Genotoxicity Tests of Poly(styrene-co-maleic anhydride)-Coated Silver Nanoparticles in vivo and in vitro
YT Chena,b, JH Wuc, FJ Tsaia,b, YW Changd, SH Hsue, JJ Line, HL Suef, JW Liaoc,*
a Genetic Center, Department of Medical Research, China Medical University Hospital,
Taichung, Taiwan.
b Department of Biomedical Informatics, Asia University, Taichung, Taiwan.
c Graduate Institute of Veterinary Pathobiology and Medicine, National Chung Hsing
University, Taichung, Taiwan.
d Institute of Toxicology, National Taiwan University, Taipei, Taiwan.
e Institute of Polymer Science and Engineering, National Taiwan University, Taipei, Taiwan.
f Department of Life Sciences, National Chung Hsing University, Taiwan.
Corresponding authors: Jiunn-Wang Liao
Tel: +886-4-2284-0894, Fax: +886-4-2286-2073, E-mail: jwliao@dragon.nchu.edu.tw
Abstract
Nano-silver is thought to hold potential for use in medical materials. The safety of newly developed poly(styrene-co-maleic anhydride)-coated silver nanoparticles (SMA-AgNPs) requires investigation. In this study, three in vitro and in vivo experiments for investigating genetic toxicity-the Ames test, a micronucleus assay, and a chromosome aberration test-were conducted. Results from Ames testing showed SMA-AgNPs to have a negative effect, either with or without S9 metabolism. In addition, SMA-AgNPs increased the number of reticulocytes and micronuclei in reticulocytes at 48 and 72 h after treatment. Indeed, SMA-AgNPs induced significant changes in the chromosomal aberration rate in CHOK1 cells. In conclusion, SMA-AgNPs did cause DNA damage in terms of chromosomal aberration and may have a potential genotoxic effect in certain applications.
1. Introduction
These days, many common products are made with silver, such as photo film. Silver is known for its antibacterial properties, and it has been applied in many areas because it is less toxic to human cells than it is to bacteria [1]. In recent years, nanoparticles have been used for a wide variety of applications in electronics, pharmaceuticals, biotechnology, and medicine [2]. Exposure to nanosilver can occur through inhalation, skin absorption, and ingestion in liquids [3]. Furthermore, medical supplies such as ointments and bandages containing nanosilver for wound healing are also available today [4]. In view of the growing popularity of nanomaterials, the Royal Society first warned in 2004 of the possible health risk of such compositions [5]. However, research knowledge regarding the mechanisms of nanosilver genotoxicity remains limited [6-7].
Poly(styrene-co-maleic anhydride) and its derivatives are a group of biocompatible polymers [8]. They have been used for conjugation with neocarzinostatin (NCS), a potent but highly toxic antitumor protein. Polymer conjugation has been shown to increase the plasma half-life of NCS and reduce its toxicity [9-10]. In another study, poly(styrene-co-maleic anhydride)-grafting poly(oxyalkylene) (SMA)-AgNPs was demonstrated in vitro to be safer and provides greater antibacterial effects [11]. However, few studies have investigated the interaction between nanosilver particles and the body after they have entered the body [12].
In this study, the potential genotoxicity of SMA-AgNPs was investigated by using the Ames test, a chromosome aberration test, and a micronucleus test. Results from this study can provide valuable information for the safe application of nanosilver in the future.
2.1. Nanosilver preparation
The procedure used in this study for SMA polymer synthesis follows the one previously described [13].The particle size of SMA-AgNPs was analyzed using transmission electron microscopy (TEM, 200EX, JEOL, Tokyo, Japan). The shape of SMA-AgNPs was spherical, and the diameter was found to be 8.6±0.2 nm (Fig. 1) [11]. The zeta potential value, as determined by the Delsa Nano particle analyzer (Beckman and Coulter, USA), was -40±4 mV. The chemical composition was 19.6% silver and 80.4% SMA, as determined by thermogravimetrical analysis (Perkin Elmer TGA7, USA).
