein in relation to coronary heart disease are comparable with previous cohort studies among the Caucasian and African American population(9, 10) but are not consistent to the positive association reported in the nested case-control study(23). Rifai and colleagues conducted a nested case-control study and collected Lp(a) lipoprotein and apolipoprotein(a) information from 195 men who developed severe coronary atherosclerosis during a 5-years’follow up and 195 matched controlfrom the Physicians’HeartStudy(23). Although two metaanalyses indicated significant association of Lp(a) lipoprotein and coronary heart disease risk, the results may not be valid for different populations because of heterogeneity of different participant characteristics and study(24, 25).
The inconsistency of findings among different studies is troublesome and begs
explanation(26). One proposed explanation has been that Lp(a) lipoprotein has an interaction between other risk factors for further cardiovascular events. Different genders, young or older ages, and presence of high low density lipoprotein cholesterol and triglyceride levels were reported to modify the association of Lp(a) lipoprotein and cardiovascular events(3, 7, 9, 11, 20, 23). Our subgroup analysis did not provide significant interaction of Lp(a) lipoprotein and age as well as gender for cardiovascular risk. Our data suggested a potential relationship among older male Chinese, which was compatible with the findings from the Cardiovascular Health Study data(9). However, our subgroup results should be considered exploratory and require further investigation in larger studies. Specific high-risk population, including type 2 diabetes and hypercholesterolemia, may provide new evidence for the role of Lp(a) lipoprotein on cardiovascular disease(27).
Another possible explanation for the controversial results has been that Lp(a) lipoprotein may be only an indicator, not a hazard, for progressive atherosclerotic burden status.
Researchers have demonstrated the presence of Lp(a) lipoprotein within the early atherosclerotic plaques in aorta and cerebral arteries(28). Blood levels of Lp(a) lipoprotein also correlated with subclincial atherosclerosis(29, 30), and thus reflected early pathophysiologic process that related
to vascular events.
To our knowledge, this is the first extensive investigation of Lp(a) lipoprotein and the risks of stroke, coronary heart disease and all-cause death among ethnic Chinese. Because of the prospective cohort design, the baseline measurements of all cohort members were unlikely to be affected by storage and laboratory issues that might be raised in some nested case-control studies. The use of a homogeneous community-based population could reduce the possibility of selection bias. We also included important socioeconomic status and lifestyle factors in the models to control the potential confounding factors. Finally, because few participants (<1%) reported taking cholesterol-lowering medications, our results were minimally affected by statins and other cholesterol-lowering drugs.
Our study had several potential limitations. First, the incident cases of stroke and
coronary heart disease were relatively few, even with median 13.8-years’follow-up, which would reduce the power to detect the subtle effects between Lp(a) lipoprotein levels and make the relative risk estimation unstable. Second, because Lp(a) lipoprotein concentrations were measured only once, our results might be attenuated by intraindividual variations.
In conclusion, our data did not support the hypothesis that an increase in Lp(a) lipoprotein, over most of its range, was significantly associated with stroke, coronary heart disease and
all-cause death among ethnic Chinese adults. At the same time, a small proportion of people with extremely high Lp(a) lipoprotein were at excess risk. These findings limit the use of Lp(a) lipoprotein as a biomarker in comprehensive evaluation of risk of cardiovascular disease in Chinese. However, a single measure of Lp(a) lipoprotein to identify the small subgroup with extreme values may have some utility.
參考文獻
1. Loscalzo J. Lipoprotein(a). A unique risk factor for atherothrombotic disease. Arteriosclerosis 1990;10:672-9.
2. Gaubatz JW, Heideman C, Gotto AM, Jr., Morrisett JD, Dahlen GH. Human plasma lipoprotein [a]. Structural properties. J Biol Chem 1983;258:4582-9.
3. Frohlich J, Dobiasova M, Adler L, Francis M. Gender differences in plasma levels of lipoprotein (a) in patients with angiographically proven coronary artery disease. Physiol Res 2004;53:481-6.
4. Jovicic A, Ivanisevic V, Ivanovic I. Lipoprotein(a) in patients with carotid atherosclerosis and ischemic cerebrovascular disorders. Atherosclerosis 1993;98:59-65.
