Synthesis of the N(CH
2CH
2NdCH(o-C
6H
4)PPh
2)
3Ligand
and Its Complexation with Tungsten Carbonyls To Form
a 47-Membered Cryptand-Like Metallatricycle
Wen-Yann Yeh* and Shu-Ching Hsiao
Department of Chemistry, National Sun Yat-Sen University, Kaohsiung 804, Taiwan
Shie-Ming Peng and Gene-Hsiang Lee
Department of Chemistry, National Taiwan University, Taipei 106, Taiwan Received April 13, 2005
Summary: Schiff base condensation of N(CH2CH2NH2)3
and Ph2P(o-C6H4)C(dO)H produces the potentially
hep-tadentate ligand N(CH2CH2NdCH(o-C6H4)PPh2)3, which
reacts with tungsten carbonyls to form two tripodal complexes and a tricyclic cage complex.
Recent work on assembly of metal complexes (or ions) with suitable polydentate ligands allows the preparation of well-defined inorganic 2-D and 3-D supramolecular architectures in solution.1,2Several factors, such as the
design of ligands, the mode of coordination by the metal atom, the metal-to-ligand ratio, and the presence of guest molecules, are crucial to the structure and proper-ties of the final assemblies.3-10 Thus far, most of the
effort has been directed to the use of mononuclear metal ions (especially Pd2+and Pt2+)3,4or dinuclear ion centers
(Rh24+, Mo24+),6,11while the zero-valence organometallic
species, in particular the metal carbonyls, are seldom selected as the geometry-setting metal components.12-15
Herein we present the synthesis of a new tripodal ligand16 and its coordination to tungsten carbonyls,
leading to a cryptand-like cage complex. The results are summarized in Scheme 1.
Schiff base17condensation of N(CH
2CH2NH2)3and 3
equiv of Ph2P(o-C6H4)C(dO)H in refluxing benzene gave
the expected tripodal compound N(CH2CH2
NdCH(o-C6H4)PPh2)3 (1; 82%)18 as an air-stable, pale yellow
crystalline solid after crystallization from n-hexane. A few pellets of 3 Å molecular sieves must be added to remove the water generated from the condensation reaction; otherwise, low yields of 1 resulted. Compound 1 contains three iminophosphino groups connected to a nonrigid triethyleneamine spacer. The IR spectrum in KBr depicts the NdC stretching at 1641 cm-1. The1H
NMR spectrum shows a doublet signal at δ 8.80 for the imine protons with 4J
P-H ) 5 Hz, a multiplet in the (1) Lehn, J.-M. Supramolecular Chemistry: Concepts and
Perspec-tives; VCH: Weinheim, Germany, 1995.
(2) Steed, J. W.; Atwood, J. L. Supramolecular Chemistry; Wiley: New York, 2000.
(3) Leininger, S.; Olenyuk, B.; Stang, P. J. Chem. Rev. 2000, 100, 853.
(4) Swiegers, G. F.; Malefetse, T. J. Chem. Rev. 2000, 100, 3483. (5) Fujita, M.; Umemoto, K.; Yoshizawa, M.; Fujita, N.; Kusukawa, T.; Biradha, K. Chem. Commun. 2001, 509.
(6) Cotton, F. A.; Lin, C.; Murillo, C. A. Acc. Chem. Res. 2001, 34, 759.
(7) Holliday, B. J.; Mirkin, C. A. Angew. Chem., Int. Ed. 2001, 40, 2022.
(8) Hof, F.; Craig, S. L.; Nuckolls, C.; Rebek, J., Jr. Angew. Chem.,
Int. Ed. 2002, 41, 1488.
(9) Swiegers, G. F.; Malefetse, T. J. Coord. Chem. Rev. 2002, 225, 91.
(10) Brammer, L. Chem. Soc. Rev. 2004, 33, 476.
(11) Cotton, F. A.; Lin, C.; Murillo, C. A. Proc. Natl. Acad. Sci. U.S.A. 2002, 99, 4810.
(12) Haiduc, I.; Edelmann, F. T. Supramolecular Organometallic
Chemistry; Wiley-VCH: Weinheim, Germany, 1999.
(13) Contakes, S. M.; Rauchfuss, T. B. Angew. Chem., Int. Ed. 2000,
39, 1984.
(14) Tanase, T.; Goto, E.; Takenaka, H.; Horiuchi, T.; Yamamoto, Y.; Kuwabara, J.; Osakada, K. Organometallics 2005, 24, 234.
(15) Oh, M.; Carpenter, G. B.; Sweigart, D. A. Angew. Chem., Int.
Ed. 2002, 41, 3650.
(16) Hierso, J.-C.; Amardeil, R.; Bentabet, E.; Broussier, R.; Gauth-eron, B.; Meunier, P.; Kalck, P. Coord. Chem. Rev. 2003, 236, 143.