2.2. Chemicals and reagents
The positive control (PC) mutagens were 4-nitroquinoline-N-oxide (4-NQO), sodium azide, 2-Aminoanthracene (2-AA), mitomycin C ethyl methanesulfonate, cyclophosphamide, acridine orange and colchicine, and were purchased from Sigma Co. (MO, USA). Histidine was obtained from Merck (Germany).
2.3. Ames test
The Ames test was performed as previously described [14-15]. Three test histidine-dependent Salmonella typhimurium strains (TA98, TA100, and TA1535) were used. The TA98 strain was used mainly to detect frameshift mutations; the TA100 and TA1535 strains were used to detect base-pair substitutions. The TA98 and TA100 strains (histidine needed a mutant) were purchased from the Bioresources Collection and Research Center (BCRC) (Hsinchu, Taiwan). The TA1535 strain was obtained from Discovery Partners International (DPI, CA, USA). The concentration of SMA-AgNPs with no bacterial toxicity was found at up to 0.8µg /plate for TA98, 1.6 µg /plate for TA100, and 0.32 µg /plate for TA1535 in the preliminary bacterial toxicity study. The Ames test (plate incorporation method) was
administered the SMA-AgNPs at doses of 0.05, 0.10, 0.20, 0.40, and 0.80 µg /plate for TA98, 0.10, 0.20, 0.40, 0.80, and 1.60 µg/plate for TA100, and 0.02, 0.04, 0.08, 0.16, and 0.32 µg/plate for TA1535. The tests were performed with or without the S9-fraction from Aroclor 1254-induced rat livers (36.5 mg/ml) (Moltox, TM, USA). The S9-fraction was prepared freshly by adjusting the S9-fraction to a final concentration of 10% with dilution buffer containing 4 mole nicotinamide adenine dinucleotide phosphate (sodium salt), 5 mole glucose-6-phosphate (mono sodium salt), 8 mole MgCl2 , 33 mole KCl, and 100 mole sodium phosphate buffer (pH 7.4). Briefly, 0.1 ml of distilled water (DW) -soluble sample was first added to culture tubes containing 2 ml of top agar (containing 0.5% NaCl), 0.2 ml of 0.5 mM histidine/biotin, and 0.1 ml of tester strain suspension. The sample-loaded cultures were then plated. Metabolic activation experiments were conducted using the rat liver S9-fraction at a volume of 0.5 ml in activation mixtures. Plates were incubated at 37 °C for 48 h. Control (DW) and PC experiments were also conducted. The PC mutagen incubation without S9-fraction treatment was 4-NQO (1 g /plate) for TA98 and sodium azide (5 µg /plate) for TA100 and TA1535. All tester bacterial strain incubation with S9-fraction in the PC groups otherwise used 2-AA (5 µg /plate). Test samples were assayed in triplicate at 5 separate concentrations, and 2 independent experiments were performed for each bacterial strain. 2.4. Micronucleus test
Six-week-old male mice (ICR strain, body weight, 25-35 g), obtained from Biolasco Taiwan Co., Ltd. (Yilan, Taiwan), were subjected to a general physical examination upon receipt and acclimatized for 1 wk. The animals were housed in cages (5 per cage) and provided with food (Lab Diet 5001 Rodent diet; Purina Mills LLC, St. Louis, MO, USA) and water ad libitum. The stainless steel cages were kept at 21 ± 2 °C with 50-70% humidity under a 12-h
light/12-h dark cycle. Tlight/12-his study was approved by tlight/12-he Institutional Animal Care and Use Committee (IACUC) of National Chung-Hsing University (IACUC: 96-34). The micronucleus assay was conducted as previously described [16-17]. Mice were divided into three groups, with 5 mice allocated randomly to each group. The low-dose group was administered 0.25 mg/kg SMA-AgNPs by intraperitoneal injection, and the high-dose group 1 mg/kg. The administered volume was 10 ml/kg body weight. The PC group was intraperitoneously injected with 50 mg/kg body weight of cyclophosphamide and DW as a control. After dosing, the animals were examined for mortality and clinical signs. The animals were then anesthetized using 2% isoflurane (Halocarbon Laboratories, USA), and 100 µl of orbital peripheral blood was withdrawn at 48 and 72 h. Slides were prepared for staining with 0.1% acridine orange. Reticulocytes (RETs) stained orange and micronuclei (MN) in RETs stained yellow-green on each slide were counted under a florescence microscope (BX50, Olympus, Münster, Germany). In total, 1000 RETs per animal were analyzed for the existence of MN. The ratio of RETs to normochromatic erythrocytes (NCEs) was determined based on 1000 NCEs. The to-NCE ratio was recorded while counting 1000 RETs per animal, and the MN-RETs/1000 RETs (‰) was calculated.