5. Pantoni L, Sarti C, Pracucci G, Di CA, Vanni P, Inzitari D. Lipoprotein(a) serum levels and vascular diseases in an older Caucasian population cohort. Italian Longitudinal Study on Aging (ILSA). JAmGeriatrSoc 2001;49:117-25.
6. Jones GT, van Rij AM, Cole J, Williams MJ, Bateman EH, Marcovina SM, et al. Plasma Lipoprotein(a) Indicates Risk for 4 Distinct Forms of Vascular Disease. Clin Chem 2007;53:679-85.
7. Suk Danik J, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), measured with an assay independent of apolipoprotein(a) isoform size, and risk of future cardiovascular events
among initially healthy women. JAMA 2006;296:1363-70.
8. Ridker PM, Stampfer MJ, Hennekens CH. Plasma concentration of lipoprotein(a) and the risk of future stroke. JAMA 1995;273:1269-73.
9. Ariyo AA, Thach C, Tracy R. Lp(a) lipoprotein, vascular disease, and mortality in the elderly. N Engl J Med 2003;349:2108-15.
10. Gaw A, Murray HM, Brown EA. Plasma lipoprotein(a) [Lp(a)] concentrations and
cardiovascular events in the elderly: evidence from the prospective study of pravastatin in the elderly at risk (PROSPER). Atherosclerosis 2005;180:381-8.
11. Ohira T, Schreiner PJ, Morrisett JD, Chambless LE, Rosamond WD, Folsom AR. Lipoprotein(a) and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke 2006;37:1407-12.
12. Lee YT, Lin RS, Sung FC, Yang CY, Chien KL, Chen WJ, et al. Chin-Shan Community Cardiovascular Cohort in Taiwan: baseline data and five-year follow-up morbidity and mortality. Journal of Clinical Epidemiology 2000;53:836-46.
13. Chien KL, Lee YT, Sung FC, Su TC, Hsu HC, Lin RS. Lipoprotein (a) level in the population in Taiwan: relationship to sociodemographic and atherosclerotic risk factors. Atherosclerosis 1999;143:267-73.
14. Chien KL, Hsu HC, Sung FC, Su TC, Chen MF, Lee YT. Metabolic syndrome as a risk factor for coronary heart disease and stroke: An 11-year prospective cohort in Taiwan community. Atherosclerosis 2006 Sep 13; [Epub ahead of print].
15. Chien KL, Sung FC, Hsu HC, Su TC, Chang WD, Lee YT. Relative importance of atherosclerotic risk factors for coronary heart disease in Taiwan. EurJ
CardiovascPrevRehabil 2005;12:95-101.
16. Chien KL, Sung FC, Hsu HC, Su TC, Lin RS, Lee YT. Apolipoprotein A1 & B, and stroke events in a community-based cohort in Taiwan: Report of Chin-Shan Community
Cardiovascular Study. Stroke 2002;33 39-44.
17. Chien KL, Lee YT, Sung FC, Hsu HC, Su TC, Lin RS. Hyperinsulinemia and related atherosclerotic risk factors in the population at cardiovascular risk: a community-based study. Clinical chemistry 1999;45:838-46.
18. Wieland H, Seidel D. A simple specific method for precipitation of low density lipoproteins. Journal of Lipid Research 1983;24:904-9.
19. Hosmer DW, Jr., Lemeshow S. The multiple logisitc regrssion model. Applied logistic regression, Vol. 1 ed. New York: John Wiley & Sons, 1989:25-37.
20. Rigal M, Ruidavets JB, Viguier A, Petit R, Perret B, Ferrieres J, Larrue V. Lipoprotein (a) and risk of ischemic stroke in young adults. J Neurol Sci 2007;252:39-44.
21. Nagayama M, Shinohara Y, Nagayama T. Lipoprotein(a) and ischemic cerebrovascular disease in young adults. Stroke 1994;25:74-8.
22. Glader CA, Stegmayr B, Boman J, Stenlund H, Weinehall L, Hallmans G, Dahlen GH. Chlamydia pneumoniae antibodies and high lipoprotein(a) levels do not predict ischemic cerebral infarctions. Results from a nested case-control study in Northern Sweden. Stroke 1999;30:2013-8.