(17) Vigato, P. A.; Tamburini, S. Coord. Chem. Rev. 2004, 248, 1717.
(18) Anal. Calcd for C63H57N4P3: C, 78.57; H, 5.97; N, 5.82. Found: C, 79.20; H, 6.21; N, 5.85. MS (FAB): m/z 963 (M+). IR (KBr): 1641 (NdC) cm-1.1H NMR (CDCl3, 20 °C): δ 8.80 (d, 3H,4JP-H) 5 Hz, NdCH), 7.92 (m, 3H, Ph), 7.37-7.23 (m, 36 H, Ph), 6.85 (m, 3H, Ph), 3.39 (t, 6H,3JH-H) 7 Hz, CH2), 2.45 (t, 6H,3JH-H) 7 Hz, CH2).31 P-{1H}NMR (CDCl3, 20 °C): δ -13.02 (s).13C{1H}NMR (CDCl3, 20 °C): δ 160.3 (d,3J P-C) 21 Hz, NdCH), 139.5-127.5 (m, Ph), 59.4 (s, CH2), 54.7 (s, CH2). Scheme 1 3365 Organometallics 2005, 24, 3365-3367
10.1021/om050281p CCC: $30.25 © 2005 American Chemical Society Publication on Web 06/07/2005
Downloaded by NATIONAL TAIWAN UNIV on August 19, 2009
range δ 7.92-6.85 for the phenyl protons, and two triplet signals at δ 3.39 and 2.45, with3J
H-H) 7 Hz,
for the ethylene protons. The31P{1H}NMR spectrum
presents a sharp singlet at δ -13.02 for the phosphine groups.
Treatment of 1 with 3 equiv of W(CO)4(NCMe)2 in
CH2Cl2 at ambient temperature produced a deep red
solution, from which air-stable, dark red crystals of N[CH2CH2NdCH(o-C6H4)PPh2W(CO)4]3(2; 83%) were
obtained by adding n-hexane.19The1H NMR spectrum
shows that the imine N-CH resonance is shifted upfield to δ 8.08 upon coordination, and the 31P{1H} NMR
spectrum displays the phosphine resonance at δ 24.62 concomitant with183W satellites (J
W-P) 236 Hz). The
spectral data indicate equivalence of the three [CH2
-CH2NdCH(o-C6H4)PPh2W(CO)4] units in solution, likely
through C3 rotations. The molecular structure of 2,
depicted in Figure 1, shows that each iminophosphino group chelates a W(CO)4moiety in a cis fashion. The
central amine atom N4 is constrained 0.32 Å above the plane of three tungsten atoms, with nonbonding N4‚‚‚ W distances of 4.75-5.30 Å and W‚‚‚W′ distances of 7.94-8.70 Å. The P-W-N bite angles are acute, being 79.7° on average, and the mean CdN length is 1.29 Å. Compound 2 is thermally robust and shows little reactivity toward 1, even at elevated temperatures. Thus, W(CO)3(NCMe)3was prepared to react with 1 to
afford N[CH2CH2NdCH(o-C6H4)PPh2W(CO)3(NCMe)]3
(3) in 90% yield,20where each tungsten atom is linked
to a labile acetonitrile ligand. Compound 3 forms a slightly air-sensitive, dark purple crystalline solid. The averaged CO stretching frequency at 1857 cm-1for 3 is
red-shifted by 60 cm-1 in comparison with 2 (average 1917 cm-1), suggesting a stronger π-back-donation from the tungsten atom to the carbonyl ligands of 3. The1H
resonance for the imine protons (δ 7.82) and the 31P
resonance for the phosphine groups (δ 29.76 with JW-P
) 225 Hz) are comparable with those of 2.