2.5. Chromosome aberration (CA) assay
To assess the ability of SMA-AgNPs to induce structural and numerical chromosome aberrations, an in vitro chromosome assay was applied to Chinese hamster ovary cell clone K1 (CHO-K1), according to methods described previously [18-19]. The CHO-K1 cells were obtained from the BCRC. Cells, 1.5 × 105 in a 25 cm2 flask, were seeded overnight prior to treatment. For cytotoxicity, 2.5, 5.0, 7.5, 10, and 20µg/ml of SMA-AgNPs were dissolved in culture media and incubated with cells for 24 h. Cultures treated with SMA-AgNPs were
incubated with the S9-fraction for 3 h before treatment. The PC reagent ethyl ethanesulfonate (3.14 mM) was used with S9-fraction incubation. After treatment for 18 to 21 h, the cells were harvested and then added to Colchicine (0.1 µg/ ml) for 3 h before harvesting. After trypsinization, the cells were treated with hypotonic solution (0.5% KCl) for 5 to 7 min at 37°C prior to incubation, fixed with an acetic acid and methanol (1:3) solution, dropped onto slides, air-dried, and stained with 10 % Giemsa. For the analysis of chromosomal aberrations, at least 100 metaphases were scored for each group [19-20]. The number of cells with damaged chromosomes was calculated as aberration rate (%) = (number of cells with damaged chromosomes/total number of cells examined) × 100. Two independent treatments were conducted.
2.6. Statistical analysis
Data are expressed as mean ± standard deviation. Statistical differences are evaluated by Student’s t-test. Differences are regarded as significant at p <0.05.
3. Results
3.1. Mutagenicity of SMA-AgNPs in Ames test
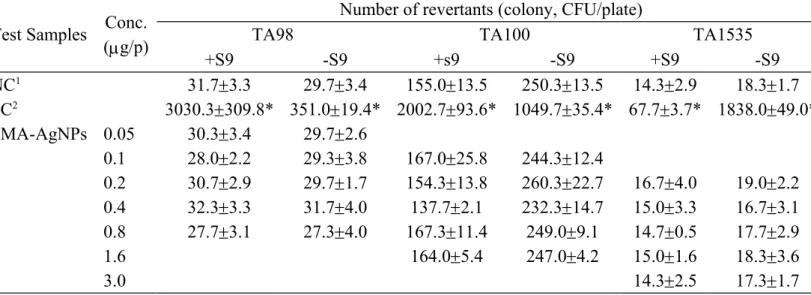
Three mutant Salmonella TA strains were tested for the SMA-AgNPs. Table 1 shows the Ames assay results for the tested SMA-AgNPs in TA98, TA100, and TA1535. SMA-AgNPs did not have mutagenic responses either with or without the S9-fraction in the 3 strain testers. The results indicate that the SMA-AgNPs did not cause mutagenic effects.
3.2. Effect of SMA-AgNPs on mouse micronuclei
In the micronucleus tests, mice were injected with SMA-AgNPs solution at 10 ml/kg body weight. Only the mice in the high-dose group (1 mg/kg) exhibited paleness and dullness. The
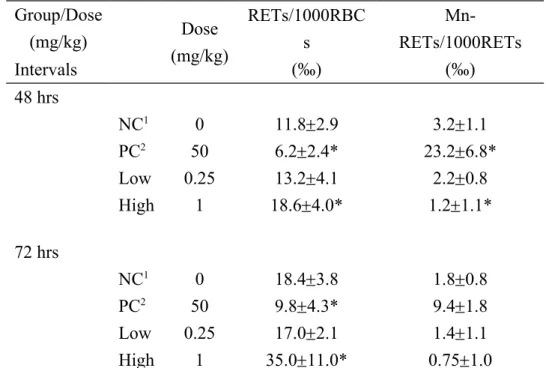
frequency of micronucleus ratios for all test animals was within the normal range of 1.8 to 3.2‰, except for that of the PCs, whose range increased to 9.4 to 23.2‰ at 72 h and 48 h. The experimental results showed that SMA-AgNPs did not induce micronucleus ratios, but increased the reticulocyte ratio in the high-dose group (Table 2).