23. Rifai N, Ma J, Sacks FM, Ridker PM, Hernandez WJ, Stampfer MJ, Marcovina SM. Apolipoprotein(a) size and lipoprotein(a) concentration and future risk of angina pectoris with evidence of severe coronary atherosclerosis in men: The Physicians' Health Study. Clin Chem 2004;50:1364-71.
24. Craig WY, Neveux LM, Palomaki GE, Cleveland MM, Haddow JE. Lipoprotein(a) as a risk factor for ischemic heart disease: metaanalysis of prospective studies. Clin Chem 1998;44:2301-6.
25. Danesh J, Collins R, Peto R. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation 2000;102:1082-5.
26. Berglund L, Ramakrishnan R. Lipoprotein(a): an elusive cardiovascular risk factor. ArteriosclerThrombVascBiol 2004;24:2219-26.
27. Shai I, Schulze MB, Manson JE, Stampfer MJ, Rifai N, Hu FB. A prospective study of lipoprotein(a) and risk of coronary heart disease among women with type 2 diabetes. Diabetologia 2005;48:1469-76.
28. Cambillau M, Simon A, Amar J, Giral P, Atger V, Segond P, et al. Serum Lp(a) as a discriminant marker of early atherosclerotic plaque at three extracoronary sites in hypercholesterolemic men. The PCVMETRA Group. Arterioscler Thromb
1992;12:1346-52.
29. Baldassarre D, Tremoli E, Franceschini G, Michelagnoli S, Sirtori CR. Plasma
lipoprotein(a) is an independent factor associated with carotid wall thickening in severely but not moderately hypercholesterolemic patients. Stroke 1996;27:1044-9.
30. Megnien JL, Sene V, Jeannin S, Hernigou A, Plainfosse MC, Merli I, et al. Coronary calcification and its relation to extracoronary atherosclerosis in asymptomatic
hypercholesterolemic men. The PCV METRA Group. Circulation 1992;85:1799-807.
計畫成果自評
In the present longitudinal study, Our data suggested that a threshold relationship with little gradient of risk across Lp(a) lipoprotein values for stroke and all-cause death among Chinese. We found marginally significant association between Lp(a) lipoprotein and stroke only in men of age 65 years and older. Moreover, the strength of association of Lp(a) and stroke was
10
可供推廣之研發成果資料表
Table 1. Distribution of Various Baseline Demographic, Lifestyle, and Socioeconomic Factors in the Study Population in the CCCC cohort (1990-91), specified by Lp(a) lipoprotein quartiles
Quartiles 1 2 3 4 N=871 (%) N=871 (%) N=871 (%) N=871 (%) P Gender 0.67 Men 48.1 47.1 45.8 45.5 Women 51.9 52.9 54.2 54.5 Current smoker (yes) 37.8 34.2 35.9 35.9 0.49 Alcohol drinking (yes) 34.3 28.1 27.6 29.3 0.008 Married status 0.10 Single 2.8 3.6 2.4 2.4
Lived with spouse 88.0 85.5 85.8 84.1 Divorced or separate 9.2 10.9 11.8 13.5 Education level 0.14 <9 yr 93.3 93.2 95.2 95.1 >=9 yr 6.7 6.8 4.8 4.9 Job status <.0001 No job 46.2 49.6 51.9 53.3 Manual 33.8 33.1 34.6 34.8 Professional 20.1 17.3 13.6 11.9 Regular exercise (yes) 14.6 13.7 17.3 14.5 0.15 Family history of CHD 11.1 9.8 8.2 9.6 0.22 Hypertension 30.8 28.4 29.7 32.2 0.37 Diabetes 15.1 12.0 12.3 13.9 0.19
Mean SD Mean SD Mean SD Mean SD