The reaction of 3 and 1 in CH2Cl2 took place at
ambient temperature to produce air-stable, dark red crystals of [N(CH2CH2NdCH(o-C6H4)PPh2)3]2[W(CO)3]3
(4) in 82% yield.21In contrast, attempts to synthesize 4
by self-assembly of 1 and W(CO)3(NCMe)3in a 2:3 molar
ratio led to a complex mixture. The ESI mass spectrum of 4 shows isotopically resolved peaks of the molecular ions in the range m/z 2726-2737. The IR spectrum in the carbonyl region displays a pattern in agreement with a fac-W(CO)3LL′L′′configuration.22It appears that
compound 4 exhibits an idealized C3 symmetry in
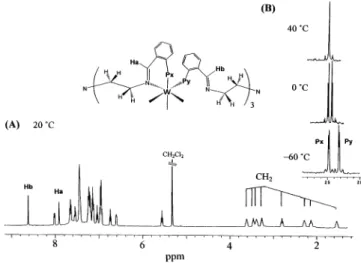
solution. Thus, the1H NMR spectrum (Figure 2A) shows
a singlet at δ 8.62 for the free N-CH protons, a singlet at δ 7.94 for the coordinated NdCH protons, several multiplets at δ 8.05-5.51 for the phenyl protons, and eight signals from δ 3.57 to 1.57 for the CH2 protons,
which are diastereotopic due to asymmetric coordination of the ligands. Noticeably, a phenyl resonance at δ 5.51 is markedly shielded relative to the others and might
(19) Anal. Calcd for C75H57O12N4P3W3: C, 48.67; H, 3.10; N, 3.03. Found: C, 48.66; H, 3.24; N, 3.09. MS (FAB): m/z 1850 (M+,184W). IR (CH2Cl2, νCO): 2000 (m), 1898 (s), 1852 (s) cm-1.1H NMR (CDCl3, 20 °C): δ 8.08 (s, 3H, NdCH), 7.58-7.34 (m, 39H, Ph), 6.83 (t, 3H, Ph), 3.75 (t, 6H,3JH-H) 7 Hz, CH2), 2.45 (t, 6H,3JH-H) 7 Hz, CH2).31 P-{1H}NMR (CDCl3, 20 °C): δ 24.62 (s; with183W satellites JW-P) 236 Hz).13C{1H}NMR (CDCl3, 20 °C): δ 210.8-202.3 (CO), 170.9 (Nd CH), 138.4-128.7 (Ph), 72.5 (CH2), 54.7 (CH2). Crystal data for 2: Mr ) 1850.71, monoclinic, space group P21/n, a ) 20.5438(4) Å, b ) 9.0415-(2) Å, c ) 38.2879(7) Å, β ) 101.1405(9)°, V ) 6977.89.0415-(2) Å3, Z ) 4, Fcalcd) 1.762 g cm-3, µ ) 5.067 mm-1, F(000) ) 3592, θ range 1.05-27.50°, 875 variables refined with 15 955 independent reflections to final R indices (I > 2σ(I)) of R1 ) 0.0528 and wR2 ) 0.1166, and GOF ) 1.064.
(20) Anal. Calcd for for C78H66N7O9P3W3: C, 49.59; H, 3.52; N, 5.19. Found: C, 50.01; H, 3.86; N, 5.43. MS (FAB): m/z 1766 (M+- 3NCMe, 184W). IR (CH3CN, νCO): 1910 (s), 1792 (s) cm-1.1H NMR (C6D6, 20 °C): δ 7.82 (s, 3H, NdCH), 7.41-6.71 (m, 42H, Ph), 3.56 (t, 6H,3J H-H ) 7 Hz, CH2), 2.45 (t, 6H,3J H-H) 7 Hz, CH2), 2.02 (s, 9H, NCMe). 31P{1H}NMR (CD3CN, 20 °C): δ 29.76 (s; with183W satellites JW-P) 225 Hz).13C{1H}NMR (C6D6, 20 °C): δ 220.3-215.4 (CO), 170.4 (Nd CH), 138.3-126.5 (Ph), 71.0 (CH2), 56.4 (CH2), 3.5 (CH3).
(21) Anal. Calcd for C135H114O9N8P6W3: C, 59.40; H, 4.21; N, 4.10. Found: C, 59.87; H, 4.27; N, 4.02. MS (ESI): m/z 2728 (M+,184W). IR (CH2Cl2, νCO): 1920 (s), 1808 (s) cm-1.1H NMR (CD2Cl2, 20 °C): δ 8.62 (s, 3H, NdCH), 7.94 (s, 3H, W-NdCH), 8.05-6.63 (m, 81H, Ph), 5.51 (t, 3H, Ph), 3.57 (m, 3H, CH2), 3.43 (m, 3H, CH2), 3.30 (m, 3H, CH2), 3.22 (m, 3H, CH2), 2.76 (m, 3H, CH2), 2.12 (m, 3H, CH2), 2.02 (m, 3H, CH2), 1.57 (m, 3H, CH2).31P{1H}NMR (CD2Cl2, -60 °C): δ 24.8 (d,2J P-P) 24 Hz; with183W satellites JW-P) 182 Hz), 23.3 (d, 2JP-P) 24 Hz; with183W satellites JW-P) 234 Hz).31P{1H}NMR (CD2-Cl2, 40 °C): δ 24.9 (s; with183W satellites JW-P) 220 Hz). Crystal data for 4: Mr) 3260.38, monoclinic, space group C2/c, a ) 33.3154(4) Å, b ) 36.4677(4) Å, c ) 28.8983(3) Å, β ) 124.3432(5)°, V ) 28989.0-(6) Å3, Z ) 8, Fcalcd) 1.494 g cm-3, µ ) 2.632 mm-1, F(000) ) 13 168,
θ range 1.12-25.00°, 1578 variables refined with 25 474 independent
reflections to final R indices (I > 2σ(I)) of R1 ) 0.0731 and wR2 ) 0.1898, and GOF ) 1.015.