3.3. Effect of SMA-AgNPs on chromosome aberration in CHO-K1 cells
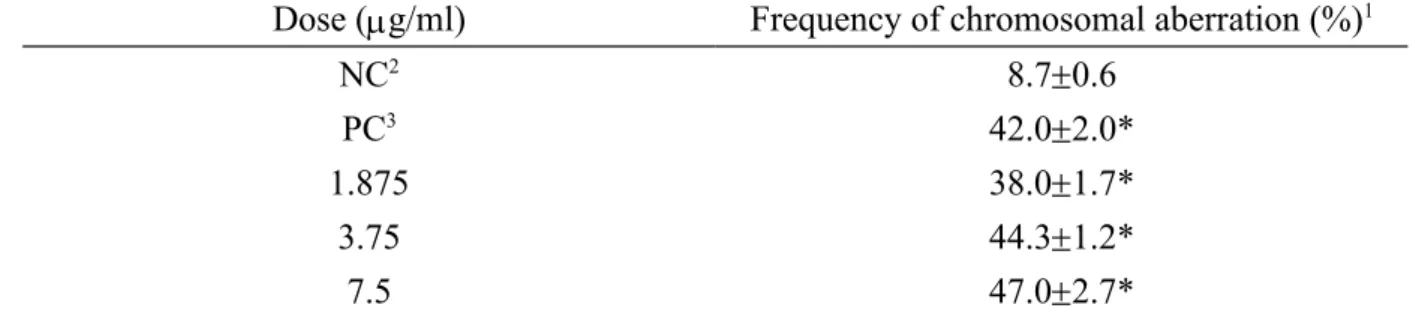
The chosen dosage of SMA-AgNPs in chromosome aberration was 7.5 µg/ml because the survival rate of CHO-K1 cells decreased to 80% when compared with control. With the S9-fraction treatment, the aberration rates of CHO-K1 cells were in the range of 38.0%, 44.3%, and 47.0%, and the PC was 42% (Table 3), indicating that the SMA-AgNPs had a detectable chromosomal aberration effect on CHO-K1 cells in vitro.
4. Discussion
Previous studies showed that nanosilver may cause acute cytotoxicity, especially in aquatic species and plant cells [21-22].Nanosilver by inhalation did not induce significant changes in the body weights of the male SD rats and showed no toxicity in either the micronucleus assay or the Ames test in this study [23]. The SMA-AgNPs particles used in this study were spherical, with a mean diameter of 15 nm, and SMA is a compound that facilitates the combination of nanosilver particles and solvents. In the micronucleus assay performed in this study, SMA-AgNPs caused clinical signs such as paleness and dullness and increased the number of RETs in the blood in only the high-dose (1 mg/kg) group; body weight in the same group decreased (data not shown) but not low-dose (0.25 mg/kg). A probable mechanism is that SMA-AgNPs injured several erythrocytes, causing a significant reduction in RBC number, which led to anemia. These phenomena may stimulate erythroblast cells in bone
marrow to produce more erythrocytes, even though the immature cells, then increased the RET ratio.
Nanosilver was found to induce cytotoxicity and genotoxicity in the cell line of Oryzias
latipes [21]. Nanosilver at 0.1 µg/ml concentration increased chromosomal aberrations
significantly in human mesenchymal stem cells (hMSCs), and the types of structural aberration are mainly chromatid deletions and exchanges [24]. In addition, the same study also demonstrated that nanosilver at 10 µg/ml increased cell migration [24]. However, the other assessed nanosilver did not induce toxic effects for human keratinocytes, using the MTT assay and Comet assay [25]. Our result showed SMA-AgNPs induced chromosome aberration at 1.875 µg/ml in CHO-K1 cells, the dosage were higher than nanosilver.