Age (yr) 53.2 11.5 54.2 12.5 55.6 12.2 56.6 12.8 <.0001 Body mass index 24.0 3.6 23.5 3.4 23.2 3.3 23.2 3.3 <.0001 TC 191.3 42.8 195.0 44.8 199.2 45.7 205.8 46.7 <.0001 Triglycerides 146.1 116.5 129.4 101.3 116.5 82.5 113.0 71.0 <.0001 HDL-Cholesterol 47.1 13.9 47.1 12.5 47.6 12.1 48.1 12.1 0.3 LDL- Cholesterol 131.2 42.0 135.0 43.2 139.6 44.3 145.4 46.0 <.0001 Non-HDL-C 143.8 43.8 147.6 45.1 151.7 45.5 157.5 47.3 <.0001 TC/HDL-C 4.39 1.62 4.40 1.52 4.42 1.42 4.53 1.50 0.2 ApoA1 136.0 54.4 132.3 28.5 133.8 26.4 132.7 26.3 0.1 ApoB 95.3 46.5 94.1 31.6 97.8 45.4 96.9 66.5 0.4 ApoB/A1 0.73 0.40 0.73 0.25 0.75 0.34 0.75 0.52 0.6 Abbreviation: Apo: apolipoprotein; CHD: coronary heart disease; SD: standard deviation; TC: total cholesterol;
Table 2. Incidence Cases, Person-year, Incidence Rates and Relative Risks (and 95% Confidence Intervals) of Stroke, CHD, and All-cause Death outcomes during median 13.6 Years of Follow-Up According to Quartiles of Baseline Lp(a) Lipoprotein
Concentrations in 1990-91 in the CCCC Study
1 2 3 4 P, trend Stroke Cases 47 42 48 68 Person-Years 11044 10986 10852 10578 Rates 4.3 3.8 4.4 6.4 Model 1 1 0.92 ( 0.60 - 1.40 ) 1.04 ( 0.69 - 1.57 ) 1.56 ( 1.07 - 2.28 ) 0.003 Model 2 1 0.85 ( 0.55 - 1.29 ) 0.87 ( 0.58 - 1.31 ) 1.19 ( 0.81 - 1.74 ) 0.12 Model 3 1 0.85 ( 0.55 - 1.30 ) 0.89 ( 0.58 - 1.34 ) 1.23 ( 0.84 - 1.81 ) 0.09 Model 4 1 0.88 ( 0.58 - 1.36 ) 0.90 ( 0.60 - 1.37 ) 1.21 ( 0.83 - 1.79 ) 0.13 Model 5 1 0.87 ( 0.56 - 1.34 ) 0.90 ( 0.59 - 1.37 ) 1.20 ( 0.81 - 1.78 ) 0.14 Coronary artery disease
Cases 33 25 30 31 Person-Years 11086 11058 10865 10693 Rates 3 2.3 2.8 2.9 Model 1 1 0.76 ( 0.45 - 1.28 ) 0.93 ( 0.57 - 1.52 ) 0.98 ( 0.60 - 1.60 ) 0.74 Model 2 1 0.71 ( 0.42 - 1.20 ) 0.82 ( 0.50 - 1.34 ) 0.81 ( 0.49 - 1.32 ) 0.68 Model 3 1 0.76 ( 0.45 - 1.27 ) 0.84 ( 0.51 - 1.40 ) 0.89 ( 0.54 - 1.47 ) 0.95 Model 4 1 0.77 ( 0.46 - 1.30 ) 0.86 ( 0.52 - 1.42 ) 0.89 ( 0.54 - 1.47 ) 0.92 Model 5 1 0.77 ( 0.45 - 1.30 ) 0.82 ( 0.49 - 1.37 ) 0.81 ( 0.48 - 1.35 ) 0.62 All-cause death Cases 175 170 193 211 Person-Years 11216 11151 10978 10764 Rates 15.6 15.2 17.6 19.6 Model 1 1 0.98 ( 0.79 - 1.21 ) 1.13 ( 0.92 - 1.39 ) 1.26 ( 1.03 - 1.54 ) 0.007 Model 2 1 0.88 ( 0.71 - 1.09 ) 0.91 ( 0.74 - 1.12 ) 0.93 ( 0.76 - 1.13 ) 0.78 Model 3 1 0.88 ( 0.71 - 1.09 ) 0.90 ( 0.73 - 1.11 ) 0.91 ( 0.74 - 1.12 ) 0.68 Model 4 1 0.89 ( 0.72 - 1.11 ) 0.91 ( 0.74 - 1.12 ) 0.91 ( 0.74 - 1.12 ) 0.61 Model 5 1 0.88 ( 0.71 - 1.09 ) 0.92 ( 0.74 - 1.13 ) 0.91 ( 0.73 - 1.12 ) 0.62 Incidence rates presented as per 1000 person yrs. Model 1: univariate; Model 2: adjusted for age groups (35-44, 45-54, 55-64, 65-74, >=75 years old) and gender, Model 3: Model 2 plus body mass index (<18, 18 to 20.9, 21 to 22.9, 23 to 24.9, or >=25 kg/m2), smoking (yes/no or abstinence), current alcohol drinking
(regular/no), marital status(single, married and living with spouse, or divorced and separate), education level (less than 9 years, at least 9 years), occupation (no work, labor, official or business), regular exercise habit (yes/no), and family history of coronary heart disease (yes/no), Model 4: plus hypertension(yes/no) and diabetes