(22) Hsu, S. C. N.; Yeh, W.-Y. J. Chem. Soc., Dalton Trans. 1998, 125.
Figure 1. Molecular structure of 2. The C6H5groups have
been artificially omitted, except for the ipso carbon atoms, for clarity.
Figure 2. 1H (A) and variable-temperature31P{1H} (B)
NMR spectra of 4 in CD2Cl2.
3366 Organometallics, Vol. 24, No. 14, 2005 Communications
Downloaded by NATIONAL TAIWAN UNIV on August 19, 2009
be attributed to the intramolecular aromatic contacts.2
The variable-temperature 31P{1H} NMR spectra are
shown in Figure 2B. At -60 °C, the two doublets at δ 24.8 and 23.3 (2J
P-P ) 24 Hz), with both signals
accompanied by 183W satellites, are assigned to the
phosphorus resonance for the η2- and η1-iminophosphino
groups, respectively. The latter signal shows a downfield shift upon warming and becomes superimposed with the former at 40 °C, and the resulting singlet signal remains unchanged to 80 °C (taken in C6D6). Since the two Nd
CH proton resonances are retained in this temperature range, coincidence of the two phosphorus resonances is likely a result of accidental overlap but is not a dynamic equilibrium involving intramolecular η1/η2site exchange
of the iminophosphino groups.23
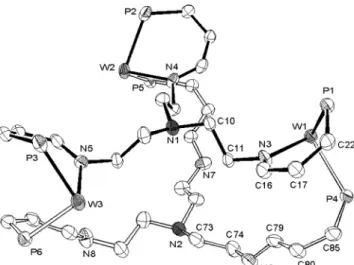
Recrystallization of 4 from CH2Cl2by slow diffusion
into diethyl ether afforded deep red crystals suitable for an X-ray diffraction study. The crystals of 4 contain a large number of solvent molecules, owing to the large cavities in the tricyclic, though rigid, molecules. The
ORTEP diagram, showing only the ring atoms, is illustrated in Figure 3. The molecule consists of two N(CH2CH2NdCH(o-C6H4)PPh2)3ligands bridged by three
W(CO)3 groups to form a metallatricyclic cage. The
coordination about each tungsten atom is a distorted octahedron with three terminal carbonyls capping a triangular face. The iminophosphino linkages connected to the N1 atom are each chelating a tungsten atom, while those connected to the N2 atom are each bonded to a tungsten atom through the phosphine group. The arrangement of N1, N2, W1, W2, and W3 atoms can be viewed as a distorted trigonal biyramid, with the apical N1 and N2 atoms being 0.34 and 4.50 Å away from the W3 plane, respectively. The nonbonding N1‚‚‚W
dis-tances are in the range 4.97-5.24 Å (average 5.11 Å), while the N4‚‚‚W distances are longer, being 6.76-6.92 Å with a mean value of 6.86 Å. The W‚‚‚W′distances are 8.82-9.14 Å (average 8.96 Å).
In summary, we have prepared the potentially hep-tadentate ligand 1, which reacts with tungsten carbo-nyls to form the tripodal complexes 2 and 3, and the tricyclic cage complex 4. Although M3L2-type cages are
relatively simple 3-D constructs, they remain uncom-mon.24-26We are currently investigating if metal cations
can assemble a chain (or a circle) of 4 through interac-tions with the free amine atoms.
Acknowledgment. We are grateful for support of this work by the National Science Council of Taiwan.
Supporting Information Available: Complete crystal-lographic data of 2 and 4; crystal data are also available as CIF files. This material is available free of charge via the Internet at http://pubs.acs.org.
OM050281P
(23) Jeffery, J. C.; Rauchfuss, T. B. Tucker, P. A. Inorg. Chem. 1980,
19, 3306.
(24) Fujita, M.; Nagao, S.; Ogura, K. J. Am. Chem. Soc. 1995, 117, 1649.
(25) Seidel, S. R.; Stang, P. J. Acc. Chem. Res. 2002, 35, 972. (26) Kuehl, C. J.; Kryschenko, Y. K.; Radhakrishnan, U.; Seidel, S. R.; Huang, S. D.; Stang, P. J. Proc. Natl. Acad. Sci. U.S.A. 2002, 99, 4932.
Figure 3. Simplified ORTEP diagram of 4. Only the ring atoms are shown for clarity.
Communications Organometallics, Vol. 24, No. 14, 2005 3367
Downloaded by NATIONAL TAIWAN UNIV on August 19, 2009