Oral nano-silver in rats did not result in the rats exhibiting clinical signs and cytotoxicity, but the nanosilver particles would exist throughout whole body tissues [26]. Size, shape, and coated compound are critical properties of nanosilver [27]. Another study observed that if nanosilver particles are coated with polysaccharides, the toxicity of the nanosilver will be increased [28]. Nanosilver disrupted the mitochondria, increased ROS production, and caused DNA damage. Although silver ion and nanosilver particles can induce ROS correlated with cyto- and genotoxicity, nanosilver induces a higher ROS level than silver ion does [29-30].Cell apoptosis occurs up to 6.25 µg/ml concentrations [31]. Such a dosage may elicit an inflammatory response, making it difficult for a wound to heal [32]. These findings provide useful information for the clinical application of nanosilver particles. To understand the properties of nanosilver fully, further studies are required. The medical application of nanosilver has developed rapidly in recent years, and the results from this study demonstrate that SMA-AgNPs are less genotoxic than nanosilver.
In summary, a CA test was conducted in this study, and the differences in concentrations were compared in the two experiments. Shedding light on nanosilver tolerance between these two cell lines facilitates the future application of nanosilver.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
This work is supported by the Department of Health for financially supporting this research under contract DOH 99-TD-N-111-012 and Ministry of Education, Taiwan, R.O.C. under the ATU plan.
References
1. A. Volker, B. Thorsten, S. Peter, W. Michael, S. Peter, D. Elvira, D. Eugen, and S, Reinhard, An in vitro assessment of the antibacterial properties and cytotoxicity of
nanoparticulate silver bone cement. Biomaterials. 25 (2004), pp. 4383-4391.
2. K.A.D. Guzman, M.P. Finnegan, and J.F. Banfield, Influence of surface potential on
aggregation and transport of titania nanoparticle. Environ. Sci. Technol. 40 (2006), pp.
7688-7693.
3. S.N. Luoma, Silver nanotechnologies and the environment: old problems or new
challenges? Project on Emerging Nanotechnologies reports PEN (2008), 15.
4. I.P. Margaret, L.L. Sau, K.M.P. Vincent, L. Ivan, and B. Andrew, Antimicrobial activities
of silver dressings: an in vitro comparison. J. Med. Microbiol. 55 (2006), pp. 59-63.
5. The Royal Society and the Royal Academy of Engineering. Environmental applications
and impacts of nanotechnology: summary of evidence presented to nanotechnology working group. 2003.
6. P.V. Asharani, G. Low KahMun, M.P. Hande, and S. Valiyaveettil. Cytotoxicity and
Genotoxicity of Silver Nanoparticles in Human Cells. American Chemical Society. 3
(2008), 279-290.
7. M. Ahamed, M.S. Alsalhi, and M.K.J. Siddiqui, Silver nanoparticle applications and
human health. Clin. Chim. Acta. 411 (2010), pp. 1841-1848.
8. I. Donati, A. Gamini, A. Vetere, C. Campa, and S. Paoletti, Synthesis, characterization,
and preliminary biological study of glycoconjugates of poly(styrene-co-maleic acid).
Biomacromolecules. 3 (2002), pp. 805-812.
poly(styrene-co-maleic acid) derivatives to the antitumor protein neocarzinostatin: pronounced improvements in pharmacological properties. J. Med. Chem. 28 (1985), pp. 455-461.
10. K. Greish, T. Sawa, J. Fang, T. Akaikea, and H. Maeda. SMA doxorubicin, a new
polymeric micellar drug for effective targeting to solid tumours. J. Cont. Release. 97
(2004), pp. 219- 230.
11. J.J. Lin, W.C. Lin, R.X. Dong, and S.H. Hsu, The cellular responses and antibacterial
activities of silver nanoparticles stabilized by different polymers. Nanotechnology.
23(2012), pp. 065102.
12. X. Chen, and H.J. Schluesener, Nanosilver: a nanoproduct in medical application. Toxicol. Lett. 176 (2008), pp. 1-12.