12
Table 3. Multivariate relative risks and 95% confidence intervals of stroke, CHD, and all-cause death outcomes during median 13.6 Years of Follow-Up According to the 90th, 95thand 99th percentile values of Baseline Lp(a) Lipoprotein Concentrations in 1990-91 in the CCCC Study
Relative risk* 95% CI P Stroke >=90th/<90th percentile 1.50 1.01 2.22 0.045 >=95th/<95th percentile 1.75 1.04 2.93 0.035 >=99th/<99th percentile 0.60 0.08 4.34 0.62 CHD >=90th/<90th percentile 1.24 0.73 2.13 0.43 >=95th/<95th percentile 1.71 0.89 3.30 0.11 >=99th/<99th percentile 2.11 0.51 8.73 0.30 All-cause death >=90th/<90th percentile 1.17 0.93 1.45 0.18 >=95th/<95th percentile 1.39 1.04 1.86 0.025 >=99th/<99th percentile 1.96 1.10 3.49 0.022
*Adjusted variables included age groups (35-44, 45-54, 55-64, 65-74, >=75 years old), gender, body mass index (<18, 18 to 20.9, 21 to 22.9, 23 to 24.9, or >=25 kg/m2), smoking (yes/no or abstinence), current alcohol drinking (regular/no), marital status(single, married and living with spouse, or divorced and separate), education level (less than 9 years, at least 9 years), occupation (no work, labor, official or business), regular exercise habit (yes/no), family history of coronary heart disease (yes/no), hypertension(yes/no), diabetes mellitus (yes/no), HDL-C and LDL-C levels.
Table 4. Stratified Analysis of Relative Risks for Stroke, CHD, and All-cause Death outcomes during median 13.6 Years of Follow-Up According to Quartiles of Baseline Lipoprotein(a) Concentrations, Stratified by Age groups (<65 years and >=65 years) and Genders
Quartiles 1 2 3 4 P for trend P for
interaction Stratified by age
Stroke 0.67
Age <65 years 1 0.78 0.70 1.02 0.71
Age >=65 years 1 1.05 1.24 1.59 0.055
Coronary artery disease 0.25
Age <65 years 1 0.80 0.88 0.53 0.13 Age >=65 years 1 0.64 0.74 1.06 0.37 All-cause death 0.32 Age <65 years 1 0.72 0.95 0.90 0.96 Age >=65 years 1 1.01 1.02 1.10 0.44 Stratified by gender Stroke 0.94 Men 1 0.87 0.81 1.17 0.31 Women 1 0.86 1.01 1.26 0.24
Coronary artery disease 0.78
Men 1 0.70 0.92 0.80 0.75
Women 1 0.88 0.67 0.83 0.72
All-cause death 0.11
Men 1 0.89 0.82 0.81 0.19
Women 1 0.87 1.15 1.15 0.20
Adjusted variables included age groups (35-44, 45-54, 55-64, 65-74, >=75 years old) [except stratified by age], gender[except stratified by gender], body mass index (<18, 18 to 20.9, 21 to 22.9, 23 to 24.9, or >=25 kg/m2), smoking (yes/no or abstinence), current alcohol drinking (regular/no), marital status(single, married and living with spouse, or divorced and separate), education level (less than 9 years, at least 9 years), occupation (no work, labor, official or business), regular exercise habit (yes/no), family history of coronary heart disease (yes/no), hypertension(yes/no), diabetes mellitus (yes/no), HDL-C and LDL-C levels.
附錄