13. J.J. Lin, Y. C. Hsu, and K.L. Wei, Mechanistic aspects of clay intercalation with
amphiphilic poly(styrene-co-maleic anhydride)-grafting polyamine salts.
Macromolecules. 40 (2007), pp. 1579-1584.
14. K. Mortelmans, and E. Zeiger, The Ames Salmonella/ microsome mutagenicity assay. Mutat. Res. 455 (2000), pp. 29-60.
15. Organization for Economic Co-operation and Development (OECD), Bacterial Reverse
Mutation Test. In: OECD Guideline for the Testing of Chemicals. Section 4: Health
Effects 2001, 474.
16. G. Krishna, and M. Hayashi, In vivo rodent micronucleus assay: protocol, conduct and
data interpretation. Mutat. Res. 455 (2000), pp. 155-166.
17. Organization for Economic Co-operation and Development (OECD), Mammalian
erythrocyte micronucleus test. In: OECD Guideline for the Testing of Chemicals. Section
18. S.R. Musk, S.B. Astley, S.M. Edwards, P. Stephenson, R.B. Hubert, and I.T. Johnson,
Cytotoxic and clastogenic effects of benzyl isothiocyanate towards cultured mammalian cells. Food. Chem. Toxicol. 33 (1995), pp. 31-70.
19. Organization for Economic Co-operation and Development (OECD), In vitro mammalian
chromosome aberration test. In: OECD Guidelines for the Testing of Chemicals. OECD
Paris, Test Guideline 1997, 473.
20. S.M. Galloway, M.J. Aardeme, M. Ishidate Jr, J.L. Ivett, D.J. Kirkland, T. Morita, P. Mosesso, and Sofuni T, Report from working group on in vitro tests for chromosomal
aberrations. Mutat. Res. 312 (1994), pp. 241-261.
21. J.P.S. Wise, B.C. Goodale, S.S. Wise, G.A. Craig, A.F. Pongan, R.B. Walter, W.D. Thompson, and A.K. Ng, Silver nanospheres are cytotoxic and genotoxic to fish cells. Aquatic. Toxicol. 97(2010), pp. 34-41.
22. M. Kumari, A. Mukherjee, N. Chandrasekaran, Genotoxicity of silver nanoparticles in
Allium cepa. Science of the Total Environment. 2009, pp. 5243-5246.
23. J.S. Kim, J.H. Sung, J.H. Ji, K.S. Song, J.H. Lee, C.S. Kang, and J.J. Yu, In vivo
genotoxicity of silver nanoparticles after 90-day silver nanoparticle inhalation exposure.
Saf. Health Work. 2 (2011), pp. 34-38.
24. S. Hackenberg, A Scherzed, M. Kessler, S. Hummel, A. Technau, K. Froelich, C Ginzkey, C. Koehler, R. Hagen, and N. Kleinsasser, Silver nanoparticles: Evaluation of
DNA damage, toxicity and functional impairment in human mesenchymal stem cells.
Toxicol. Lett. 201 (2011), pp. 27-33.
25. W. Lu, D. Senapati, S. Wang, O. Tovmachenko, A.K. Singh, H. Yu, and P.C. Ray, Effect
Chem. Phys. Lett. 487 (2010), pp. 92-96.
26. Y.S. Kim, J.S. Kim, H.S. Cho, D.S, Rha, J.M. Kim, J.D. Park, B.S. Choi, R. Lim, H.K. Chang, Y.H. Chung, I.H. Kwon, J. Jeong, B.S. Han, and I.J. Yu, Twenty-eight-day oral
toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal. Toxicol. 20 (2008), pp. 575-583.
27. M. Auffan, J. Rose, J.Y. Bottero, G.V. Lowry, J.P. Jolivet, and M.R. Wiesner, Towards a
defnition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 4 (2009), pp. 634-641.
28. M. Ahamed, M. Karns, M. Goodson, J. Rowe, S.M. Hussain, J.J. Schlager, and Y. Hong,
DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol. Appl. Pharmacol. 233 (2008), pp. 404-410.
29. P.V. Asharani, G. Low KahMun, M.P. Hande, and S. Valiyaveettil, Cytotoxicity and
genotoxicity of silver nanoparticles. ACS Nano. 3 (2009), pp. 279-290.
30. R. Foldbjerg, D.A. Dang, and H. Autrup, Cytotoxicity and genotoxicity of silver
nanoparticles in the human lung cancer cell line A549. Arch. Toxicol. 85 (2011), pp.
743-750.
31. S. Arora, J. Jain, J.M. Rajwade, and K.M. Paknikar, Cellular responses induced by silver
nanoparticles: In vitro studies. Toxicol. Lett. 179 (2008), pp. 93-100.
32. J. Tian, K.K.Y. Wong, C.M. Ho, C.N. Lok, W.Y. Yu, C.M. Chen, J.F. Chiu, K.H.T. Paul,
Topical delivery of silver nanoparticles promotes wound healing. Chem. Med. Chem. 2
Figure. 1 Transmission electron micrograph of SMA-AgNPs. The particle size was 8.6 nm in average with a spherical shape. The scale bar is 200 nm.
Table 1.Revertant changes of SMA-AgNPs in TA98, TA100, and TA1535.
Test Samples Conc. (g/p)
Number of revertants (colony, CFU/plate)
TA98 TA100 TA1535
+S9 -S9 +s9 -S9 +S9 -S9 NC1 31.73.3 29.73.4 155.013.5 250.313.5 14.32.9 18.31.7 PC2 3030.3309.8* 351.019.4* 2002.793.6* 1049.735.4* 67.73.7* 1838.049.0* SMA-AgNPs 0.05 30.33.4 29.72.6 0.1 28.02.2 29.33.8 167.025.8 244.312.4 0.2 30.72.9 29.71.7 154.313.8 260.322.7 16.74.0 19.02.2 0.4 32.33.3 31.74.0 137.72.1 232.314.7 15.03.3 16.73.1 0.8 27.73.1 27.34.0 167.311.4 249.09.1 14.70.5 17.72.9 1.6 164.05.4 247.04.2 15.01.6 18.33.6 3.0 14.32.5 17.31.7
1 negative control was distilled water (DW)
2 positive reagents were without S-9 mix reactions were 1 g/plate 4-nitroquinoline-N-oxide (4-NQO)(TA98) or 5 g/plate sodium azide (TA100 or TA1535), with s-9 mix reactions were 5 g/plate 2-aminoanthracene. * Significant difference of colonies more than two folds of blank control and treated groups at p < 0.05.
Table 2. Changes of reticulocytes with micronuclei of mice after treated with SMA-AgNPs in the peripheral blood.
Group/Dose (mg/kg) Dose (mg/kg) RETs/1000RBC s (‰) Mn-RETs/1000RETs (‰) Intervals 48 hrs NC1 0 11.82.9 3.21.1 PC2 50 6.22.4* 23.26.8* Low 0.25 13.24.1 2.20.8 High 1 18.64.0* 1.21.1* 72 hrs NC1 0 18.43.8 1.80.8 PC2 50 9.84.3* 9.41.8 Low 0.25 17.02.1 1.41.1 High 1 35.011.0* 0.751.0 1 NC: negative control, PBS.
2 PC: positive control, 50 mg/kg Cyclophosphamide.
RETs: reticulocytes; RBCs: erythrocytes ; Mn-RETs: micronucleated reticulocytes ; * Significant difference between the negative control and treated groups at p < 0.05.
Table 3. Chromosomal aberration test with mammalian cell in cultured CHO-K1 cells of SMA-AgNPs.
Dose (g/ml) Frequency of chromosomal aberration (%)1
NC2 8.70.6
PC3 42.02.0*
1.875 38.01.7*
3.75 44.31.2*
7.5 47.02.7*
1 The number of cells with damage chromosomes was recorded from which the rate of mutation was calculated. Aberration rate (%) = (number of cells with damage chromosomes/100) × 100.
2 NC: negative control, PBS.
3 PC: positive control, 3.14 mM ethyl ethanesulfonate. * Significant difference between the ne